ABSTRACT
Purpose
To investigate the incidence of and predictive factors for recurrent cytomegalovirus retinitis (CMVR) in human immunodeficiency virus (HIV)-negative patients.
Methods
A retrospective review of HIV-negative patients who were newly diagnosed with CMVR between January 2005 and February 2019.
Results
Of 28 patients (44 eyes), 35.9% of eyes had a recurrence of CMVR after discontinuation of anti-CMV therapy. The incidence of CMVR recurrence was 17 per 100 eye-years. The factors significantly associated with CMVR recurrence were eyes with retinitis area of more than 25% (P = .013), absence of vitreous haze (P = .003), neutropenia at presentation (P = .001), and absence of systemic immunosuppression therapy prior to presentation (P = .002).
Conclusion
Eyes with a large area of retinitis, absence of vitreous haze, and neutropenia at presentation are predictive of CMVR recurrence while receiving systemic immunosuppression prior to CMVR presentation has a lower risk of CMVR recurrence.
Cytomegalovirus retinitis (CMVR) is an infection of the retina caused by cytomegalovirus (CMV), a member of the Herpesviridae family. CMVR commonly occurs as an opportunistic infection in patients with human immunodeficiency virus (HIV); however, it has also been reported in individuals with other immunosuppressed conditions, including hematologic malignancies, organ transplantation, and drug-induced immunosuppression.Citation1,Citation2 Over the last two decades, the use of highly active antiretroviral therapy has significantly reduced the incidence of CMVR in patients with HIV infection; however, a rising number of CMVR cases in HIV-negative patients have been reported.Citation3,Citation4 With improved survival rates among organ transplant patients and broader acceptance of immunosuppressive therapy, the incidence of CMVR in HIV-negative patients has increased, reportedly ranging from 3.7%-11.2%.Citation5–7
Management of CMVR in HIV-negative patients is challenging owing to the complicated underlying diseases and diverse individual immune statuses.Citation8 These often influence the provider’s decision for the treatment modality.Citation8 While the studies of CMVR in HIV-negative patients are still limited, the prevalence of CMVR recurrence has been reported to range from 0–33%.Citation9–11 Hence, we conducted this study to investigate the characteristics, incidence rate of CMVR recurrence, and factors that independently predict the recurrence of CMVR in HIV-negative patients.
Material and methods
Study population
We retrospectively reviewed the medical records of all patients who were newly diagnosed with CMVR at Siriraj Hospital, Bangkok, Thailand, between January 2005 and February 2019. All patients with CMVR were initially identified using a clinical log of patients who visited the CMVR clinic. Patients with underlying HIV infection were excluded from the study. The study protocol was approved by the Siriraj Institutional Review Board (SIRB) (COA no. 860/2561[EC3]). Written informed consent was not required owing to the retrospective nature of the study. This research posed a minimal risk, and the waiver did not adversely affect the rights and welfare of the participants. This study is registered in the Thai Clinical Trials Registry: TCTR20200421002.
Data collection
Demographic data including age at presentation, sex, laterality, systemic comorbidities, history of organ transplantation, systemic immunosuppression received within 3 months prior to presentation (corticosteroids, immunosuppressive drugs, and chemotherapy), and follow-up duration were collected. Initial best-corrected visual acuity (BCVA) at the time of CMVR diagnosis and BCVA at 6 months follow-up were measured using the Early Treatment of Diabetic Retinopathy Study chart or Snellen chart. BCVA was subsequently converted to the logarithm of the minimum angle of resolution (logMAR) scale before data analysis. We assigned a logMAR unit of 2.2 for counting fingers, 2.3 for hand motion, 2.4 for light perception, and 2.5 for no light perception.Citation12 Furthermore, detailed ophthalmic slit-lamp examination findings at enrollment including grading of anterior chamber cells, vitreous haze, and vitreous cells, were collected.Citation13,Citation14
According to the Standardization of Uveitis Nomenclature (SUN) classification criteria for CMVR, the diagnosis in our study was based on characteristic clinical findings.Citation15 The confirmatory polymerase chain reaction (PCR) testing for CMV was used in eyes with atypical presentation or upon the clinician’s request. The location of the CMVR lesion was classified into three zones.Citation16 Zone 1 is described as the area within 1500 μm of the optic nerve or within 3000 μm of the fovea; zone 2 as an extension beyond zone 1 to the vortex veins; and zone 3 locates anterior to the vortex veins. The lesion size was graded using information from retinal drawing records and/or fundus photographs and categorized as <25% or ≥25% of the retina involved by CMVR lesions.Citation17
Initial laboratory test results that were available closest to the time of enrollment were recorded, including hematocrit (Hct), absolute neutrophil count (ANC), absolute lymphocyte count (ALC), platelet count, serum glomerular filtration rate (GFR), serum albumin, and hemoglobin A1C (HbA1C). Furthermore, we defined the cut-off value of abnormal laboratory results as follows; anemia as Hct < 33%; neutropenia as ANC < 1,000 cells/µL; lymphopenia as ALC < 1,000 cells/µL; thrombocytopenia as platelet < 100,000 cells/µL; impaired kidney function as GFR < 60 mL/min/1.73 mm2; hypoalbuminemia as albumin < 3.5 g/dL; poorly controlled diabetes mellitus as HbA1C > 7%.
Treatment mainly consisted of systemic anti-CMV agents, such as ganciclovir, valganciclovir, or foscarnet. Initially, patients were administered with a higher dosage of medication during the 2- or 3-week induction phase, followed by a maintenance dosage until CMVR lesions appeared quiescent. The dose of systemic medications was adjusted for patients with impaired kidney function or those who developed drug toxicity. Intravitreal injections (IVI), including ganciclovir 2 mg/0.04 mL and foscarnet 2.4 mg/0.1 mL, were used as an adjunctive therapy or monotherapy, depending on the severity and extension of CMVR and the judgment of the provider. The ocular and systemic complications that occurred during the follow-up period were also recorded.
Regarding the classification of the CMVR clinical activity in our study, the resolution of CMVR was defined as “all lesions becoming atrophic scars without evidence of active retinitis.” Remission was recorded if the inactive disease was noted for at least 3 months after discontinuation of anti-CMV agents.Citation13 Recurrence of CMVR was defined as a reappearance of active retinitis lesions after a period of disease resolution and discontinuation of anti-CMV therapy for at least 4 weeks.Citation18
Outcome measures
The primary outcome was the incidence rate of recurrent CMVR in non-HIV patients. The secondary outcomes were the predictive factors for the recurrence of CMVR.
Statistical analyses
All data analyses were performed using R program version 4.1.0 (The R Group, Vienna, Austria) with Epicalc software. Patient demographics and clinical characteristics were summarized using descriptive statistics. Pearson’s chi-square test or Fisher’s exact test was used to compare the categorical data, and the Mann-Whitney U test was used to compare the continuous data. Categorical data are reported as numbers and percentages. Continuous data were shown as mean ± standard deviation (SD) or as median (interquartile range; IQR) based on data distribution. The probability of CMVR recurrence was assessed using Kaplan-Meier survival analysis, censoring eyes without events at the last known follow-up. A Cox proportional hazards model with robust estimation was used to identify variables independently associated with the recurrence of CMVR. Variables with P < .05 in univariate analyses were included in the multivariate model. Backward elimination was used to exclude variables from the model unless they improved the fit of the model. P < .05 was considered to be statistically significant.
Results
Study population
Thirty HIV-negative patients with CMVR were initially identified from a review of medical records during the study period. Of these patients, we excluded one patient with previously treated CMVR and another patient who had incomplete medical records. Therefore, a total of 28 patients (44 eyes) were included in this study.
Demographic data and underlying comorbidities
shows the demographics and underlying systemic comorbidities of the 28 patients.
Table 1. Demographic and systemic comorbidities of HIV-negative cytomegalovirus retinitis (CMVR) patients.
The male-to-female ratio was 1:1.75, and more than half of the patients had bilateral disease (57.1%). Hematologic malignancies were the most frequently associated comorbidities (46.4%), of which non-Hodgkin lymphoma was the most common diagnosis (69.2%). Six of seven patients with diabetes mellitus had other coexisting comorbidities (four with hematologic malignancies, one with kidney disease, and one with transverse myelitis). Seven patients (25%) had prior organ transplantation (two with solid organ transplantation and five with hematopoietic stem cell transplantation). One patient (aged 46 years) reported no evidence of specific underlying diseases after a complete laboratory workup for malignancies and autoimmune disorders. At the time of CMVR diagnosis at our clinic, 20 patients (71.4%) had previously received systemic corticosteroids, immunosuppressive drugs, or chemotherapy within 3 months prior to the visit.
Clinical characteristics and laboratory results
Among the 28 patients, the frequent complaints were decreased vision (89.3%), followed by floaters (7.1%). The median duration of symptoms before diagnosis was 0.92 months (IQR 0.2–2.5). Thirty-nine eyes of 25 patients were reported to have a resolution of retinitis during the study period. Of these, 14 eyes of ten patients were reported to have CMVR recurrence (35.9%). The median onset of CMVR recurrence after discontinuation of anti-CMV medications was 2.6 months (IQR 1.4–4.6). The overall median follow-up duration was 19.25 months (IQR 8.2–52.7). presents the clinical characteristics of the 44 eyes at the time of CMVR diagnosis classified according to disease recurrence. At presentation, 75%, 63.6%, and 56.8% of the eyes presented with anterior chamber inflammation, vitreous cells, and vitreous haze, respectively. A higher proportion of patients without recurrence of CMVR initially presented with the more severe anterior chamber and vitreous inflammation. At the initial visit, the mean VA was 0.84 logMAR, which improved to 0.27 logMAR at the 6-month follow-up. During the 6-month follow-up period, an additional two eyes from two patients had decreased VA to ≤20/200 after CMVR recurrence, compared to the initial visit. In the group without CMVR recurrence, three patients (five eyes) were lost to follow-up at 6 months, while the proportion of eyes with VA ≤20/200 decreased, and one eye experienced an improvement in VA to >20/50 compared to the initial visit ().
Table 2. Characteristics at presentation of eyes with HIV-negative cytomegalovirus retinitis patients.
shows laboratory results near or at the time of CMVR diagnosis. There was no difference in laboratory test results between the patients with and without CMVR recurrence. Among patients with recurrent CMVR, only one patient had prior organ transplantation. Aqueous PCR for CMV deoxyribonucleic acid (DNA) was collected in 19 patients (19 eyes), and all specimens showed positive results for CMV. CD4+ T-cell count at the time of CMVR diagnosis was recorded in three patients and showed the value of 44, 673, and 747 cells/µL.
Table 3. Baseline laboratory results of eyes with HIV-negative cytomegalovirus retinitis patients.
Treatment
summarizes the treatment modalities for eyes with HIV-negative cytomegalovirus retinitis patients. Eighteen patients (26 eyes) had received combined systemic and intravitreal anti-CMV therapy, five patients (eight eyes) had received systemic anti-CMV agents alone, and eight eyes from five patients had been treated with IVI monotherapy. Two eyes from two patients (4.5%) did not receive systemic or IVI therapy because of small granular retinitis in the periphery (zone 3) with spontaneous resolution of retinitis when observed. No significant difference in treatment routes was found between patients with recurrence of CMVR and those without recurrence (P = .107). The mean duration of treatment with systemic anti-CMV agents was 3.67 ± 6.0 months, and the mean total IVI was 10.1 ± 13.9 injections. The patients with recurrence of CMVR had a significantly higher mean total number of IVI than those without recurrence (22.2 ± 18.8 and 4.4 ± 5.0 injections, respectively, P < .001). The mean duration of anti-CMV therapy for all patients was 7.7 ± 12.8 months.
Table 4. Treatment modalities of eyes with HIV-negative patients before resolution or developing recurrence of cytomegalovirus retinitis (CMVR).
Complications
Optic atrophy was the most common ocular complication recorded, occurring in 22.7% of cases. It developed during the course of the disease and before the recurrence episode in all eyes with CMVR recurrence. Rhegmatogenous retinal detachment was recorded in three eyes (6.8%), and cataract formation requiring cataract surgery during the follow-up period was noted in six eyes (13.6%). In addition, 11 patients developed neutropenia while receiving either systemic ganciclovir or valganciclovir. Among these patients, three required cessation of systemic anti-CMV medication and IVI was used as the main therapy. During the follow-up period, three patients died from septic shock (one had aplastic anemia with graft-versus-host disease, one had relapsed diffuse large B-cell lymphoma, and one had relapsed mantle cell lymphoma). The data for all the patients are summarized in Supplemental Table 1.
CMVR recurrence
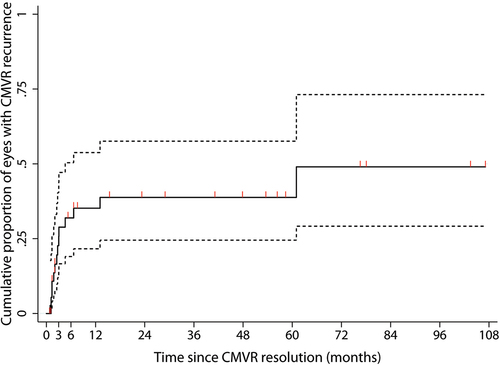
Five eyes from three patients were recorded as having active retinitis until the last follow-up date (one patient died from disseminated CMV and septic shock, and two patients were lost to follow-up). Thirty-nine eyes had CMVR resolution and 14 eyes developed CMVR recurrence. demonstrates that the cumulative incidence of CMVR recurrence at 3, 6, 12, and 24 months was 22.7% (95% confidence interval [CI], 12–40.4), 32% (95% CI, 19.1–50.4), 35.2% (95% CI, 21.6–53.8), and 38.8% (95% CI, 24.5–57.6), respectively. Then, the cumulative percentage of recurrence was stable for 5 years before increasing to 49% (95%CI, 29.2–73.1) until the last follow-up. The calculated incidence rate of CMV recurrence was 0.17/eye-year. Regarding the treatment modalities, eyes treated with combined systemic and IVI anti-CMV therapy had a recurrence rate of 0.22/eye-year and eyes treated with IVI monotherapy had a recurrence rate of 0.20/eye-year. Two eyes without treatment and eight eyes treated with systemic anti-CMV therapy did not show CMVR recurrence during the follow-up period.
Predictors of CMVR recurrence
In total 39 eyes with CMVR resolution were analyzed using the Cox regression model. summarizes all the variables analyzed in the univariate model. According to the univariate analysis, eyes with a retinitis area of more than 25%, absence of vitreous haze, neutropenia at presentation, and absence of systemic immunosuppression therapy within 3 months before presentation were significantly associated with CMVR recurrence (P = .025, .011, .001, and .014, consecutively). Significant variables were subsequently analyzed in the multivariate logistic regression model and all variables remained significantly associated with the recurrence of CMVR, including eyes with retinitis area of more than 25% (P = .013; adjusted hazard ratio [aHR], 5.74; 95% CI, 1.45–22.76), absence of vitreous haze (P = .003; aHR, 5.23; 95% CI, 1.78–15.31), neutropenia at presentation (P = .001; aHR, 6.99; 95% CI, 2.18–22.38), and absence of systemic immunosuppression therapy within 3 months prior to presentation (P = .002; aHR, 5.36; 95% CI, 1.83–15.69).
Table 5. Predictive factors of eyes with recurrent cytomegalovirus retinitis (CMVR) in HIV-negative patients.
Discussion
The present study revealed that one-third of patients developed recurrence of CMVR after discontinuation of anti-CMV therapy with an incidence rate of 0.17/eye-year. Patients who developed CMVR recurrence tended to present with less intraocular inflammation and less improvement in VA at 6 months compared to those without recurrence. Multivariable analyses showed that predictive factors for CMVR recurrence were significantly associated with large size of retinitis, absence of vitreous haze, and neutropenia at presentation. Receiving systemic immunosuppression within three months before the presentation was significantly associated with a lower risk of CMVR recurrence.
Regarding CMVR in HIV-infected patients, the decision to discontinue anti-CMV therapy depends on the clinical quiescence of the disease and persistent immune recovery with CD4 + T-cell counts ≥100 cells/µL for at least 6 months.Citation19 On the contrary, no consensus on the optimal treatment guideline for discontinuing anti-CMV therapy in non-HIV patients.Citation1,Citation4 Our study showed the overall rate of CMVR recurrence after cessation of anti-CMV therapy was quite high compared to other studies.Citation9–11 Jeon et al. reported no CMVR recurrence in 23 eyes of 15 HIV-negative patients during the follow-up period of at least 1 year following the treatment with combined systemic and intravitreal anti-CMV therapy,Citation5 whereas Iu et al. reported up to 33.3% of CMVR recurrence from 20 eyes of 13 patients treated with the same treatment modality as that of Jeon et al.Citation10 Agrawal et al. used intravitreal monotherapy to decrease the side effects from systemic anti-CMV therapy and demonstrated that CMVR recurrence occurred in only one eye out of 18 eyes (10 patients).Citation11 These findings suggest that different routes of anti-CMV therapy may not affect CMVR recurrence, which is consistent with our study, in which no significant differences in CMVR recurrence were found among the different routes of anti-CMV therapy.
Iu et al. investigated the risk factors of CMVR recurrence using logistic regression analyses.Citation10 The results showed no significant association between CMVR recurrence and duration of systemic or IVI therapy, number of IVI, the severity of vitreous haze, or size of retinitis. However, the present study showed that eyes with a more extensive area of retinitis and an absence of vitreous haze were associated with a greater risk of CMVR recurrence. A large area of CMVR lesion was previously reported to be significantly related to poor visual prognosis in both HIV and non-HIV patients.Citation17,Citation20 We speculate that not only the large area of retinitis might reflect a more severe disease, but it might also represent the more area of the retina infected with CMV, thus increasing the risk of CMVR reactivation, particularly in patients with immunosuppressed conditions.
Regarding intraocular inflammation, vitritis was reported to vary from absent to present in all patients.Citation8,Citation10,Citation21,Citation22 The proportion of vitreous haze in our study was similar to the study of Iu et al.Citation10 The degree of intraocular inflammation of CMVR in non-HIV patients depends on individuals presenting with different pre-existing conditions of immunosuppression. The absence of vitreous haze represents an impaired immune response, that may facilitate further CMV reactivation.
Patients with neutropenia at presentation also had a significantly higher risk of CMVR recurrence. Neutropenia could delay the immune reconstitution of the patients and limit the use of systemic ganciclovir or valganciclovir.Citation4 These may lead to inadequate control of CMV infection in some patients and result in disease reactivation.
Interestingly, patients who had received systemic corticosteroids, immunosuppressive drugs, or chemotherapy within 3 months before presentation were found to have a lower risk of CMV recurrence. After analyzing the data, we discovered that these patients’ immunosuppressive agents were discontinued or reduced and chemotherapy was postponed once active CMVR was diagnosed. Our findings align with those of Kuo et al., who reported that HIV-negative patients who were able to stop immunosuppressive therapy had no subsequent CMVR recurrence.Citation22 The immune function of non-HIV patients increased when the dose of immunosuppressive therapy is adjusted or tapered off.Citation11 This may explain the reduction in CMVR recurrence in these patients.
This study has several limitations. First, the retrospective nature of the study, in which the follow-up duration and treatment protocol were not standardized. Second, the medical records of the patients were complicated by diverse systemic comorbidities with individualized management. Third, in terms of predictive factors, our study did not analyze some specific factors, including aqueous CMV DNA load and aqueous interleukin (IL)-8, which might be quantitative indicators related to the treatment duration and CMVR reactivation reported in previous studies.Citation23,Citation24 Although CMVR in HIV-negative cases is not common, our study recruited a relatively large number of cases and showed the strength of the results with statistical significance. With a pattern of CMVR changing toward a rising number of non-HIV patients, we believe the results of the present study could help understand cases of recurrent CMVR and the predictors of recurrence to further improve the practice outcome.
In conclusion, our study found that CMVR recurrence can occur in up to one-third of HIV-negative patients and may impact the final visual outcome. Close monitoring of patients presenting with large retinitis, absence of vitreous haze, or neutropenia, particularly within the first three months after stopping anti-CMV therapy, is important. Additionally, adjusting immunosuppressive agents to restore immune function in these patients before discontinuing anti-CMV therapy may help reduce the risk of recurrence. Further research is needed to identify effective strategies for reducing the risk of CMVR recurrence and improving patient outcomes.
Supplemental Material
Download Zip (33.1 KB)Acknowledgments
The authors gratefully acknowledge Ms. Parichat Damthongsuk for her assistance with statistical analysis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Data are available upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/09273948.2023.2170250
Additional information
Funding
References
- Munro M, Yadavalli T, Fonteh C, Arfeen S, Lobo-Chan A-M. Cytomegalovirus retinitis in HIV and non-HIV individuals. Microorganisms. 2019;8:E55. doi:10.3390/microorganisms8010055.
- Gupta S, Vemulakonda GA, Suhler EB, et al. Cytomegalovirus retinitis in the absence of AIDS. Can J Ophthalmol. 2013;48:126–129. doi:10.1016/j.jcjo.2012.12.002.
- Whitcup SM. Cytomegalovirus retinitis in the era of highly active antiretroviral therapy. JAMA. 2000;283:653–657. doi:10.1001/jama.283.5.653.
- Shapira Y, Mimouni M, Vishnevskia-Dai V. Cytomegalovirus retinitis in HIV-negative patients-associated conditions, clinical presentation, diagnostic methods and treatment strategy. Acta Ophthalmol (Copenh). 2018;96:e761–7. doi:10.1111/aos.13553.
- Jeon S, Lee WK, Lee Y, Lee DG, Lee JW. Risk factors for cytomegalovirus retinitis in patients with cytomegalovirus viremia after hematopoietic stem cell transplantation. Ophthalmology. 2012;119:1892–1898. doi:10.1016/j.ophtha.2012.03.032.
- Yan C-H, Wang Y, Mo X-D, et al. Incidence, risk factors, and outcomes of cytomegalovirus retinitis after haploidentical hematopoietic stem cell transplantation. Bone Marrow Transplant. 2020;55:1147–1160. doi:10.1038/s41409-020-0790-z.
- Shoeibi N, Abrishami M, Mohammad Esmaeil E, Hosseini SM. Visual prognosis, clinical features, and predisposing factors in non-HIV patients with cytomegalovirus retinitis. Int Ophthalmol. 2019;39:1709–1715. doi:10.1007/s10792-018-0991-2.
- Vishnevskia-Dai V, Shapira Y, Rahav G, Shimoni A, Somech R, Moisseiev J. Cytomegalovirus retinitis in HIV-negative patients: a practical management approach. Ophthalmology. 2015; 122(866–868.e3): doi: 10.1016/j.ophtha.2014.11.010.
- Jeon S, Lee WK. Cytomegalovirus retinitis in a human immunodeficiency virus-negative cohort: long-term management and complications. Ocul Immunol Inflamm. 2015;23:392–399. doi:10.3109/09273948.2014.985385.
- Iu LP, Fan MC, Lau JK, Chan TS, Kwong Y-L, Wong IY. Long-term follow-up of cytomegalovirus retinitis in non-HIV immunocompromised patients: clinical features and visual prognosis. Am J Ophthalmol. 2016;165:145–153. doi:10.1016/j.ajo.2016.03.015.
- Agarwal A, Kumari N, Trehan A, et al. Outcome of cytomegalovirus retinitis in immunocompromised patients without Human Immunodeficiency Virus treated with intravitreal ganciclovir injection. Graefes Arch Clin Exp Ophthalmol. 2014;252:1393–1401. doi:10.1007/s00417-014-2587-5.
- Pistilli M, Joffe MM, Gangaputra SS, et al. Visual acuity outcome over time in non-infectious uveitis. Ocul Immunol Inflamm. 2019;27:1–8. doi:10.1080/09273948.2019.1571775.
- Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–516.
- Multicenter Uveitis Steroid Treatment Trial Research Group, Kempen JH, Altaweel MM, Holbrook JT, Jabs DA, Sugar EA. The multicenter uveitis steroid treatment trial: rationale, design, and baseline characteristics. Am J Ophthalmol. 2010;149:550–561.e10.
- Standardization of Uveitis Nomenclature (SUN) Working Group. Classification criteria for cytomegalovirus retinitis. Am J Ophthalmol. 2021;228:245–254. doi:10.1016/j.ajo.2021.03.051.
- Holland GN, Buhles WC, Mastre B, Kaplan HJ. A controlled retrospective study of ganciclovir treatment for cytomegalovirus retinopathy. Use of a standardized system for the assessment of disease outcome. UCLA CMV retinopathy. Study Group. Arch Ophthalmol. 1989;107:1759–1766. doi:10.1001/archopht.1989.01070020841024.
- Sittivarakul W, Seepongphun U. Incidence rates and risk factors for vision loss among AIDS-related cytomegalovirus retinitis patients in southern Thailand. Ocul Immunol Inflamm. 2018;26:82–89. doi:10.1080/09273948.2017.1283044.
- Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017;64:87–91. doi:10.1093/cid/ciw668.
- Holbrook JT, Colvin R, van Natta ML, et al. Evaluation of the United States public health service guidelines for discontinuation of anticytomegalovirus therapy after immune recovery in patients with cytomegalovirus retinitis. Am J Ophthalmol. 2011;152(628–637.e1):628–637.e1. doi:10.1016/j.ajo.2011.04.007.
- Qian Z, Chen X, Tao Y, Li W, Gu W. Prognostic factors of cytomegalovirus infection associated retinitis in HIV-negative patients: a retrospective cohort study. Ocul Immunol Inflamm. 2021;29:154–159. doi:10.1080/09273948.2019.1659978.
- Pathanapitoon K, Tesavibul N, Choopong P, et al. Clinical manifestations of cytomegalovirus-associated posterior uveitis and panuveitis in patients without human immunodeficiency virus infection. JAMA Ophthalmol. 2013;131:638–645. doi:10.1001/jamaophthalmol.2013.2860.
- Kuo IC, Kempen JH, Dunn JP, Vogelsang G, Jabs DA. Clinical characteristics and outcomes of cytomegalovirus retinitis in persons without human immunodeficiency virus infection. Am J Ophthalmol. 2004;138:338–346. doi:10.1016/j.ajo.2004.04.015.
- Qian Z, Fan H, Chen X, Tao Y. The predictive value of interleukin-8 in the development of cytomegalovirus retinitis in HIV-negative patients. Ophthalmic Res. 2020. doi:10.1159/000513791.
- Miao H, Hou J. Aqueous cytomegalovirus DNA and interleukin-8 determination in management of cytomegalovirus retinitis. Acta Ophthalmol (Copenh). 2019;97:e483–4. doi:10.1111/aos.13787.