ABSTRACT
Purpose
To evaluate and compare the blood lipid profile in retinal artery occlusion (RAO) and retinal vein occlusion (RVO).
Methods
We included 82 RAO patients and 95 RVO patients in this retrospective case–control study. Controls were matched to RAO or RVO patients at a 1:1 ratio, respectively. Associated lipid variates were analyzed in multivariable logistic regression models.
Results
LDL-C (OR = 1.69), non-HDL-C (OR = 1.87), and ApoB (OR = 11.72) individually significantly increased the risk of RAO. ApoA1 was associated with RVO (OR = 0.02), and with 75.8% sensitivity and 67.4% specificity. TG (OR = 1.61), LDL-C (OR = 1.69), non-HDL-C (OR = 1.91), and ApoB (OR = 12.12) each significantly increased the risk of RAO when compared with RVO.
Conclusions
ApoB, non-HDL-C, and LDL-C may be potential biomarkers in RAO patients. Low ApoA1 is an independent risk factor for the development of RVO.
Retinal vascular occlusions, which include retinal artery occlusion (RAO) and retinal vein occlusion (RVO), are common causes of severe vision loss.Citation1 RAO is an ophthalmic emergency with a devastating visual functional outcome and needs urgent treatment.Citation2 RVO is the second most common retinal vascular disease after diabetic retinopathy, causing painless visual impairment and blindness.Citation3 Both RAO and RVO are associated with increased cardiovascular morbidity and mortality.Citation4 Although retinal vascular occlusions share similar risk factors, such as atherosclerosis, hypertension and diabetes, RAO and RVO had markedly different pathogenesis, clinical characteristics, treatment, and prognosis.Citation1,Citation5 Prevalence between RAO and RVO is also different, with RVO having a higher prevalence and a better prognosis.
Although lipoprotein particles play an important role in the process of atherogenesisCitation6 and chronic inflammatory status,Citation7 fewer reports have analyzed the relationship between blood lipid profiles and RAO or RVO.Citation8–10 In addition, compared data about the precise role of dyslipidemia between RVO and RAO are unavailable. Detecting the different lipid profiles may be useful for understanding the different pathogenesis and providing reasonable precautions for RVO and RAO. Hence, the purpose of this study was to evaluate and compare the blood lipid profile biomarkers in retinal vascular occlusion (RAO and RVO) patients.
Materials and methods
This retrospective case–control study was conducted at Renmin Hospital of Wuhan University. Digital medical records of RAO or RVO patients from January 2020 to July 2021 were analyzed. The diagnoses of RAO and RVO were following previously published guidelines.Citation1 Patients aged more than 18 years old with signs of an acute stage of RAO or RVO were included in the study. Cataract patients from the same period, with no history of ocular diseases, were chosen as the control group (defined as A). The controls (defined as B) matched RAO patients at a 1:1 ratio based on age, sex, concomitant diabetes mellitus, and hypertension, with propensity scores matching method, were randomly selected from A. The 1:1 controls of RVO patients were randomly selected from the rest controls (calculated by A minus B) and also matched with age, sex, combined diabetes mellitus, and hypertension, with propensity scores matching method. Participants with a history of autoimmune diseases, liver disease, cardiovascular disease, chronic obstructive pulmonary disease, renal failure, acute or chronic infection, malignancy, ocular trauma, any surgery within 3 months, and drugs used such as steroid, nonsteroidal anti-inflammatory drugs, anticoagulant medications, and oral contraceptives were excluded.
All three groups underwent a comprehensive ocular examination including best-corrected visual acuity, slit-lamp examination, intraocular pressure measurement, and dilated fundus examination. Information on baseline demographics, history of operation or trauma, medical condition, drug usage, and the status of smoking and alcohol consumption was also collected. As a routine part of our clinical practice, venous blood samples were taken from all patients after overnight fasting in the morning at first admission. The lipid profiles (triglycerides [TG], total cholesterol, high-density lipoprotein cholesterol [HDL-C], low-density lipoprotein cholesterol [LDL-C], apolipoprotein A1 [ApoA1], and apolipoprotein B [ApoB]) were measured. Non-HDL-C was calculated as total cholesterol minus HDL-C. Patients with hypertension were defined as those with blood pressure above 140/90 mmHg measured on two or more separate occasions or who had a hypertension history. Diabetes was defined as having a history of diabetes. Smoking/drinking status was divided into current/no smokers/drinkers. Our study was approved by the Ethical Committee Board of Renmin Hospital of Wuhan University (approval number 2020-X-58) and followed the tenets of the Declaration of Helsinki. Because this was a retrospective observational study, the requirement for informed consent from eligible patients was waived by the Ethics Committee of the local institution.
Statistical analysis
SAS software (SAS 9.4; SAS Institute Inc., Cary, North Carolina, USA) was used for statistical analysis. Continuous data were described as median ± SD and compared with t-test or Wilcoxon test. Frequency variables were recorded as numbers and percentages and compared by χ2 test. Receiving operating characteristic (ROC) curve was performed to calculate the sensitivity and specificity of the significant blood lipid profile analyzed in the univariate analysis, and investigate the optimal cutoff value for RAO and RVO predictions. The optimal cutoff value for sensitivity and specificity was calculated according to the maximal Youden Index. The areas under the ROC (AUROC) curves were used to demonstrate the predictive validity. Spearman’s rank correlation coefficient (γ) between lipid subclasses was also calculated. Multivariable logistic regression models adjusting for age, sex, hypertension, diabetes, and smoking status were used to evaluate the relation between RAO and RAO and lipid profiles. Odds ratios (OR) and 95% confidence intervals (CI) were calculated. A p value (two-sided) ≤0.05 was considered statistical significance.
Results
Blood lipid biomarkers in RAO
Our study included 82 newly diagnosed RAO patients and 82 matched controls. Demographic characteristics and blood parameters are summarized in . The mean age of RAO group and control group was 58.8 ± 11.7 and 58.8 ± 18.1 years old, respectively (p = .148). The two groups were similar regarding current smokers (p = 1.000) and current drinkers (p = .755).
Table 1. Characteristics of patients with retinal artery occlusion (RAO) and control.
In univariate analysis, TG (p = .009), non-HDL-C (p = .002), and ApoB (p = .005) were significantly higher in RAO patients than in the control group. LDL-C and Apo A1 were statistically similar between the two groups (all comparisons p > .05).
The Spearman correlation coefficients for the lipid profiles in RAO patients are shown in the Supplement. ApoB, LDL-C, and non-HDL-C were highly correlated (γ ≥ 0.79). TG was mediumly correlated with ApoB (γ = 0.40) and non-HDL-C (γ = 0.56), while not correlated with HDL-C (γ = 0.12).
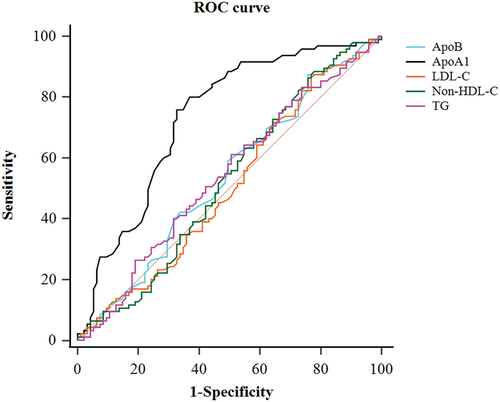
ROC curve analysis results for the lipid parameters are presented in . Non-HDL-C has the biggest AUROC (0.640), with 58.5% sensitivity and 69.5% specificity (). Compared among these AUROCs, a significant difference was only detected between non-HDL-C and LDL-C (p = .034).
Figure 1. Receiver operating characteristics curve (ROC) analysis of key lipid parameters for retinal artery occlusion.
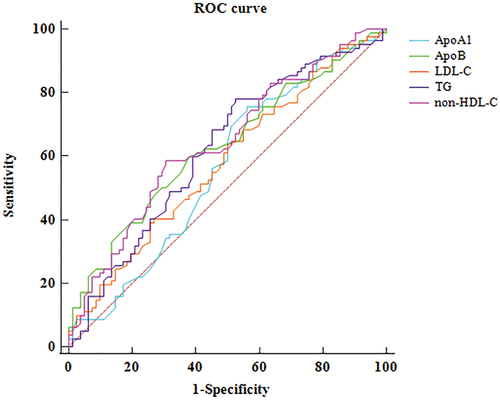
Table 2. Receiver operator characteristic (ROC) curve analysis for lipid profiles in different groups.
Multivariate logistic regression analysis, adjusting age, sex, hypertension, diabetes, and smoking status, indicated that LDL-C (OR = 1.69), Non-HDL-C (OR = 1.87), and ApoB (OR = 11.72) individually significantly increased the risk of RAO ().
Table 3. Logistic regression analysis of independent lipid profile for identification of retinal vascular occlusions.
Blood lipid biomarkers in RVO
Ninety-five newly diagnosed RVO patients with a mean age of 58.1 ± 12.3 years old and 95 matched cataract patients with a mean age of 54.7 ± 20.7 years old were included in this study. The demographic data and blood lipid parameters are shown in . No gender-, history of hypertension and diabetes-, current smokers-, and current drinkers-related significant differences were detected between RVO and control group (all comparisons p > .05).
Table 4. Characteristics of patients with retinal vein occlusion (RVO) and control.
Univariate analysis showed that among the analyzed lipid variables, only ApoA1 was significantly lower in RVO patients than in the control group (p < .0001) (). We also measured hematologic index and found that monocyte (p = .01) and platelet (p = .02) were lower in RVO patients than in the control group.
The Spearman correlation coefficients for the lipid profiles in RVO patients are shown in the Supplement. ApoB, LDL-C, and non-HDL-C were highly correlated (γ ≥ 0.88). TG was weakly correlated with ApoB (γ = 0.30) and non-HDL-C (γ = 0.39). LDL-C was weakly negatively correlated with ApoA1 (γ = 0.22).
ROC curve analysis indicated that ApoA1 has the biggest AUROC with significant statistical difference (all comparisons <0.05) (). The ideal cutoff value of ApoA1 for RVO was less than or equal to 1.35, with 75.8% sensitivity and 67.4% specificity. The AUROC was 0.732 (). Multivariate analysis, adjusted age, sex, hypertension, diabetes, and smoking status, showed that only ApoA1 was significantly associated with RVO (OR = 0.02) ().
Compared blood lipid parameters between RAO and RVO
Univariate analysis indicated that TG, TC, LDL-C, non-HDL-C, and ApoB were significantly higher in RAO patients than RVO patients. ApoA1 was similar between RAO and RVO groups (). ROC analysis for key lipid subclasses is shown in . The difference between AUROCs of non-HDL-C and LDL-C was significant (p = .013). Results of multivariate logistic regression analysis, adjusted age, sex, hypertension, diabetes, and smoking status are shown in . TG (OR = 1.61), LDL-C (OR = 1.69), non-HDL-C (OR = 1.91), and ApoB (OR = 12.12) each was individually associated with RAO ()..
Table 5. Characteristics of patients with retinal artery occlusion (RAO) and retinal vein occlusion (RVO).
Discussion
Cardiovascular events and retinal vascular diseases share common atherosclerosis and risk factors.Citation4 A Multi-Ethnic Study of Atherosclerosis (MESA) has confirmed that retinal venular caliber is associated with atherosclerosis markers such as ankle-arm index and carotid plaque score.Citation11 Studies have also found that in age-related macular degeneration (AMD), Bruch’s membrane acts as a specialized vascular wall of the eye where lipid deposits lead to abnormal activation of the complement system and inflammatory immune response, thereby accelerating disease progression.Citation12 In RVO, thrombosis is one of its pathogenic mechanisms, and its formation is similar to the histopathology of thrombosis in atherosclerotic diseases.Citation9 High cardiovascular events risk in retinal vascular occlusion patients has also been widely accepted.Citation13–15 Lipid markers are obviously associated with atherosclerotic cardiovascular diseases risk.Citation16–18 Based on the evidence above, we hypothesize that lipid parameters may take part in the pathogenesis of retinal vascular occlusions. Thus, we analyzed the lipid profiles in RAO and RVO patients, respectively, and further compared the differences between the two groups.
We know that ApoB, LDL-C, and non-HDL-C are important atherogenic lipoproteins. ApoB-containing lipoproteins are spherical particles, and the sums of the concentrations of LDL lipoproteins, TG-rich lipoproteins, and their remnants.Citation19 Recent studies have found that ApoB48 secreted by the intestine is not only found in chylomicron (CM) but also in very low-density lipoproteins (VLDL). The ApoB48 -containing particles of VLDL are cleared at a slower rate in the plasma. When the body is under a high fat load, the plasma concentration of residual lipoprotein increases and leads to the development of atherosclerotic disease.Citation20 With this in mind, it is reasonable to speculate that whether reduced clearance of ApoB in plasma contributes to the development of RAO and RVO. Fortunately, ApoB can be used as a direct measure of the aggregate amount of atherogenic lipoproteins as each atherogenic particle contains only a single ApoB100.Citation19 Moreover, the American Association of Clinical Chemistry has stated that ApoB can be measured more accurately than LDL-C and non-HDL-C.Citation21 Non-HDL-C can sum the total atherogenic concentration by measuring the aggregate number of “cholesterol” in all contributive particlesCitation13 and has been suggested as an ideal surrogate for ApoB.Citation12 Few studies have specially reported the associations between these key lipid parameters and RAO. Higher LDL-C has been found in RAO patients, with most results coming from univariate analysis without considering other possible confounding covariates.Citation8,Citation22 Only one study reported higher ApoB in RAO patients.Citation9 The relationship between non-HDL-C and RAO has not been found in the reported study. In this study, ApoB, LDL-C, and non-HDL-C were all positively correlated with RAO events regardless of clinical phenotypes (hypertension and diabetes) and lifestyle (smoke status) confounders. Moreover, the OR and AUC values for non-HDL-C were relatively higher than those for LDL-C, indicating that non-HDL-C is a more accurate risk marker of RAO than LDL-C. The same phenomenon was also reported in cardiovascular risk analysis.Citation13
ApoA1 is the principal protein (about 70% of pr) of HDL and secreted by liver and intestine normally.Citation23 It interacts with different receptors and transporters, and is essential for reverse cholesterol transport.Citation24 By reversing cholesterol transport, ApoA1 can prevent the accumulation of cholesterol in the arterial wall and hinders the progression of atherosclerosis.Citation25 Moreover, ApoA1 neutralizes the procoagulant properties of anionic phospholipids and thus reduces thrombosis.Citation26 Previous studies have also reported that ApoA1 exerts atheroprotective function through modulating inflammation and oxidative stress.Citation24 A 14-year population-based cohort study has shown that high levels of ApoA1 in serum are an independent risk factor for AMD progression to advanced stages.Citation27 Interestingly, ApoA1 levels have dissimilar effects on different retinal diseases. Another clinical research confirmed that serum ApoA1 concentration is negatively correlated with DR severity.Citation28 In RVO patients, although we did not detect the positive effect of ApoB, non-HDL-C, and LDL-C in RVO patients, a low ApoA1 level was found significantly increasing the risk of RVO after adjusting for relevant covariates. In addition, the AUC values for ApoA1 were relatively high compared with the other lipid variables, indicating that ApoA1 was a powerful independent predictor for RVO.
We also found that monocyte and platelet were associated with RAO in our study. Circulating monocytes serve as a class of inflammatory markers, they promote the release of oxidative cytokines that exacerbate the inflammatory response and promote thrombosis. Monocyte counts have been shown to play an important role in the pathogenesis of atherosclerosis.Citation29 The monocyte/HDL ratio (MHR) was found to be significantly higher in retinal branch vein obstruction (BRVO).Citation30 Among our results, we surprisingly found that the monocyte counts were significantly lower in patients with RVO than in controls. Given the above findings and our results, we suggest that monocytes are important in RVO, and their counts may differ in different types of RVO. The role of monocytes in distinguishing different types of RVO can be further investigated. Platelets are involved in physiological hemostasis. In pathological states, they participate in thrombosis and promote the development of atherosclerosis.Citation31 Among the platelet factors, platelet function, mean platelet volume (MPV), platelet distribution width (PDW), and platelet large cell ratio (PLCR) correlate with RVO.Citation32 However, the association between platelet count and RVO is currently unclear. Our study found that platelet count is lower in patients with RVO, while a meta-analysis concluded that platelet count was not significantly associated with RVO.Citation33 Therefore, it appears that further studies are required to explore the pathological significance of platelet levels in retinal vein obstruction disease.
For the comparison between RAO and RVO, ApoB, non-HDL-C, and LDL-c were also useful biomarkers to distinguish RAO from RVO. These effects remained after adjusting for the presence of possible confounding effects. Non-HDL-C was still a good predictor than LDL-C in distinguishing RAO from RVO. Our results verified that RAO and RVO are retinal vascular occlusions with different pathogenesis. Studies have reported that RAO was embolism and RVO is preferable to thrombosis. Our study provided a clue that the same lipid profile may play a role in the development of different retinal vascular occlusions through different lipid metabolism.
There were some limitations in our study. First, our study was a retrospective case–control design, which can only provide information of contemporary relationship between exposure and diseases and cannot provide a reliable analysis of the cause–effect relationship between lipid profiles and retinal vascular occlusions. Second, we did not get the accurate data for lipid-lowering therapy. Third, we were unable to conduct the subgroup analysis for different types of retinal vascular occlusions (e.g. branch and central RAO or RVO) as the small sample.
In conclusion, our study suggested that abnormal lipid levels are correlated with the development of retinal vascular occlusions, and there are differences in lipid profiles between RAO and RVO patients. ApoB, non-HDL-C, and LDL-C may be potential disease biomarkers in RAO patients and were more prominent in RAO patients than RVO patients. Non-HDL-C was a more accurate marker of the risk of RAO than LDL-C. ApoA1 was an independent factor associated with RVO. Our results may provide clinical evidence of adjusting the risk lipid parameters for lowering the risk of retinal vascular occlusions. To further understand the link between retinal vascular occlusions and lipid biomarkers, prospective cohort studies are required.
Supplemental Material
Download Zip (19.5 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/09273948.2023.2173245
Additional information
Funding
References
- Scott IU, Campochiaro PA, Newman NJ, Biousse V. Retinal vascular occlusions[J]. Lancet. 2020;396:1927–1940. doi:10.1016/S0140-6736(20)31559-2.
- Mac Grory B, Schrag M, Biousse V, et al. Management of central retinal artery occlusion: a scientific statement from the American Heart Association[J]. Stroke. 2021;52:e282–e294. doi:10.1161/STR.0000000000000366.
- Rogers SL, Mcintosh RL, Lim L, et al. Natural history of branch retinal vein occlusion: an evidence-based systematic review[J]. Ophthalmology. 2010;117:1094–1101.e5. doi:10.1016/j.ophtha.2010.01.058.
- Mirshahi A, Feltgen N, Hansen LL, Hattenbach LO. Retinal vascular occlusions: an interdisciplinary challenge[J]. Dtsch Arztebl Int. 2008;105:474–479. doi:10.3238/arztebl.2008.0474.
- Hayreh SS. Prevalent misconceptions about acute retinal vascular occlusive disorders[J]. Prog Retin Eye Res. 2005;24:493–519. doi:10.1016/j.preteyeres.2004.12.001.
- Geovanini GR, Libby P. Atherosclerosis and inflammation: overview and updates[J]. Clin Sci (Lond). 2018;132:1243–1252. doi:10.1042/CS20180306.
- Wolf D, Ley K. Immunity and inflammation in atherosclerosis[J]. Circ Res. 2019;124:315–327. doi:10.1161/CIRCRESAHA.118.313591.
- Guven S, Kilic D. Neutrophil to Lymphocyte Ratio (NLR) is a better tool rather than Monocyte to High-Density Lipoprotein Ratio (MHR) and Platelet to Lymphocyte Ratio (PLR) in central retinal artery occlusions[J]. Ocul Immunol Inflamm. 2021;29:997–1001. doi:10.1080/09273948.2020.1712433.
- Stojakovic T, Scharnagl H, März W, et al. Low density lipoprotein triglycerides and lipoprotein(a) are risk factors for retinal vascular occlusion[J]. Clin Chim Acta. 2007;382:77–81. doi:10.1016/j.cca.2007.03.024.
- Hwang S, Kang SW, Choi KJ, et al. High-density lipoprotein cholesterol and the risk of future retinal artery occlusion development: a nationwide cohort study[J]. Am J Ophthalmol. 2022;235:188–196. doi:10.1016/j.ajo.2021.09.027.
- Nguyen TT, Islam FM, Farouque HM, et al. Retinal vascular caliber and brachial flow-mediated dilation: the multi-ethnic study of atherosclerosis[J]. Stroke. 2010;41:1343–1348. doi:10.1161/STROKEAHA.110.581017.
- Curcio CA, Johnson M, Rudolf M, Huang JD. The oil spill in ageing Bruch membrane[J]. Br J Ophthalmol. 2011;95:1638–1645. doi:10.1136/bjophthalmol-2011-300344.
- Woo SC, Lip GY, Lip PL. Associations of retinal artery occlusion and retinal vein occlusion to mortality, stroke, and myocardial infarction: a systematic review[J]. Eye (Lond). 2016;30:1031–1038. doi:10.1038/eye.2016.111.
- Ponto KA, Scharrer I, Binder H, et al. Hypertension and multiple cardiovascular risk factors increase the risk for retinal vein occlusions: results from the Gutenberg retinal vein occlusion study[J]. J Hypertens. 2019;37:1372–1383. doi:10.1097/HJH.0000000000002057.
- Callizo J, Feltgen N, Pantenburg S, et al. Cardiovascular risk factors in central retinal artery occlusion: results of a prospective and standardized medical examination[J]. Ophthalmology. 2015;122:1881–1888. doi:10.1016/j.ophtha.2015.05.044.
- Cao J, Nomura SO, Steffen BT, et al. Apolipoprotein B discordance with low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol in relation to coronary artery calcification in the multi-ethnic study of atherosclerosis (mesa)[J]. J Clin Lipidol. 2020;14:109–121.e5. doi:10.1016/j.jacl.2019.11.005.
- St-Pierre AC, Cantin B, Dagenais GR, et al. Apolipoprotein-B, low-density lipoprotein cholesterol, and the long-term risk of coronary heart disease in men[J]. Am J Cardiol. 2006;97:997–1001. doi:10.1016/j.amjcard.2005.10.060.
- Ramjee V, Sperling LS, Jacobson TA. Non-high-density lipoprotein cholesterol versus apolipoprotein B in cardiovascular risk stratification: do the math[J]. J Am Coll Cardiol. 2011;58:457–463. doi:10.1016/j.jacc.2011.05.009.
- Stock J. Triglycerides and cardiovascular risk: apolipoprotein B holds the key[J]. Atherosclerosis. 2019;284:221–222. doi:10.1016/j.atherosclerosis.2019.03.004.
- Björnson E, Packard CJ, Adiels M, et al. Apolipoprotein B48 metabolism in chylomicrons and very low-density lipoproteins and its role in triglyceride transport in normo- and hypertriglyceridemic human subjects[J]. J Intern Med. 2020;288:422–438. doi:10.1111/joim.13017.
- Cole TG, Contois JH, Csako G, et al. Association of apolipoprotein B and nuclear magnetic resonance spectroscopy-derived LDL particle number with outcomes in 25 clinical studies: assessment by the AACC lipoprotein and vascular diseases division working group on best practices[J]. Clin Chem. 2013;59:752–770. doi:10.1373/clinchem.2012.196733.
- Elbeyli A, Kurtul BE, Ozcan DO, et al. Assessment of red cell distribution width, platelet/lymphocyte ratio, systemic immune-inflammation index, and neutrophil/lymphocyte ratio values in patients with central retinal artery occlusion[J]. Ocul Immunol Inflamm. 2022;30:1940–1944. doi:10.1080/09273948.2021.1976219.
- Ben-Aicha S, Badimon L, Vilahur G. Advances in HDL: much more than lipid transporters[J]. Int J Mol Sci. 2020;21:732. doi:10.3390/ijms21030732.
- Xu X, Song Z, Mao B, Xu G. Apolipoprotein A1-related proteins and reverse cholesterol transport in antiatherosclerosis therapy: recent progress and future perspectives[J]. Cardiovasc Ther. 2022;2022:4610834. doi:10.1155/2022/4610834.
- Ouimet M, Barrett TJ, Fisher EA. HDL and reverse cholesterol transport[J]. Circ Res. 2019;124:1505–1518. doi:10.1161/CIRCRESAHA.119.312617.
- Oslakovic C, Krisinger MJ, Andersson A, et al. Anionic phospholipids lose their procoagulant properties when incorporated into high density lipoproteins[J]. J Biol Chem. 2009;284:5896–5904. doi:10.1074/jbc.M807286200.
- Buch H, Vinding T, La Cour M, et al. Risk factors for age-related maculopathy in a 14-year follow-up study: the Copenhagen City Eye Study[J]. Acta Ophthalmol Scand. 2005;83:409–418. doi:10.1111/j.1600-0420.2005.00492.x.
- Sasongko MB, Wong TY, Nguyen TT, et al. Serum apolipoprotein AI and B are stronger biomarkers of diabetic retinopathy than traditional lipids[J]. Diabetes Care. 2011;34:474–479. doi:10.2337/dc10-0793.
- Gratchev A, Sobenin I, Orekhov A, Kzhyshkowska J. Monocytes as a diagnostic marker of cardiovascular diseases[J]. Immunobiology. 2012;217:476–482. doi:10.1016/j.imbio.2012.01.008.
- Duru Z, Altunel O, Alabay B, et al. Elevated monocyte-to-high-density lipoprotein ratio as an indicator of systemic inflammation in patients with branch retinal vein occlusion[J]. Beyoglu Eye J. 2021;6:212–216. doi:10.14744/bej.2021.94547.
- Marcinkowska A, Wolska N, Luzak B, et al. Platelet-derived procoagulant microvesicles are elevated in patients with Retinal Vein Occlusion (RVO)[J]. J Clin Med. 2022;11:5099. doi:10.3390/jcm11175099.
- Marcinkowska A, Cisiecki S, Rozalski M. Platelet and thrombophilia-related risk factors of retinal vein occlusion[J]. J Clin Med. 2021;10:3080. doi:10.3390/jcm10143080.
- Liu Z, Perry LA, Edwards TL. Association between platelet indices and retinal vein occlusion: a systematic review and meta-analysis[J]. Retina. 2021;41:238–248. doi:10.1097/IAE.0000000000003022.