ABSTRACT
Purpose
To analyze radial peripapillary capillaris (RPC) and intra-papillary capillaris (IPC) using optical coherence tomography angiography (OCTA) in acute retinal necrosis (ARN) with good outcome.
Methods
RPC and IPC were analyzed by OCTA in patients diagnosed with ARN and treated with pharmacotherapy alone without surgery at the Tokyo Medical University Hospital.
Results
A total of 13 patients were studied. Ophthalmoscopic examination showed no abnormality in the optic disc in 12 of the 13 patients. However, OCTA findings of the affected eye compared with the unaffected fellow eye revealed morphological abnormalities in RPC in nine cases (69%) and decrease in capillary network in RPC or IPC in eight cases (62%).
Conclusion
In ARN, RPC and IPC were impaired even in eyes that were healed with medical treatment only without requiring surgical intervention and had no abnormal findings on ophthalmoscopic examination. This result suggests the presence of some degrees of optic neuropathy even in mild cases with good visual prognosis.
Acute retinal necrosis (ARN) typically presents with severe inflammation of the retina caused by herpes simplex virus (HSV) or varicella zoster virus (VZV) infection.Citation1,Citation2 ARN has poor visual prognosis, often complicated by retinal detachment.Citation3 On the other hand, in eyes with limited lesions, relatively good visual prognosis may be obtained if treated aggressively.Citation4 Optic neuropathy as well as retinal detachment have been reported to be poor visual prognostic factors in ARN.Citation5 Several mechanisms may lead to optic neuropathy associated with ARN, including vasculitis, optic nerve ischemia, and direct invasion of the optic nerve by herpes virus.Citation6
Compared to conventional fluorescein angiography (FA) and indocyanine green angiography, optical coherence tomography angiography (OCTA) allows non-invasive, quick, and repeatable examination without the risk of complications such as allergic reactions due to intravenous injection of contrast medium. OCTA provides visualization of detailed retinal choroidal vascular structures such as microaneurysms, abnormal intraretinal microvasculature, and neovascularization. Recently, OCTA has been used to analyse the structures of intraretinal vascular layers, as well as radial peripapillary capillaris (RPC) and intra-papillary capillaris (IPC) layers. Some studies that used OCTA reported lower retinal vascular densities in the optic nerve head, peripapillary region, and macula in glaucoma compared with normal eyes,Citation7,Citation8 parapapillary deep-layer microvasculature dropout (MvD) in glaucoma,Citation9 and correlation of decrease in vascular density with severity of visual field disorder.Citation10
Although optic nerve damage has been reported to be a poor visual prognostic factor in ARN,Citation5,Citation6 there is no report of using OCTA to evaluate the optic nerve in ARN. Many cases of ARN require vitrectomy due to retinal detachment, but vitrectomy may cause mechanical damage to the retina and optic neuron.Citation11 Thus, it is possible that optic neuropathy observed in patients who have undergone vitrectomy for ARN may be caused by the surgical procedure. In other words, evaluation of optic neuropathy in ARN cases that do not require vitrectomy is important. However, there are no reports of using OCTA to evaluate the optic nerve papilla in patients with ARN not requiring vitrectomy.
The purpose of this study was to examine the capillary structural changes using OCTA in ARN patients in remission after pharmacotherapy alone without undergoing vitreous surgery and were not suspected of optic neuropathy on ophthalmoscopic examination.
Methods
We conducted a retrospective, non-interventional single-institution, observational study. This study was conducted in compliance with the Helsinki principles. Ethical approval was obtained from the Medical Research Ethics Committee of Tokyo Medical University Hospital. Since the Japanese government recommends obtaining informed consent for scientific studies of three or more cases even for retrospective study, all patients in this retrospective study signed an informed consent form before analysis.
The medical records of 127 patients diagnosed with ARN at the Department of Ophthalmology, Tokyo Medical University Hospital between April 2004 and March 2020 were reviewed. Among these patients, 114 were excluded because vitrectomy was performed or OCTA was not conducted. The remaining 13 patients with ARN (onset between January 2007 and March 2020) healed by pharmacotherapy alone and did not require vitreous surgery who had OCTA imaging data during remission were included in this analysis. A diagnosis of ARN was based on the Japanese criteria for ARN published in 2015Citation12 or the criteria of American Uveitis Society.Citation13 All patients were treated with systemic acyclovir and corticosteroids. After treatment, RPC and IPC were evaluated in both eyes (affected eye and unaffected fellow eye) using OCTA (Topcon DRI Swept Source OCT, Triton; Topcon Corporation, Tokyo, Japan). Circumpapillary retinal nerve fiber layer was measured by OCT (RS3000; NIDEK corporation, Ltd., Aichi, Japan, or CIRRUS6000, Carl Zeiss Meditec Inc, USA). Visual field test was performed in both eyes after treatment, using Humphrey visual field analyzer (30–2 program) or Goldman perimetry. Since OCTA was introduced into clinical use at our hospital in 2015, the time from pharmacotherapy to OCT examination varied among cases ().
Table 1. Summary of clinical data of 13 patients with acute retinal necrosis.
Results
Demographics of patients diagnosed with ARN not requiring vitrectomy is shown in . There were seven males and six females. The mean age was 41.9 ± 18.1 (range: 20 to 88) years. Polymerase chain reaction (PCR) test using aqueous humor samples detected HSV in two cases (15%) and VZV in 11 cases (85%), with mean viral copy numbers of 1.74 × 10Citation4 to 5.3 × 10Citation6 for HSV and 1.2 × 10Citation6 ± 1.5 × 10Citation6 for VZV. Mean best corrected visual acuity (BCVA) at the first visit was logMAR 0.21 ± 0.34 (−0.18 to 1), and that at the final visit after treatment was logMAR −0.09 ± 0.11 (−3.0 to 0.15). Decreased visual field sensitivity was found in two patients (15%). All patients did not develop retinal detachment and did not require vitrectomy, and visual acuity improved by pharmacotherapy alone. OCTA imaging for RPC and IPC was performed at an average of 13.2 month (1–120 months) after pharmacotherapy. In nine cases (69%), morphological abnormalities in RPC or IPC were observed in the affected eye on OCTA compared with the unaffected fellow eye. Eight cases (62%) had decreased capillary network in RPC or IPC. Ophthalmoscopic examination found no abnormalities in the optic disc in 12 of the 13 cases. Three representative cases are presented below.
Case 1
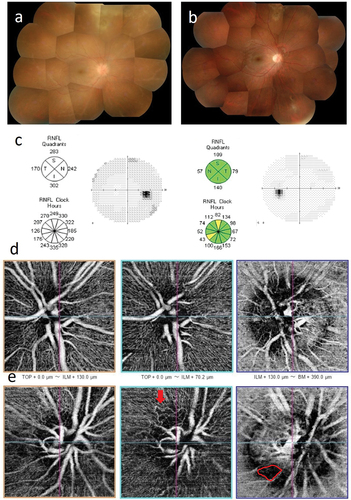
A 20-year-old female first visited an ophthalmologist in August 2015 because of decreased visual acuity in the right eye, and was subsequently referred to our hospital. At presentation, BCVA of her right eye was 20/32. Small white keratic precipitates, focal posterior synechia, and anterior chamber cells (2+) were observed in the right eye. Vitreous opacities, scattered yellowish white retinal exudates, and occlusive vasculitis fused at the periphery were also depicted (). ARN was suspected from these ophthalmologic findings. PCR test using anterior aqueous sample from the right eye detected HSV−2 (1.74 × 10Citation4 copies/ml), confirming a diagnosis of ARN. Treatment with intravenous acyclovir (2,250 mg/day) and corticosteroids (betamethasone 6 mg/day and then switched to oral prednisolone) was immediately started and continued for 18 days. Improvement was achieved with antiviral and corticosteroid therapy alone (), and vitrectomy was not required. No abnormalities were found in optic disc and visual field examinations after treatment (). After 3 years, ophthalmoscopic examination revealed no abnormalities, and BCVA in the right eye improved to 20/16. OCTA examination found no abnormalities in the unaffected fellow left eye (), but detected abnormal looped capillary in RPC and parapapillary choroidal microvasculature dropout (MvD) in IPC in the right eye ()
Figure 1. Case 1: Acute retinal necrosis in the right eye. (a) Fundus image of the right eye at presentation. A yellowish lesion in superior retina, white sheathed vessels in peripheral retina, and optic disc swelling are observed. (b) Fundus image of the right eye at 3 months after treatment. The yellowish lesion in superior retina has shrunk and become scarred. (c) Optic nerve head analysis by OCT and visual field examination by HFA at 15 months after treatment. No abnormalities are detected in both examinations. (d) and (e) OCTA examination of the optic nerve at 15 months after treatment. OCTA images of the optic nerve head, radial peripapillary capillaris (RPC), and choroid are shown sequentially from the left. (d) No abnormalities are observed in the left eye. (e) in the right eye, a loop-like capillary network is visible in RPC (red arrow), and parapapillary choroidal microvasculature dropout is observed (area enclosed by red line).
Case 2
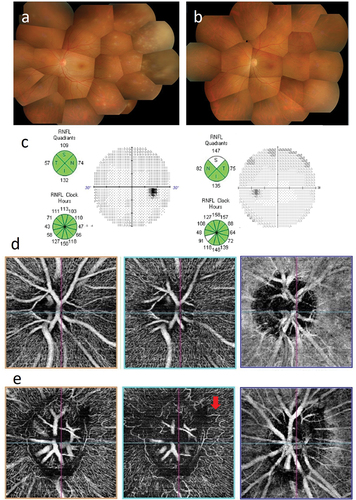
A 42-year-old female first visited an ophthalmologist because of foggy vison in her left eye in September 2015, and was referred to our department. At presentation, left eye BCVA was 20/20. Small white keratic precipitates and anterior chamber cells (2+) were observed in the left eye. A fused yellowish white retinal lesion was found in the peripheral fundus (). VZV (2.7 × 10Citation6 copies/ml) was detected by PCR test using anterior aqueous humor sample from the left eye, confirming a diagnosis of ARN. Treatment with the same drugs and dosing regimen as Case 1 was started. Improvement was achieved with these medical treatments only (), and vitrectomy was not required. No remarkable abnormalities were found in optic disc analysis and visual field examination after treatment (). At 3-year follow-up, no deterioration in the fundus findings was observed, and left eye BCVA was 20/16. On OCTA, there was no abnormality in the unaffected fellow right eye (), but abnormal looped capillary in RPC was observed in the left eye ().
Figure 2. Case 2: Acute retinal necrosis in the left eye. (a) Fundus image of the left eye at presentation. Vitreous opacification and obstructive phlebitis are observed. A yellowish white lesion with fusion expansion is visible in peripheral retina. The optic disc appears normal. (b) Fundus image of the left eye at 3 months after treatment. Vitreous opacification and obstructive phlebitis have improved. The yellowish white lesion has disappeared completely. (c) Optic nerve head analysis by OCT and visual field examination by HFA at 8 months after treatment. show no remarkable abnormalities in both examinations. (d) and (e) OCTA examination of the optic nerve at 8 months after treatment. OCTA images of the optic nerve head, radial peripapillary capillaris (RPC), and choroid are shown sequentially from the left. (d) No abnormalities are observed in the unaffected right eye. (e) in the left eye, looped capillary network is observed in RPC (red arrow).
Case 3
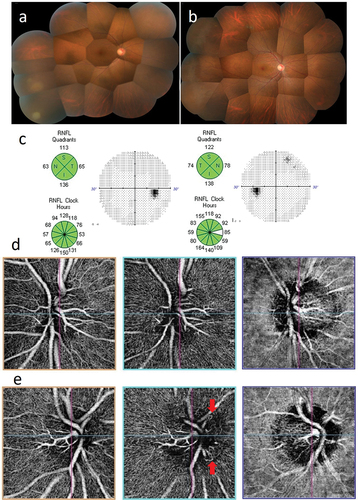
A 33-year-old female first visited an ophthalmologist because of foggy vison in her right eye in January 2017, and was referred to our department. At presentation, right eye BCVA was 20/20. Small white keratic precipitates and anterior chamber cells (2+) were observed in the right eye. Vitreous opacification and a yellowish white lesion were found in the peripheral fundus (). VZV (5.8 × 10Citation5 copies/ml) was detected by PCR method using anterior aqueous humor sample obtained from the right eye, confirming a diagnosis of ARN. The same treatment as in Case 1 and Case 2 was started. Improvement was achieved with drug treatment alone (), and vitrectomy was not required. The right eye BCVA was 30/20. At 1-year follow-up, no remarkable abnormalities were detected in optic disc analysis and visual field examination (). On OCTA, no abnormalities were observed in the unaffected fellow left eye (), while abnormal looped capillary network in RPC was observed in the right eye ().
Figure 3. Case 3: Acute retinal necrosis in the right eye. (a) Fundus image of the right eye at presentation. A yellowish white lesion with fusion expansion is observed in inferior temporal retina, and the optic papilla appears reddish. (b) Fundus image of the right eye at 3 months after treatment. The yellowish white lesion has completely disappeared, and the optic papilla appears normal. (c) Optic nerve head analysis by OCT and visual field examination by HFA at 3 months after treatment. show no remarkable abnormalities in both examinations. d and (e) OCTA examination of the optic nerve at 3 months after treatment. OCTA images of the optic nerve head, radial peripapillary capillaris (RPC), and choroid are shown sequentially from the left. (d) No abnormalities are observed in the left eye. (e) in the right eye, looped capillary network is found in RPC (red arrows).
Discussion
Clinical evidence has shown that herpes viruses may cause optic neuritis.Citation13,Citation14 A previous study using an ARN model demonstrated infection of the optic nerve by herpes simplex virus.Citation15 Moreover, histological study demonstrated viral invasion of the optic nerve in one case each of cytomegalovirus and VZV retinitis.Citation16,Citation17 On the other hand, the causes of optic neuropathy include inflammation, ischemia of the optic nerve, and direct invasion of the optic nerve by herpes virus.Citation6,Citation11 Whether optic neuropathy is caused by mechanical damage associated with vitrectomy remains debatable, because many ARN cases are complicated with retinal detachment and hence require vitrectomy. Therefore, it is necessary to evaluate optic neuropathy in cases that has not undergone vitrectomy.
OCTA has received considerable attention as an imaging modality for the evaluation of retinal diseases including uveitis, and has the potential to revolutionize the approach to the diagnosis and management of uveitis. OCTA may allow optimal, non-invasive, and effective management of uveitis. OCTA is capable of depicting lesions and their spatial distribution, calculating microvascular blood flow area and vessel density in three dimensions, and detecting microvascular changes that would otherwise go unnoticed by conventional angiography.Citation18–20 Additional evaluation by OCTA may lead to early detection of etiology of uveitis and prediction of recurrence before overt morphological changes and clinical signs appear. Anterior segment OCTA has been used to examine iris vessel density in acute anterior uveitis and scleral vessel density in scleritis.Citation21,Citation22 Posterior segment OCTA has elucidated foveal avascular zone expansion in Behcet’s disease,Citation23 detected capillary hypoperfusion or small round areas of hyperreflectivity in superficial (SCP) and deep capillary plexuses (DCP) in sarcoidosis,Citation24,Citation25 showed decreased DCP vascular density in Vogt-Koyanagi-Harada disease,Citation26 and demonstrated decreased SPC and DPC vascular densities in ARN.Citation27 In addition, OCTA revealed microvascular blood flow disorder in patients with non-arteritic anterior ischemic optic neuropathy,Citation28 and reduced densities of retinal microvasculature in neuromyelitis optica spectrum disorder.Citation29 However, there is no report of using OCTA to evaluate the optic nerve in ARN. This is the first report of evaluating optic neuropathy using OCTA in ARN patients who did not require surgery.
In the present study, 9 cases (69%) of ARN had morphologically abnormal capillaries in RPC or IPC. Eight cases (62%) had decreased capillary network in RPC or IPC. There were no cases without morphological abnormality or decrease of capillary network. In other words, even mild ARN cases that had remitted after antiviral and corticosteroid therapy alone without undergoing vitreous surgery showed vascular disorders in RPC and IPC, suggesting the possibility of latent optic neuropathy in these patients.
Since a decrease in peripapillary vascular density has also been reported in optic neuritis,Citation30 it is possible that severe inflammation induces a decrease in peripapillary vascular density in our cases. Moreover, the vascular changes observed in patients with ARN may reflect collateral vessels that arise to compensate for the reduced blood flow caused by inflammation. On the other hand, serial evaluation of morphological abnormalities or decrease of capillary network from initial onset was not possible in any of our cases, due to vitreous opacities or anterior chamber inflammation. Further studies are needed to determine whether the morphological abnormalities or decrease of capillary network are primary changes due to ARN or secondary changes due to severe inflammation.
The present preliminary study has some limitations. First and foremost, the number of cases is small. Thus, the frequencies of fine capillary abnormalities in RPC and IPC and their causal relationship with the pathogenesis of optic neuropathy are unknown. In addition, since the vascular structure around the optic nerve was not evaluated serially from disease onset, it is not clear when the fine capillary abnormalities in RPC and IPC develop. This study also did not quantify vessel density or blood vessel abnormalities. Some abnormal vessels were dramatically altered, while others showed mild changes. It is desirable to quantify the decrease in vessel density in the future, since quantification may more accurately reveal small decreases in vessel density which cannot be perceived visually.
The mechanism of optic nerve damage in ARN is unclear. However, we found that patients with ARN who had good visual prognosis and no obvious abnormalities on OCT had fine capillary changes in RPC and IPC on OCTA.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Usui Y, Goto H. Overview and diagnosis of acute retinal necrosis syndrome. Semin Ophthalmol. 2008;23(4):275–283. doi:10.1080/08820530802111325.
- Cochrane TF, Silvestri G, McDowell C, Foot B, McAvooy CE. Acute retinal necrosis in the United Kingdom. Eye (Lond). 2012;26(3):370–377. doi:10.1038/eye.2011.338.
- Usui Y, Takeuchi M, Goto H, et al. Acute retinal necrosis in Japan. Ophthalmology. 2008;115(9):1632–1633. doi:10.1016/j.ophtha.2008.03.005.
- Crapotta JA, Freeman WR, Feldman RM, et al. Visual outcome in acute retinal necrosis. Retina. 1993;13(3):208–213. doi:10.1097/00006982-199313030-00004.
- Takase H, Goto H, Namba K, et al. Clinical characteristics, management, and factors associated with poor visual prognosis of acute retinal necrosis. Ocul Immunol Inflamm. 2020;30(1):48–53. doi:10.1080/09273948.2020.1789179.
- Witmer MT, Pavan PR, Fouraker BD, Levy-Clarke GA. Acute retinal necrosis associated optic neuropathy. Acta Ophthalmol. 2011;89(7):599–607. doi:10.1111/j.1755-3768.2010.01911.x.
- Akil H, Huang AS, Francis BA, Sadda SR, Chopra V, Frishman L. Retinal vessel density from optical coherence tomography angiography to differentiate early glaucoma, pre-perimetric glaucoma and normal eyes. PLoS One. February 2, 2017;12(2):e0170476. doi:10.1371/journal.pone.0170476.
- Dastiridow A, Chopra V. Potential applications of optical coherence tomography angiography in glaucoma. Curr Opin Ophthalmol. 2018;29(3):226–233. doi:10.1097/ICU.0000000000000475.
- Lee EJ, Lee SH, Kim JA, Kim TW. Parapapillary Deep-Layer Microvasculature dropout in glaucoma: topographic association with glaucomatous damage. Invest Ophthalmol Vis Sci. 2017;58(7):3004–3010. doi:10.1167/iovs.17-21918.
- Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Relationship between optical coherence tomography angiography vessel density and severity of visual field loss in glaucoma. Ophthalmology. 2016;123(12):2498–2508. doi:10.1016/j.ophtha.2016.08.041.
- Bansal AS, Hsu J, Garg SJ, et al. Optic neuropathy after vitrectomy for retinal detachment: clinical features and analysis of risk factors. Ophthalmology. 2012;119(11):2364–2370. doi:10.1016/j.ophtha.2012.06.002.
- Takase H, Okada AA, Goto H, et al. Development and validation of new diagnostic criteria for acute retinal necrosis. Jpn J Ophthalmol. 2015;59(1):14–20. doi:10.1007/s10384-014-0362-0.
- Sergott RC, Belmont JB, Savino PJ, Fischer DH, Bosley TM, Schatz NJ. Optic nerve involvement in the acute retinal necrosis syndrome. Arch Ophthalmol. August, 1985;103(8):1160–1162. doi:10.1001/archopht.1985.01050080072023. PMID: 4026646.
- Ordoñez G, Rivas V, Santos M, et al. Herpes viruses in optic neuritis: similar to Bell’s palsy. Clin Neurol Neurosurg. January, 2020;188:105588. doi:10.1016/j.clineuro.2019.105588. Epub 2019 Nov 5. PMID: 31715425.
- Zheng M, Fields MA, Liu Y, Cathcart H, Richter E, Atherton SS. Neutrophils protect the retina of the injected eye from infection after anterior chamber inoculation of HSV-1 in BALB/c mice. Invest Ophthalmol Vis Sci. September, 2008;49(9):4018–4025. doi:10.1167/iovs.08-1914. Epub 2008 May 16. PMID: 18487377; PMCID: PMC4018730.
- Marmor MF, Egbert PR, Egbert BM, Marmor JB. Optic nerve head involvement with cytomegalovirus in an adult with lymphoma. Arch Ophthalmol. July, 1978;96(7):1252–1254. doi:10.1001/archopht.1978.03910060078016. PMID: 208496.
- Greven CM, Singh T, Stanton CA, Martin TJ. Optic chiasm, optic nerve, and retinal involvement secondary to varicella-zoster virus. Arch Ophthalmol. April, 2001;119(4):608–610. doi:10.1001/archopht.119.4.608. PMID: 11296030.
- Pichi F, Srvivastava SK, Chexal S, et al. En face optical coherence tomography and optical coherence tomography angiography of multiple evanescent white dot syndrome: new Insights into Pathogenesis. Retina. 2016;1:178–188. doi:10.1097/IAE.0000000000001255.
- Kinouchi R, Nishikawa N, Ishibazawa A, Yoshida A. Vascular rarefaction at the choriocapillaris in acute posterior multifocal placoid pigment epitheliopathy viewed on OCT angiography. Int Ophthalmol. 2017;37(3):733–736. doi:10.1007/s10792-016-0308-2.
- Wons J, Dinges J, Becker MD, Michels S. Optical coherence tomography angiography (OCT-A) in a patient with occult retinal dysfunction. Case Rep Ophthalmol Med. 2019;2019:4349692. doi:10.1155/2019/4349692. PMID: 31341688; PMCID: PMC6612972.
- Pichi F, Sarraf D, Arepalli S, et al. The application of optical coherence tomography angiography in uveitis and inflammatory eye diseases. Prog Retin Eye Res. 2017;59:178–201. doi:10.1016/j.preteyeres.2017.04.005.
- Hau SC, Devarajan K, Ang M. Anterior segment optical coherence tomography angiography and optical coherence tomography in the evaluation of episcleritis and scleritis. Ocul Immunol Inflamm. 2019;12(2):1–8. doi:10.1080/09273948.2019.1682617.
- Karalezli A, Kaderli ST, Sul S, et al. Preclinical ocular features in patients with Behçet’s disease detected by optical coherence tomography angiography. Eye (Lond). 2020;35(10):2719–2726. doi:10.1038/s41433-020-01294-z.
- Cerquaglia A, Iaccheri B, Fiore T, et al. New insights on ocular sarcoidosis: an optical coherence tomography angiography study. Ocul Immunol Inflamm. 2019;27(7):1057–1066. doi:10.1080/09273948.2018.1497665.
- Usui Y, Goto H. Granuloma-like formation in deeper retinal plexus in ocular sarcoidosis. Clin Ophthalmol. May 27, 2019;13:895–896. doi: 10.2147/OPTH.S200519. PMID: 31213760; PMCID: PMC6549785.
- Liang A, Zhao C, Jia S, et al. Retinal microcirculation defects on OCTA Correlate with active inflammation and vision in Vogt–Koyanagi–Harada disease. Ocul Immunol Inflamm. 2020;14(7–8):1–7. doi:10.1080/09273948.2020.1751212.
- Costa de Andrade G, Marchesi Mello LG, Martines GC, Maia A. Optical coherence tomography angiography findings in acute retinal necrosis. Retin Cases Brief Rep. 2021;15(3):256–260. doi:10.1097/ICB.0000000000000778.
- Wright Mayes E, Cole ED, Dang S, et al. Optical coherence tomography angiography in nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 2017;37(4):358–364. doi:10.1097/WNO.0000000000000493.
- Kleerekooper I, Houston S, Dubis AM, Trip SA, Petzold A. Optical coherence tomography angiography (OCTA) in multiple sclerosis and neuromyelitis optica spectrum disorder. Front Neurol. December 10, 2020;11:604049. doi:10.3389/fneur.2020.604049.
- Fard MA, Yadegari S, Ghahvechian H, Moghimi S, Soltani-Moghaddam R, Subramanian PS. Optical coherence tomography angiography of a pale optic disc in demyelinating optic neuritis and ischemic optic neuropathy. J Neuroophthalmol. 2019;39(3):339–344. doi:10.1097/WNO.0000000000000775. PMID: 30893268.
