ABSTRACT
Objectives
We aimed to establish the degree of consensus among clinicians on descriptors of KP morphology.
Methods
A web-based exercise in which respondents associated KP descriptors, as identified through a scoping review of the published literature, to images from different disorders. Inter-observer agreement was assessed using the Krippendorff kappa alpha metric.
Results
Of the 76 descriptive terms identified by the scoping review, the most used included “mutton-fat” (n = 93 articles, 36%), “fine/dust” (n = 76, 29%), “stellate” (n = 40, 15%), “large” (n = 33, 12%), and “medium” (n = 33, 12%). The survey of specialists (n = 26) identified inter-observer agreement for these descriptors to be poor (“stellate,” kappa: 0.15, 95% confidence interval 0.13–0.17), limited (“medium”: 0.27, 95% CI 0.25–0.29; “dust/fine”: 0.36, 95% CI 0.34–0.37), or moderate (“mutton fat”: 0.40, 95% CI 0.36–0.43; “large”: 0.43, 95% CI 0.39–0.46).
Conclusion
The clinical utility of KP morphology as an indicator of disease classification is limited by low inter-observer agreement.
Uveitis is a significant cause of blindness.Citation1 A descriptive term rather than a diagnosis, uveitis can be associated with a range of diverse conditions united by the manifestation of inflammatory change.Citation2 The heterogeneity and multiplicity of these associated conditions may be an obstacle to the prompt diagnosis of potential underlying disorders in patients presenting with ocular inflammation.
Keratic precipitates (KPs), seen on slit-lamp biomicroscopic ophthalmic examination as aggregated deposits of inflammatory cells on the corneal endothelium, are a marker of the presence of intraocular inflammatory material.Citation2,Citation3 Leukocyte populations in aqueous humour can differ by uveitis aetiology, with, for example, HLA-B27 uveitis being characterized by an accumulation of lymphocytes, while neutrophils are uncommon in samples from eyes with viral uveitis.Citation4 KP morphology, as an indicator of leukocyte population, has been used as an informative adjunct for decision-making on potential disease classification, and through that a facilitator of diagnosis.Citation5,Citation6 This is particularly important in the paediatric population, for whom uveitis may be the first presenting sign of disorders, such as Juvenile Idiopathic Arthritis, Sarcoidosis, and Behçet disease.Citation1,Citation2 The extraocular phenotype may not emerge for years after uveitis onset,Citation1,Citation2 preventing the elicitation of suggestive systemic symptoms or signs at presentation with ocular disease.
Multicenter observational studies, which aim to describe disease natural history and the determinants of outcome across and within complex inflammatory conditions, are reliant on harmonized phenotype and disease taxonomy.Citation7 Within the recent Standardization of Uveitis Nomenclature (SUN) group work on disease classification, KP morphology formed part of the classification criteria for specific disorders, with stellate KP included in the criteria for Fuchs Uveitis Syndrome.Citation8 Conversely, the work of the SUN group, which relied on retrospectively collected clinical data, also highlighted the apparent variability of KP morphology within the different autoimmune, autoinflammatory, and infective uveitides ().
Table 1. Distribution of different KP morphologies within different uveitis populations. Derived from the standardization of uveitis nomenclature working groupCitation7
Given the absence of an agreed international classification system for KP morphology, we sought to define the degree of consensus around, and inter-observer agreement of the most used descriptors, within the context of a national prospective inception cohort study of childhood onset uveitis.
Materials and methods
We undertook a cross-sectional consensus survey study informed by a review of the evidence base.
Scoping review
To identify the terms used to describe keratic precipitate morphology, we conducted a review of available literature following PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analyses) guidelines for scoping reviews.Citation9 We searched for relevant articles published between database inception and 19 November 2020 (inclusive) in the electronic databases MEDLINE and PubMed. In the absence of a MESH term for keratic precipitate,Citation10 we used free search terms to develop the search strategy. The following search term was used: “keratic precipitates.” These search terms were limited to “Human.” Eligible studies were those which used descriptive terms for keratic precipitate morphology directly observed through slit-lamp examination by the investigators in human eyes with intraocular inflammation. All study types were eligible. We excluded articles not available in English. Two independent reviewers (ALS and OC) screened the title and abstract of all publications identified by the literature search. Any discrepancies were resolved by consensus after re-reviewing the article. Microsoft Excel was used to manage the search output. Reviewers (OC, ALS) extracted from each eligible article the following: the date of publications, the KP descriptor used by the investigators, and the associated uveitis diagnosis.
Survey
We developed an image-based survey consisting of 29 slit-lamp images of KPs from different disorders self-administered individually and independently by survey participants. Survey content development was aimed at representation of a range of disorders ensuring inclusion of child and adult cases, and infectious and non-infectious diseases (details of image sources available in supplementary table S1). Survey participants were presented with each image and asked to indicate the types of precipitates present in the image by selecting one, or more, descriptive term. Terms which were used as KP descriptors in at least 10% of articles within the scoping review were offered as potential descriptors within the survey. Participants comprised members of the UK’s Paediatric Ocular Inflammation Group (POIG), which was established in 2019 in order to develop an evidence base to support clinical practice and health policy for children, young people, and adults with childhood onset ocular inflammatory diseases.Citation11,Citation12 Eligible POIG members who were those consultant (“attending”) ophthalmologists who had been identified as relatively high volume practitioners through their role as collaborators on the Uveitis in childhood prospective national cohort study (UNICORN) study.Citation13 Members received an email invitation to take part in the survey, followed by a reminder email 4 weeks later.
This work, as part of the UNICORN study, was approved by the London-Bloomsbury Research Ethics Committee (REC reference 20/LO/0661). Formal written consent was not taken, as there was no research enrolment, and activities were limited to a research survey deployed via an online survey platform.
Analyses
Descriptive analysis of scoping review results was undertaken to report the most used descriptive terms, with subgroup analysis to report frequency of use by diagnosis and year of publication. Analyses of survey findings sought to describe the degree of agreement through two methods: to assess the degree of consensus for each keratic precipitate image and morphological term, an arbitrary threshold of 80% agreement was set. In addition to quantifying consensus, we also examined inter-observer agreement (i.e., reliability) using Krippendorff’s alpha coefficient, chosen for its flexibility regarding missing values, and since the response variables, i.e., the morphological terms, were not mutually exclusive. Values were computed using the SPSS software and the KALPHA macro developed by Hayes et al.Citation14 The interpretation of the Krippendorff’s Alpha values was adapted from Landis and Koch’s definitions of Fleiss’ Kappa, with values less than zero reflecting poor, 0–0.20 slight, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 substantial, and 0.81–1 almost perfect agreement.Citation15
Results
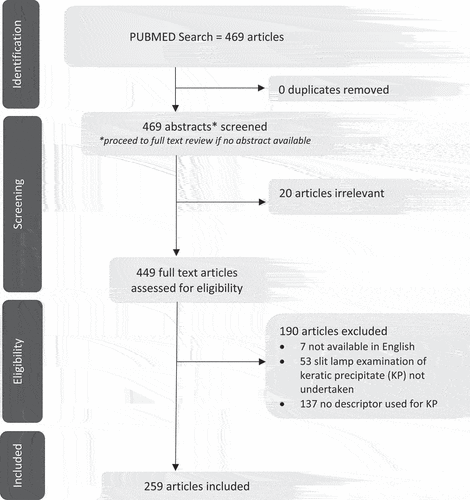
Of the 469 articles identified from the literature search, 259 eligible articles were included for review of the descriptive morphological terms for KPs (). Across these articles, 41 descriptive terms were identified across different disorders (). Frequency of use ranged from 93 to 1 incidence, with a total of 15 terms being used only once in the literature. The most used terms were “mutton-fat” (n = 93 articles, 36%), “fine” (n = 76, 29%), “stellate” (n = 40, 15%), “large” (n = 33, 12%), and “medium” (n = 33, 12%). Tracking of term use over time revealed increased use of different descriptive terms, with nine different descriptors used between 1951 and 1999, versus 41 terms used from 2000 to 2021 (). Almost half of the identified terms (19/41, 46%) had only been in use over the preceding decade.
Table 2. Frequency of use of keratic precipitate descriptors in identified articles.
Survey
The terms chosen for the online survey of clinicians were “mutton fat,” “stellate,” “fine,” “medium,” and “large.” Despite the relatively high frequency of their use, terms relating to colour of KPs, specifically “pigmented” and “white,” were not selected in recognition of the variable impact across viewing platforms (different PCs, tablets, smartphones) on the colour spectrum of the presented image. Of the 32 clinicians invited to take part in the survey, we received completed responses from 26 (response rate 81%).
Three terms surpassed the arbitrary threshold of 80% mean agreement across images (): “large” (consensus threshold reached in 25/29 images), “mutton-fat” (23/29), and “stellate” (25/29). The terms “fine/dust” (12/29) and “medium” (11/29) did not reach the threshold. With regard to inter-observer agreement, the overall Krippendorff’s alpha for all descriptive terms was 0.229 (95% confidence interval, CI 0.207 to 0.262), and for each term, the results showed either poor, slight, fair, or moderate inter-observer agreement ().
Table 3. Agreement metrics for each KP morphological term.
Discussion
From our scoping review, we report multiple descriptors of keratic precipitate morphology in use in the literature, particularly over the last decade. A review-informed survey of clinicians managing uveitis in children and adults identified variation in use for the most common descriptors of KP morphology. Terms such as “mutton-fat” and “large” had moderate inter-observer agreement, while the remaining terms, including descriptors such as “fine” or “stellate” had only poor or limited agreement.
This work has some limitations. By design, the review aimed to identify the articles in which clinical examination was reported. This resulted in the inclusion of many case reports. As novelty and rarity are key determining factors for successful publication of case reports, there may be an over-representation of the rarer disorders within the scoping review, resulting in a literature database in which atypical keratic precipitate morphology dominates. The most used terms identified by the review were, however, those which are commonly used within the Standardisation of Nomenclature (SUN) literature,Citation5,Citation7 suggesting an appropriate representation and good external validity. We did not control for the viewing parameters for survey respondents, and it is possible that the magnification of the photograph differed among respondents with resultant impact on decisions on descriptors. However, keratic precipitates, when viewed on a slit lamp, are not viewed in isolation, but instead their dimensions are judged relative to their surroundings, for example corneal diameter and curvature, and pupil size. Thus, the impact of magnification is likely to have been minimized for this clinical sign. The survey design, with the use of a representative but not exhaustive collection of images of keratic precipitates, may have resulted in insufficiently characteristic images of keratic precipitates presented to respondents. However, more recent work, again undertaken by the SUN group,Citation5,Citation7 has identified that a range of KP types may be present within individual inflammatory disorders, suggesting that a “platonic ideal” KP morphology type is uncommon, and strengthening the broader representativeness of the images used in our work. It is possible that a wider sample of clinicians may have resulted in stronger inter-observer variability, but inclusion of practitioners from different subspecialities may also have resulted in lower agreement. Our measures of agreement (Krippendorff’s alpha) were robust, as opposed to a single arbitrary threshold for percent-agreement (e.g., 80% level consensus, as used in previous studies).Citation11 Percent-agreement metrics are intuitive, simple to calculate, easy to explain, but can overinflate agreement, particularly where there is a low incidence of a specific response in a consensus exercise. This may have been the case with the “stellate” descriptor within the survey responses. Measures such as Krippendorff’s alpha correct for the element of chance agreement among raters. Uveitis is a heterogeneous disease area, and our study respondents (a mix of adult and paediatric practitioners, from high volume centres across England, Northern Ireland, Ireland, Scotland, and Wales) are a good reflection of the range of clinicians who are typically involved in multicenter inflammatory eye disease studies, undertaking collaborative work with a need for harmonized, reliable, and repeatable phenotypic classification. Our findings may benefit from comparison to those from larger populations of clinicians internationally.
Morphological analyses of KPs have been reported as helpful in determining the underlying cause of intraocular inflammation. In terms of pathophysiology, KPs can consist of polymorphonuclear cells, lymphocytes, and epithelioid cells in varying proportions, this diversity being thought to give rise to a wide range of their physical presentation.Citation16 While KPs in the non-granulomatous uveitides tend to be described as round, and pale in colour,Citation16,Citation17 the KPs seen in granulomatous disease tend to be larger, with less defined shapes, and a “mutton fat” appearance.Citation18 Although the correct usage of the term “granulomatous” is for the description of histopathological features seen in uveal biopsies from the eyes of patients with, for example, ocular sarcoidosis, tuberculous uveitis, or Vogt–Koyanagi–Harada,Citation19–21 the description remains to facilitate clinical terminology, characterising the phenotype of the anterior segment manifestations of such disorders. The differentiation of anterior uveitis into non-granulomatous and granulomatous clinical phenotypes, as suggested by SUN,Citation7 is supported by our evidence of strong inter-observer agreement around “large” and “mutton fat” as KP descriptors. This differentiation also appears to remain helpful to aid the direction of investigation.Citation3,Citation7,Citation19–21 As the SUN group themselves suggested, however, a KP descriptor reference resource, based on a series of standardized images, is needed.Citation7
Inter-observer variability is an arguably unavoidable aspect of clinical examination, appearing across clinical fields and across different examination modalities. The negative impact of this variability is diminished at patient level, where clinical signs are considered in the context of the associated history, signs and symptoms, and outcomes of investigations. At population level, however, the precision, reliability, and reproducibility of disease phenotype underpins the ability to undertake meaningful research.Citation22 Our study demonstrates the low agreement between expert clinicians regarding the terms used to describe the morphology of KPs, other than for “large” or “mutton-fat.” Future work should aim to develop consensus-based definitions of the other morphological descriptors, with their use demonstrating strong inter-observer (consistency of use between different observers) and intra-observer (consistency of use by a single observer) reliability. An imaging-assisted classification system, using confocal microscopy or optical coherence tomography to limit subjectivity and bring a greater degree of standardisation and repeatability, may be most appropriate, particularly as this may elicit additional features which are not appreciated on slit-lamp examination.Citation3 Our work should support future attempts at classifying the morphological terms used to describe KPs and build towards more reliable diagnosis and management of uveitis. Until there is an international consensus on definitions for these heterogeneous clinical features, the clinical utility of KP morphology as an indicator of uveitis type, and underlying systemic or ocular diagnosis, is limited. These terms should be approached with caution by clinicians and researchers seeking to apply the existing evidence base to guide their clinical practice or to map clinical phenotypes.
Financial Support
AL Solebo and S Kellett are supported by an NIHR Clinician Scientist award (CS-2018-18-ST2-005). JS Rahi and AD Dick are supported in part by the NIHR BRC based at Moorfields Eye Hospital NHS Foundation Trust and by the UCL Institute of Ophthalmology, and an NIHR Senior Investigator award. This work was undertaken at UCL Institute of Child Health/Great Ormond Street Hospital for children which received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centers funding scheme. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Supplemental Material
Download Zip (22.2 KB)Acknowledgments
Members of the POIG UNICORNs Group: Jane Ashworth Manchester Academic Health Science Centre, Manchester Royal Eye Hospital, Manchester, UK & Manchester Royal Eye Hospital, Manchester University Hospitals NHS Trust, Kate Bush, Department of Ophthalmology, Royal Bournemouth Hospital, Bournemouth, UK, Sarah Chamney, Department of Ophthalmology, Children’s Health Ireland at Temple Street, Dublin, Ireland, Jessy Choi, Department of Ophthalmology, Sheffield Children Hospital NHS Foundation Trust and Sheffield Teaching Hospital NHS Foundation Trust, Sheffield, UK, Erika Damato, Birmingham Midland Eye Centre, Birmingham, UK, Alastair K Denniston, Academic Unit of Ophthalmology, University of Birmingham, Birmingham, UK, Clive Edelsten, Department of Ophthalmology, Great Ormond Street Hospital, London, UK, Eibhlin McLoone, Eye and Ear Clinic, Royal Victoria Hospital, Belfast, UK, Jose Gonzalez-Martin, Alder Hey Hospital, Liverpool, UK, Brinda Muthusamy, Department of Ophthalmology, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK, Una O’Colmain, Department of Ophthalmology, Ninewells Hospital, Dundee, UK, Harry Petrushkin, Department of Ophthalmology, Great Ormond Street Hospital, London, UK, and Moorfields Eye Hospital, London, UK, Patrick Watts, Departments of Ophthalmology, University Hospital Wales, Cardiff, UK, Katya Tambe, Nottingham University Hospitals NHS Trust, Nottingham, UK, Ailsa E Ritchie, Department of Ophthalmology, St. Thomas’ Hospital, London, UK, Adam Bates, Department of Ophthalmology, Maidstone Hospital, Kent, UK, Rosemary Lambley, Nottingham University Hospitals NHS Trust, Nottingham, UK Department of Ophthalmology, Ed Hughes, Brighton and Sussex University Hospitals NHS Trust, Sussex Eye Hospital, Brighton, UK, Srilakshmi M Sharma, Oxford Eye Hospital, Oxford University Hospitals NHS Foundation Trust, Oxford, UK, Anne Cees Houtman, Department of Ophthalmology, Glasgow Children’s Hospital, Glasgow, UK, Rachel Pilling, Department of Ophthalmology, Bradford Teaching Hospital, Bradford, UK.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/09273948.2023.2242946
Additional information
Funding
References
- de Smet MD, Taylor SRJ, Bodaghi B, Miserocchi E, Murray PI, Pleyer U, et al. Understanding uveitis: The impact of research on visual outcomes. Prog Retin Eye Res. 2011;30(6):452–470. doi:10.1016/j.preteyeres.2011.06.005.
- Thorne JE, Suhler E, Skup M, Tari S, Macaulay D, Chao J, et al. Prevalence of noninfectious uveitis in the United States: A claims-based analysis. JAMA Ophthalmol. 2016;134(11):1237–1245. doi:10.1001/jamaophthalmol.2016.3229.
- Chan NS, Chee SP. Keratic precipitates: the underutilized diagnostic clue. Ocul Immunol Inflamm. 2021;29(4):776–785. doi:10.1080/09273948.2020.1836236.
- Denniston AO, Curnow SJ. 2009. What can the aqueous humour tell us about uveitis? In: Uveitis and Immunological Disorders. Essentials in Ophthalmology. Berlin, Heidelberg: Springer; 19–27. doi:10.1007/978-3-540-69459-5_3.
- Van Gelder RN, Sen HN, Tufail A, Lee AY. Here comes the SUN (Part 2): standardization of uveitis nomenclature for disease classification criteria. Am J Ophthalmol. 2021;228:A2–A6. doi:10.1016/j.ajo.2021.05.006.
- Deschenes J, Murray PI, Rao NA, Nussenblatt RB. International uveitis study group. International Uveitis Study Group (IUSG): clinical classification of uveitis. Ocul Immunol Inflamm. 2008;16(1):1–2. doi:10.1080/09273940801899822.
- Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) working group. Standardization of Uveitis Nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140:509–516.
- Standardization of Uveitis Nomenclature (SUN) Working Group. Development of classification criteria for the uveitides. Am J Ophthalmol. 2021;228:96–105. doi:10.1016/j.ajo.2021.03.061.
- Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D. et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Int Med. 2018;169(7):467–473. doi:10.7326/M18-0850.
- Medical Subject Headings. https://www.nlm.nih.gov/mesh/meshhome.html. Accessed January 2, 2023.
- Solebo AL, Rahi JS, Edelsten C, Ashworth JL, Dick AD. Management of paediatric ocular inflammatory disease in the UK: national survey of practice. Eye. 2020;34(3):591–592. doi:10.1038/s41433-019-0518-8.
- Solebo AL, Rahi JS, Dick AD, Ramanan AV, Ashworth J, Edelsten C. Areas of agreement in the management of childhood non-infectious chronic anterior uveitis in the UK. Br J Ophthalmol. 2020;104(1):11–16. doi:10.1136/bjophthalmol-2018-313789.
- Kellett S, Rahi JS, Dick AD, Knowles R, Tadic V, Solebo AL. UNICORNS: Uveitis in childhood prospective national cohort study protocol. F1000res. 2020;9:1196. doi:10.12688/f1000research.26689.1.
- Hayes AF, Krippendorff K. Answering the call for a standard reliability measure for coding data. Commun Methods Meas. 2007;1(1):77–89. doi:10.1080/19312450709336664.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159. doi:10.2307/2529310.
- Rothova A, Buitenhuis HJ, Christiaans BJ, Linssen A, Van der Gaag R, Kijlstra A, et al. Acute Anterior Uveitis (AAU) and HLA-B27. Rheumatology. 1983;XXII(2):144–145. doi:10.1093/rheumatology/XXII.suppl_2.144.
- Bacchiega ABS, Balbi GGM, Ochtrop MLG, de Andrade FA, Levy RA, Baraliakos X. Ocular involvement in patients with spondyloarthritis. Rheumatology. 2017;56(12):2060–2067. doi:10.1093/rheumatology/kex057.
- Mocan M, Kadayifcilar S, Irkec M. Keratic precipitate morphology in uveitic syndromes including Behçet’s disease as evaluated with in vivo confocal microscopy. Eye. 2009;23(5):1221–1227. doi:10.1038/eye.2008.239.
- Locke LW, Schlesinger LS, Crouser ED. Current sarcoidosis models and the importance of focusing on the granuloma. Front Immunol. 2020;11:1719. doi:10.3389/fimmu.2020.01719.
- Basu S, Wakefield D, Biswas J, Rao NA. Pathogenesis and pathology of intraocular tuberculosis. Ocul Immunol Inflamm. 2015;23(4):353–357. doi:10.3109/09273948.2015.1056536.
- Rao NA. Pathology of Vogt-Koyanagi-Harada disease. Int Ophthalmol. 2007;27(2–3):81–85. doi:10.1007/s10792-006-9029-2.
- Distler O, Ludwig RJ, Niemann S, Riemekasten G, Schreiber S. Editorial: precision medicine in chronic inflammation. Front Immunol. 2021;12:770462. doi:10.3389/fimmu.2021.770462.