ABSTRACT
Purpose: Following surveys in 2004–2006 in 50 high-risk districts of mainland Tanzania, trachoma was still suspected to be widespread elsewhere. We report on baseline surveys undertaken from 2012 to 2014.
Methods: A total of 31 districts were surveyed. In 2012 and 2013, 12 at-risk districts were selected based on proximity to known trachoma endemic districts, while in 2014, trachoma rapid assessments were undertaken, and 19 of 55 districts prioritized for baseline surveys. A multi-stage cluster random sampling methodology was applied whereby 20 villages (clusters) and 36 households per cluster were surveyed. Eligible participants, children aged 1–9 years and people aged 15 years and older, were examined for trachoma using the World Health Organization simplified grading system.
Results: A total of 23,171 households were surveyed and 104,959 participants (92.3% of those enumerated) examined for trachoma signs. A total of 44,511 children aged 1–9 years and 65,255 people aged 15 years and older were examined for trachomatous inflammation–follicular (TF) and trichiasis, respectively. Prevalence of TF varied by district, ranging from 0.0% (95% confidence interval, CI 0.0–0.1%) in Mbinga to 11.8% (95% CI 6.8–16.5%) in Chunya. Trichiasis prevalence was lowest in Urambo (0.03%, 95% CI 0.00–0.24%) and highest in Kibaha (1.08%, 95% CI 0.74–1.43%).
Conclusion: Only three districts qualified for mass drug administration with azithromycin. Trichiasis is still a public health problem in many districts, thus community-based trichiasis surgery should be considered to prevent blindness due to trachoma. These findings will facilitate achievement of trachoma elimination objectives.
Background
Trachoma, a neglected tropical disease, is the most common infectious cause of blindness, responsible for visual impairment in about 2.2 million people, of whom 1.2 million are irreversibly blind.Citation1 The World Health Organization (WHO) Alliance for the Global Elimination of Blinding Trachoma by 2020 (GET2020) is an initiative endorsed by the World Health Assembly in 1998 with the goal of eliminating trachoma through the SAFE strategy.Citation2,Citation3 The SAFE strategy comprises: (1) Surgery for trichiasis to correct in-turned eyelashes, stopping pain and minimizing progression of corneal damage;Citation4 (2) Antibiotics to clear conjunctival Chlamydia trachomatis infection using annual single-dose oral azithromycin;Citation5 (3) Facial cleanliness through sustained behavior change;Citation6 and (4) Environmental improvement to increase access to water and sanitation.Citation7 Prior to SAFE implementation, baseline surveys of trachoma prevalence are needed to guide programs about where to implement interventions.Citation8
In mainland Tanzania, trachoma has been documented as a serious public health problem in large parts of the country. While there are no nationally representative data on blindness, a survey in 1990 in Central Tanzania estimated that 26% of blindness was due to trachoma.Citation9 Surveys of trachoma undertaken in 2004–2006 in 50 districts showed that 43 districts had trachomatous inflammation–follicular (TF) prevalences of 10% and above, and were thus eligible for implementation of the A, F and E components of the SAFE strategy.Citation10 By 2012, large parts of the country were still suspected to be endemic for trachoma; therefore, further baseline surveys were required to plan for implementation of the SAFE strategy with the target of achieving GET2020 objectives. We report results of baseline prevalence surveys undertaken in 31 districts of mainland Tanzania in 2012–2014.
Materials and methods
Study setting
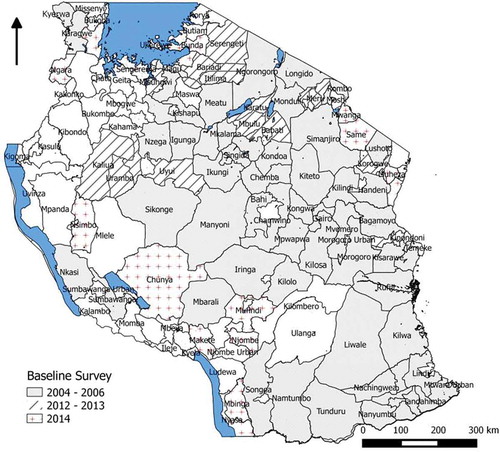
Surveys were undertaken in 9, 3, and 19 districts in 2012, 2013, and 2014, respectively (). In 2012 and 2013, the survey districts were selected because they were adjacent to districts known to be trachoma endemic from the 2004–2006 surveys,Citation10 and had low water and sanitation coverage (based on routine data collection). However, since none of the districts surveyed in 2012 or 2013 were found to be endemic for trachoma, trachoma rapid assessments (TRA)Citation11 were undertaken in 55 un-surveyed rural districts to prioritize those in which population-based surveys were warranted. A total of 19 districts were selected for surveys in 2014 on the basis of ≥30 trichiasis surgeries performed over the previous 5 years, ≥15% proportion of active trachoma (TF and/or trachomatous inflammation-intense) in examined children aged 1–9 years, and/or ≥5% proportion of trichiasis in examined people aged 15 years and older, based on TRA results.
Sample size estimation
To estimate the district prevalence of TF among children aged 1–9 years, the sample size was calculated assuming an expected prevalence of 20% with an absolute precision of ± 5%, 95% confidence level, a design effect of 4 and 10% non-response rate. A minimum sample size of 1082 children aged 1–9 years was required in each district. Assuming that 30% of the population was aged between 1 and 9 years, and an average household size of 4.8 persons,Citation12 it was necessary to sample a total of 721 households. The number of clusters per district was set at 20, therefore a total of 36 households were to be sampled per cluster, in order to reach the required sample size.
The sample size for trichiasis was calculated assuming an expected prevalence of 2%, with an absolute precision of 1%, 95% confidence level, 5% level of significance and 10% non-response rate. Based on these parameters, a total of 1657 adults aged 15 years and older in each district were required to be sampled. With 50% of the population estimated to be aged 15 years and older and an average household size of 4.8 persons,Citation12 it was necessary to sample 663 households per district in order to examine the required number of adults for trichiasis. This resulted in 33 households per cluster. Therefore, sampling the larger sample of 721 households ensured that the sample size for both TF and trichiasis were achieved. For the Global Trachoma Mapping Project (GTMP)-supported surveys conducted in 2014, these sample size calculations were accepted as providing outcomes equivalent to the templates used elsewhere for the GTMP.Citation13
Sample selection
Selection of clusters
In mainland Tanzania, districts are sub-divided into divisions, wards and villages. Villages have populations ranging from 2000 to 5000 people;Citation14 they are sub-divided into hamlets (kitongoji), each with an average size of 100 households. A multi-stage cluster random sampling design was used. In the first stage, 20 clusters (villages) were randomly selected per district. The complete list of villages in each district was obtained from the National Bureau of Statistics. Village selection was stratified by ward to ensure adequate representation of the full geographical range of district residents. The number of villages selected per ward was proportional to the ward population, with at least one village selected from each ward. Villages were systematically selected in each ward. In each selected village, two hamlets were randomly selected.
Selection of households
A household was defined as persons living together and sharing meals. In the second sampling stage, in each of the two randomly selected hamlets, systematic random sampling was used to select 18 households making a total of 36 households per village. Village leaders were requested to prepare the list of households for each selected hamlet. The total number of households in a hamlet was divided by the required number of households per hamlet to obtain the sampling interval for systematic sampling. Thereafter, a table of random numbers was used to randomly select the starting household and subsequent households systematically identified by adding the sampling interval.
Selection of participants
In the third stage, within the selected households, all eligible household participants (children aged 1–9 years and people aged 15 years and older) were examined for trachoma signs.
Household interviews
Household interviews on water sanitation and hygiene (WASH) indicators were undertaken by trained interviewers. Heads of households were interviewed on types of water sources and distance to water source, while types of sanitation facilities used by the household were verified through observation. WASH questions differed slightly between the survey waves with the 2014 surveys using the standard GTMP questionnaire.Citation13
Trachoma grading
Graders participating in the surveys had obtained kappa ≥0.7 for inter-grader agreement for TF compared to a qualified senior grader, as per the GTMP standards.Citation15 Eyelids and tarsal conjunctivae were examined using a 2.5× magnifying loupe and torch, looking for signs of active trachoma and its complications.
Data management and analysis
In 2012, data were collected using paper-based questionnaires and processed using the TELEFORM System (http://www.cardiff.com/products/teleform/) that enabled scanning of completed questionnaires into a database. In 2013 and 2014, data were collected electronically using Android tablets and smartphones and the LINKS system (https://gtmp.linkssystem.org/tanzania) developed for the GTMP, and processed as described elsewhere.Citation13,Citation15 In 2012, data analysis was undertaken using Stata 12 (Stata Corporation, College Park, Texas, USA). Age- and sex-specific weights were calculated based on the 2012 population census and applied to all survey participants. Point prevalence estimates and confidence intervals (CIs) accounted for clustering of trachoma, standardization by age and sex, and the survey design.Citation16 Associations of trachoma signs and WASH indicators were explored using Spearman’s rank test.
Ethical consideration
The surveys were undertaken as part of routine programmatic implementation of the SAFE strategy, therefore ethical clearance was not required a priori. Permission to conduct all surveys was obtained from the Ministry of Health and Social Welfare. Approval for the GTMP-supported surveys in 2014 was obtained from the ethics committee of the London School of Hygiene & Tropical Medicine (reference 6319). Written consent forms (in Swahili) were signed by heads of households selected to participate in the survey. Personal identifiers were removed from the datasets before analyses were undertaken. Permission to publish these data was granted by the Director General, National Institute for Medical Research, Tanzania.
Results
Survey population characteristics
summarizes the characteristics of the population examined by district. A total of 104,959 participants (92.3% of those enumerated) in 23,171 households from 620 clusters in 31 districts were examined. The proportion of male participants was 50.0% among children 1–9 years and 44.8% among people aged 15 years and older. The mean age was 5.2 (standard deviation, SD, 2.6) years among children aged 1–9 years and 37.0 (SD 18.4) years among people aged 15 years and older.
Table 1. Characteristics of the survey population, population-based trachoma surveys, Tanzania, 2012–2014.
Prevalence of trachoma signs
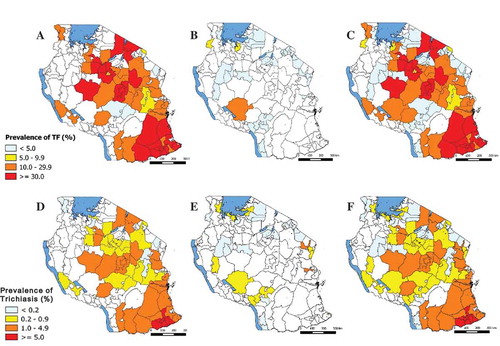
The prevalence of trachoma signs is shown in and . A total of 43,568 children aged 1–9 years and 61,373 people aged 15 years and older were examined for TF and trichiasis, respectively. Prevalence of TF varied by district, ranging from 0.0% (95% CI 0.0–0.1%) in Mbinga, to 11.8% (95% CI 6.8–16.5%) in Chunya. Among people aged 15 years and older, prevalence of trichiasis was lowest in Urambo (0.09%, 95% CI 0.00–0.24%) and highest in Kibaha (1.08%, 95% CI 0.74–1.43%).
Table 2. Prevalence of trachomatous inflammation–follicular (TF) among children aged 1–9 years and trichiasis among people aged 15 years and older, Tanzania, 2012–2014.
Table 3. Summary of key water and sanitation hygiene indicators by district, population-based trachoma surveys, Tanzania, 2012–2014.
Figure 2. Prevalence of trachomatous inflammation–follicular (TF) in children aged 1–9 years and trichiasis in people aged 15 years and older, Tanzania, 2012–2014. (A) TF prevalence 2004–2006 surveys; (B) TF prevalence 2012–2014 surveys; (C) TF prevalence 2004–2014 surveys; (D) Trichiasis prevalence 2004–2006 surveys; (E) Trichiasis prevalence 2012–2014 surveys; (F) Trichiasis prevalence 2004–2014 surveys.
Prevalence of access to water sanitation and hygiene
summarizes key WASH indicators by district. The overall proportion of households that reported using an improved drinking water source was 46.9% (range by district, 9.7% in Muleba to 94.4% in Siha). Across the survey districts, 63.0% (range by district, 30.6% in Ngara to 93.2% in Siha) of households reported that their drinking water source was in the household’s yard or within 1 km. Overall, 83.0% (range by district, 53.3% in Serengeti to 99.1% in Moshi District Council) had access to sanitation facilities. District-level TF prevalence was associated with the proportion of households with a drinking water source in the yard or within 1 km (spearman rho = −0.5; p = 0.004), however, there was no association of TF prevalence with the proportion of households with an improved drinking water source (p = 0.7) or the proportion of households with sanitation facilities (p = 0.07). Trichiasis prevalence by district was not associated with any of the WASH indicators; the proportion of households with an improved drinking water source (p = 0.3), proportion of households with a drinking water source in the yard or within 1 km (p = 0.1), or proportion of households with sanitation facilities (p = 0.5) were not significantly associated with trichiasis prevalence.
Discussion
With five years remaining before the GET2020 deadline, timely baseline surveys of trachoma in suspected endemic districts are important for planning SAFE interventions. Our data revealed that in 29 of 31 districts surveyed, the prevalence of TF was below 5% and therefore mass drug administration with azithromycin is not warranted in these districts. In Chunya, TF prevalence was above 10%, while in Ngara and Misungwi, TF prevalence was between 5% and 9.9%; therefore these two districts require implementation of mass drug administration, and community-based implementation of the F and E components of the SAFE intervention, before impact surveys are undertaken.Citation17 In Korogwe and Kibaha, prevalence of trichiasis was above the 1.0% threshold at which community-based trichiasis surgery services become a public health priority, while a further 19 districts had trichiasis prevalences in adults at or above the 0.2% elimination threshold (1 case per 1000 total population). The surveys found that, while access to WASH varied markedly by district, overall, nearly half of all households reported using an improved drinking water source, 6/10 households reported using a drinking water source in the yard or within 1 km distance, and more than 4/5 households had a sanitation facility. Increased distance to water source was associated with increasing prevalence of TF.
The 2012 and 2013 survey districts were selected because they were suspected to be trachoma endemic, due to proximity to known endemic districts surveyed nearly a decade previously. This selection criterion turned out to be uninformative in predicting districts that required SAFE implementation. In 2014, trachoma rapid assessments were undertaken to prioritize districts for further surveys, however, only 3/19 selected districts had trachoma as a serious public health problem. Nonetheless, these survey findings are important, facilitating planning of SAFE interventions where needed, and de-prioritizing attention to trachoma where they are not.
The survey estimated key WASH indicators including proportion of households with an improved drinking water source, proportion of households with a drinking water source in the yard or within 1 km, and proportion of households with sanitation facilities. The findings on WASH were consistent with those reported in the most recent (2010) demographic and health survey for Tanzania, which reported that of surveyed households, 56.2% used an improved source of drinking water, 54.6% had a drinking water source in the yard or within 1 km distance and 86.3% had a toilet/latrine facility.Citation18
Our surveys used methods recommended by the WHO for sampling of populations and examination for trachoma. The overall proportion of eligible participants absent from surveyed households was 7.7%. The majority of those absent at the time of the survey team’s visit were adult men. This may have potentially biased the prevalence estimates for trichiasis. Nonetheless, adjustment of trichiasis prevalence estimates for age and sex enabled calculation of more precise prevalence estimates. Recent evidence from Ethiopia suggests that trichiasis is frequently attributable to metaplastic or misdirected eyelashes,Citation19 often from etiologies other than trachoma. We did not examine eyes with trichiasis for trachomatous scarring, so we have reported prevalence of trichiasis. Sub-division of districts after surveys were conducted remains a potential limitation when generalizing findings from “mother” districts to respective “child” districts. Following the surveys, a number of districts have been sub-divided as follows; Arumeru district split into Arusha District Council and Meru District Council, Urambo district split into Urambo and Kaliua, and Bariadi split into Bariadi and Itilima. To overcome this challenge, the neglected tropical diseases program in Tanzania has adopted an approach whereby the prevalence from a “mother” district is applied to “child” districts, but for all subsequent surveys, the “child” districts are to be surveyed as independent domains.
The findings from these surveys are important and will facilitate progress of mainland Tanzania towards GET2020 targets. Our data suggest that only two of the districts surveyed require mass drug administration with azithromycin and implementation of the F and E components of the SAFE strategy. Nonetheless, trichiasis is still a public health problem in many districts, indicating urgent consideration of the best way to deliver surgery for trichiasis to the populations of these districts.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Funding
The survey field work was made possible through support provided to RTI International via the ENVISION Project [Cooperative Agreement no. AID-OAA-A-11-00048] by the U.S. Agency for International Development (USAID). The 2012 surveys were also funded by Sightsavers, Hellen Keller International (HKI) and World Health Organization (WHO). Core support to the GTMP was provided by a grant from the United Kingdom’s Department for International Development (DFID) (ARIES: 203145) to Sightsavers, which led a consortium of non-governmental organizations and academic institutions to complete baseline trachoma mapping worldwide. A committee established in March 2012 to examine issues surrounding completion of global trachoma mapping was initially supported by a grant from Pfizer to the International Trachoma Initiative. AWS was a Wellcome Trust Intermediate Clinical Fellow (098521) at the London School of Hygiene & Tropical Medicine. The funders of this study had no role in the study design, data collection, data analysis, data interpretation, or the writing of the report.
Additional information
Funding
References
- Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol 2012;96:614–618.
- World Health Organization. Future Approaches to Trachoma Control: Report of a Global Scientific Meeting, Geneva, 17–20 June 1996. Geneva. WHO/PBL/96.56: WHO; 1997. Accessed 15 December 2015 from: http://www.who.int/iris/handle/10665/63413
- World Health Assembly. Global elimination of blinding trachoma. In: 51st World Health Assembly. Geneva, 16 May 1998, Resolution WHA51.11: 1998. Accessed 15 December 2015 from: http://www.who.int/blindness/causes/WHA51.11/en/
- Reacher MH, Muñoz B, Alghassany A, et al. A controlled trial of surgery for trachomatous trichiasis of the upper lid. Arch Ophthalmol 1992;110:667–674.
- Schachter J, West SK, Mabey D, et al. Azithromycin in control of trachoma. Lancet 1999;354(9179):630–635.
- West S, Muñoz B, Lynch M, et al. Impact of face-washing on trachoma in Kongwa, Tanzania. Lancet 1995;345(8943):155–158.
- Emerson PM, Cairncross S, Bailey RL, et al. Review of the evidence base for the “F” and “E” components of the SAFE strategy for trachoma control. Trop Med Int Health 2000;5:515–527.
- Ngondi J, Reacher M, Matthews F, et al. Trachoma survey methods: a literature review. Bull World Health Organ 2009;87:143–151.
- Rapoza PA, West SK, Katala SJ, et al. Prevalence and causes of vision loss in central Tanzania. Int Ophthalmol 1991;15:123–129.
- Masesa D, Moshiro C, Masanja H, et al. Trachoma prevalence in Tanzania. EA J Ophthal 2007;13:34–38. Accessed 15 December 2015 from: http://www.coecsa.org/ojs-2.4.2/index.php/JOECSA/article/view/8
- Négrel A, Taylor HR, West SK. Guidelines for rapid assessment for blinding trachoma. 2000. Accessed 15 December 2015 from: http://apps.who.int/iris/handle/10665/66842#sthash.DzEHVqrJ.dpuf
- National Bureau of Statistics (NBS), Office of Chief Government Statistician (OCGS), Zanzibar. 2012 Population and Housing Census: Population Distribution by Administrative Areas. 2013. Accessed 15 December 2015 from: http://ihi.eprints.org//1344/
- Solomon AW, Pavluck AL, Courtright P, et al. The Global Trachoma Mapping Project: methodology of a 34-country population-based study. Ophthalmic Epidemiol 2015;22:214–225.
- Ministry of Health. District Health Management Training. Module one: health sector reforms and district health systems. 2001. Accessed 10 February 2016 from: http://ihi.eprints.org/419/1/ihi_(31).pdf
- Courtright P, Gass K, Lewallen S, et al. Global Trachoma Mapping Project: training for mapping of trachoma. 2012. Accessed 15 December 2015 from: http://www.trachomacoalition.org/resources/global-trachoma-mapping-project-training-mapping-trachoma
- StataCorp LP. Stata survey data reference manual: release 13. 2013. http://www.stata.com/manuals13/svy.pdf
- International Coalition for Trachoma Control. Preferred Practices for Zithromax® Mass Drug Administration. 2013. Accessed 15 December 2015 from: http://trachoma.org/sites/default/files/guidesandmanuals/ICTC_MDAToolkitEN_0.pdf
- National Bureau of Statistics (NBS) [Tanzania] and ICF Macro. Tanzania Demographic and Health Survey 2010. Dar es Salaam, Tanzania: NBS and ICF Macro; 2011. Accessed 15 December 2015 from: http://www.nbs.go.tz/takwimu/references/2010TDHS.pdf
- Rajak SN, Habtamu E, Weiss HA, et al. The clinical phenotype of trachomatous trichiasis in Ethiopia: not all trichiasis is due to entropion. Invest Ophthalmol Vis Sci 2011;52:7974–7980.
Appendix
The Global Trachoma Mapping Project Investigators are: Agatha Aboe (1,11), Liknaw Adamu (4), Wondu Alemayehu (4,5), Menbere Alemu (4), Neal D. E. Alexander (9), Berhanu Bero (4), Simon J. Brooker (1,6), Simon Bush (7,8), Brian K. Chu (2,9), Paul Courtright (1,3,4,7,11), Michael Dejene (3), Paul M. Emerson (1,6,7), Rebecca M. Flueckiger (2), Allen Foster (1,7), Solomon Gadisa (4), Katherine Gass (6,9), Teshome Gebre (4), Zelalem Habtamu (4), Danny Haddad (1,6,7,8), Erik Harvey (1,6,10), Dominic Haslam (8), Khumbo Kalua (5), Amir B. Kello (4,5), Jonathan D. King (6,10,11), Richard Le Mesurier (4,7), Susan Lewallen (4,11), Thomas M. Lietman (10), Chad MacArthur (6,11), Colin Macleod (3,9), Silvio P. Mariotti (7,11), Anna Massey (8), Els Mathieu (6,11), Siobhain McCullagh (8), Addis Mekasha (4), Tom Millar (4,8), Caleb Mpyet (3,5), Beatriz Muñoz (6,9), Jeremiah Ngondi (1,3,6,11), Stephanie Ogden (6), Alex Pavluck (2,4,10), Joseph Pearce (10), Serge Resnikoff (1), Virginia Sarah (4), Boubacar Sarr (5), Alemayehu Sisay (4), Jennifer L. Smith (11), Anthony W. Solomon (1,2,3,4,5,6,7,8,9,10,11), Jo Thomson (4); Sheila K. West (1,10,11), Rebecca Willis (2,9).
Key: (1) Advisory Committee, (2) Information Technology, Geographical Information Systems, and Data Processing, (3) Epidemiological Support, (4) Ethiopia Pilot Team, (5) Master Grader Trainers, (6) Methodologies Working Group, (7) Prioritisation Working Group, (8) Proposal Development, Finances and Logistics, (9) Statistics and Data Analysis, (10) Tools Working Group, (11) Training Working Group.