ABSTRACT
Purpose: To investigate the genus distribution of bacteria and fungi associated with keratitis in a large eye center located in Southern China and to compare the results with existing data from other areas in China.
Methods: All results of corneal microbiological examinations from 2009 to 2013 of patients who had been clinically diagnosed with bacterial or fungal keratitis were obtained chronologically and anonymously from the microbiology database at Zhongshan Ophthalmic Center. Smear/culture data were reviewed and analyzed. Antibiotic resistance of the harvested bacteria was also evaluated.
Results: Of 2973 samples, the microbial detection rate was 46.05%; in which 759 eyes (25.5%) were positive for bacteria, 796 eyes (26.8%) were positive for fungi, and 186 eyes (6.3%) were co-infected with both fungi and bacteria. The most common type of bacteria isolated was Staphylococcus epidermidis (31.9%), followed by Pseudomonas aeruginosa (12.4%). The most common type of fungus was Fusarium species (29.3%), followed by Aspergillus species (24.1%). For the bacteria harvested, mean antibiotic resistance was chloromycetin (34.6%), cephalosporins (20.0%), fluoroquinolones (18.6%), and aminoglycosides (10.5%).
Conclusion: The genus distribution of organisms detected in keratitis cases in the largest eye center located in Southern China differs from those in other areas in China. In Southern China during the time period studied, S. epidermidis and Fusarium sp. were the most common pathogens of infectious keratitis. Monitoring the changing trend of pathogens as well as antibiotic resistance are warranted.
Introduction
Infectious keratitis is a refractory ocular disease that has high potential for blindness; it can lead to corneal ulceration and purulent reactions.Citation1 Infectious keratitis can progress rapidly and, depending on the causative agent, it can be resistant to anti-microbial treatment. Therefore, to aid in the clinical diagnosis of this disease and to ensure its proper management, it is critical to determine the genus distribution of the pathogens and identify their susceptibility to antibiotic agents. The distribution and epidemiology of pathogens vary significantly by region and also evolve over time.Citation2–Citation6 Given that China is a large country with a land area of 9.6 million kmCitation2, infectious disease epidemiology may be quite distinct in different districts of the country.
In contrast to western medical systems, medical care in China is primarily provided by public hospitals supported by the government. These hospitals are organized according to a 3-tier system.Citation7 In brief, hospitals in China are designated as primary, secondary or tertiary institutions. A primary hospital is typically a township hospital that aims to offer preventive care and minimal health care. Secondary hospitals tend to be affiliated with a medium size city, county or district and are responsible for providing comprehensive health services. Tertiary hospitals are usually located at local, provincial or national level cities and are responsible for providing specialist health services and medical research. Until recently, epidemiological data on infectious keratitis have been provided by local tertiary hospitals from Northern or Central China,Citation2–Citation6 but to the best of our knowledge, there have been no published studies pertaining to Southern China. In light of this, we analyzed samples from patients with infectious keratitis in Zhongshan Ophthalmic Center, the largest eye institute in Southern China and compared the results with reports from other areas in China.
Materials and methods
All results of corneal microbiological examinations of patients from 2009 to 2013, who had been clinically diagnosed with bacterial or fungal keratitis, were obtained chronologically and anonymously from the microbiology database at Zhongshan Ophthalmic Center. All researchers connected with this study were not involved in the patient care or collection of the corneal samples. The study was approved by the Ethics Committee of Zhongshan Ophthalmic Center. All of the protocols and interpretation of data were conducted in accordance with the Clinical and Laboratory Standards Institute (CLSI).Citation8 As all reported results were collected anonymously, patient consent was waived.
All specimens were obtained by scraping the corneal lesion with a platinum spatula. Specimens were then smeared onto two slides, one for Gram staining and the other for 10% KOH staining. Subsequently the slides were examined directly under a light microscope to determine the presence of bacteria, hyphae and spores.
Bacterial culture
Specimens were inoculated into media containing blood agar, chocolate agar, brain heart infusion broth, and thioglycollate (liquid) as described previously.Citation9 All bacterial colonies were subjected to species identification and antibiotic susceptibility testing, using the VITEK 2 compact automated system (BioMérieux, Marcy l’Etoile, France). The antibiotics used for the susceptibility test included cephalosporins (cefazolin, ceftazidime, and cefuroxime sodium), fluoroquinolones (ofloxacin and levofloxacin), chloromycetin, and aminoglycosides (tobramycin and neomycin). The procedures were performed as recommended by the manufacturers’ instructions, and the results were interpreted according to CLSI terms.
Fungal culture
Specimens were inoculated into Sabouraud agar and potato glucose agar media as described previously.Citation10 The genus of fungi, including Helminthosporium maydis, were all identified by experienced technicians according to colony characteristics on potato dextrose agar, as well as microscopic characteristics of hyphae and spores in lacto-phenol cotton blue. No susceptibility tests were conducted for the fungal cultures.
Statistical analysis
The data presented in this study are categorical variables expressed as percentages. Therefore, differences between groups were compared using the Chi-squared test. All analyses were performed with commercially available software (SPSS 16.0; SPSS Inc, Chicago, IL, USA). A 2-tailed Student’s t-test was used to test statistical significance, and the level of significance was set at p < 0.05. A positive culture was defined by the ultimate gold diagnostic criteria for either bacterial or fungal infection. With this criteria, the sensitivity (true positive rate) and specificity (true negative rate) of the microscopic examination for bacteria or fungi were calculated accordingly.
To evaluate the temporal trends of prevalence of the organisms detected, data were divided into two periods as 2009–2010 and 2011–2013. Chi-square analysis was then employed to compare the distribution between these two periods. Post hoc multiple tests were conducted with the procedure described previouslyCitation11,Citation12 if differences were statistically significant, to reveal the significant change of a certain organism.
Results
A total of 2973 infectious corneal specimens were reviewed in the study. Positive culture was found in 1369 (46.05%) specimens, among which 759 (25.5%) were positive for bacteria, 796 (26.8%) were positive for fungi and 186 (6.3%) were co-infected with both bacteria and fungi. The genus distribution of bacterial and fungal keratitis specimens in the study are shown in and , respectively.
Table 1. Genus distribution of bacterial keratitis in Southern China.
Table 2. Genus distribution of fungal keratitis in Southern China.
The most common bacterial organism detected was Staphylococcus epidermidis (31.88%), followed by Pseudomonas aeruginosa (12.39%) and S. simulans (5.53%). Most of the detected gram-positive cocci were coagulase-negative staphylococci (CNS). Compared with organism culture, sensitivity and specificity of Gram staining for bacterial detection was 11.7% and 89.4%, respectively.
The most common fungal organism was Fusarium species (29.27%), followed by Aspergillus species (24.12%) and H. maydis (18.22%). Compared with organism culture, sensitivity and specificity of KOH staining for fungal detection was 45.8% and 81.8%, respectively.
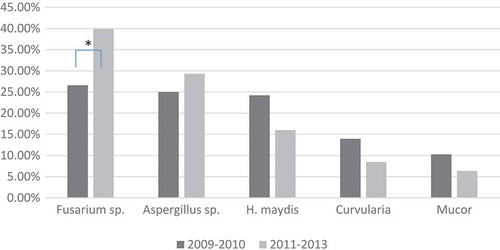
Overall, gram-positive cocci were the major type of bacteria in both periods (2009–2011 and 2011–2013), with a percentage of around 71% in both (). There was also no significant change in the general genus distribution of the bacterial classifications between these two periods (i.e. gram positive, gram negative, etc; χCitation2 = 0.287, p = 0.866). However, there was a significant change in the percentage of the five most frequent bacteria (χCitation2 = 18.498, p = 0.001). Post hoc analysis revealed that the percentage of S. saprophyticus decreased significantly from 14.56% in 2009–2010 to only 3.35% in 2011–2013 (p < 0.001; ). Likewise, there was a significant change in the percentage of the five most frequent fungi (χCitation2 = 23.490, p < 0.001). Post hoc analysis revealed a significant increase of Fusarium sp. from 26.58% in 2009–2010 to 39.88% in 2011–2013 (p < 0.001; ).
Table 3. Change in bacterial subtypes associated with keratitis, 2009–2010 to 2011–2013, Southern China.
Figure 1. Time trend of bacteria most frequently associated with keratitis, Southern China. To evaluate temporal trends in prevalence of organisms detected, data were divided into two periods, 2009–2010 and 2011–2013. Chi-square analysis revealed a significant change in the percentage of the five most frequent bacteria between these two periods (χCitation2 = 18.498, p = 0.001). Post hoc analysis revealed that the percentage of Staphylococcus saprophyticus decreased significantly from 14.56% in 2009–2010 to only 3.35% in 2011–2013 (p < 0.001). *p < 0.05; S, Staphylococcus; P, Pseudomonas.
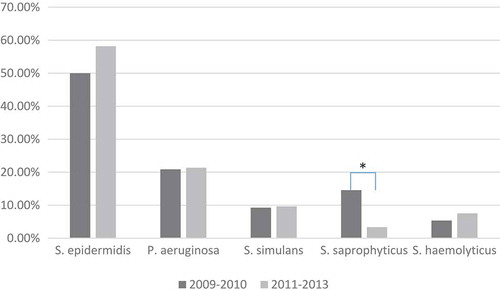
Figure 2. Time trend of fungi most frequently associated with keratitis, Southern China. To evaluate temporal trends in prevalence of organisms detected, data were divided into two periods, 2009–2010 and 2011–2013. Chi-square analysis revealed a significant change in the percentage of the five most frequent fungi between these two periods (χCitation2 = 23.490, p = 0.001). Post hoc analysis revealed a significant increase of Fusarium species, from 26.58% in 2009–2010 to 39.88% in 2011–2013 (p < 0.001). *p < 0.05; H, Helminthosporium.
Of the 2973 eyes sampled, 186 eyes (6.3%) had both bacterial and fungal infection. The most common dual infection was S. epidermidis combined with Fusarium sp. (19 eyes), followed by S. epidermidis combined with H. maydis (16 eyes), and S. epidermidis combined with Aspergillus sp. (16 eyes). As shown in , there were no statistical differences among the dual infection rates for the different species of fungi (p = 0.99). Similarly, there were no statistical differences among the dual infection rates of gram-negative bacilli, gram-positive bacilli, or gram-positive cocci (P=0.15; ).
Table 4. Incidence of mixed infection for fungal keratitis, Southern China.
Table 5. Incidence of mixed infection for bacterial keratitis, Southern China.
With regards to antibiotic resistance of bacteria (), the highest resistance rate was demonstrated for chloromycetin (34.58%) in general, while the lowest resistance rate was for aminoglycosides (10.48%). The resistance rates for the tested antibiotic agents of the gram-positive cocci (from high to low) were; chloromycetin (27.3%), fluoroquinolones (19.1%), cephalosporins (10.5%), and aminoglycosides (8.0%). Similarly, the resistance rates for gram-negative bacillus were; chloromycetin (60.2%), cephalosporins (45.2%), aminoglycosides (18.3%), and fluoroquinolones (16.9%). Resistance rates for gram-positive bacillus were; cephalosporins (40.9%), fluoroquinolones (19.4%), Chloromycetin (12.9%), and andaminoglycosides (8.1%).
Table 6. Antibiotic resistance rates of bacterial cultures for keratitis infection, Southern China.
Discussion
Early identification of causative pathogens is critical for managing infectious keratitis. Recently, new but expensive diagnostic technologies have been established, such as polymerase chain reaction (PCR) and in-vivo confocal microscopy (IVCM).Citation13,Citation14 Unfortunately, the use of PCR in bacterial keratitis is limited due to the large variety of bacterial species. The resolution of IVCM is inadequate to detect bacteria.Citation15,Citation16 Therefore, diagnosis of infectious keratitis by microscopic detection using staining and culture is still the mainstay.Citation17
In the present study, the sensitivity of microscopic detection with Gram staining for bacteria was only found to be 11.7%, lower than that of a study from India at 36%,Citation17 a country with similar economic level and climate to Southern China. Selection bias might be the reason for this difference. As mentioned earlier, samples collected in our study were from the largest eye hospital in Southern China, but the majority of infection is community-acquired and patients tend to visit the bigger center once treatment from community hospitals has failed. Therefore, the pathogens may have been inhibited by previous treatment. The situation in India may be different. Therefore, although microscopic detection with Gram staining is recommended by the American Academy of Ophthalmology in the Preferred Practice Pattern guidelines for infectious keratitis,Citation18 it is only classified as “insufficient” level and graded as “discretionary,” but not “strong.”
In contrast to bacteria, microscopy combined with KOH staining was found to be much more valuable for fungus detection, as it revealed almost half of the culture-proven samples. It is easier to tell fungus from inflammatory cells, given their typical morphology. For the same reason, the microscopic detection rate for fungal keratitis was also high in India.Citation19–Citation21
Overall, the distribution of bacterial genera in the present study was similar to those reported within China and many other foreign regions.Citation2,Citation20,Citation22–Citation25 Gram-positive cocci and gram-negative bacilli were the most common types of isolated bacteria, while gram-positive bacilli and gram-negative cocci accounted for no more than 5%. However, there were some variations in different areas. The latest study reported that the most common pathogens for bacterial keratitis in Northern China was P. aeruginosa (26.29%), followed by CNS (18.38%) and Coryneba-cterium species (10.04%). In contrast, S. epidermidis was the most prevalent bacteria (31.88%) in our study, followed by P. aeruginosa (12.39%). Nevert-heless, it should be noted that S. epidermidis, as a CNS, is the predominant microflora in the conjunctival sac and eye lid in normal populations. CNS tend to be low virulence and considered opportunistic pathogens. Therefore, even though it was detected as a common organism in keratitis in Northern ChinaCitation26 and also in Western developed countries,Citation27–Citation29 its pathogenesis and significance as the main cause of keratitis remains open to debate. Close monitoring of such bacteria is warranted. Likewise, sufficient attention should be paid to P. aeruginosa for its severe consequences and high prevalence in our study as well as other reported studies.Citation2,Citation24
The distribution of fungal infections in Southern China was different to other areas of China. For example, although filamentous fungi were predominant and Fusarium sp. was still the most common pathogen in fungal keratitis, consistent with previous studies, the proportion of Fusarium sp. was much lower in Southern China (29.3%) compared with Central (56.9%) and Northern China (73.3%).Citation4,Citation5 We were surprised to find that H. maydis accounted for 18.2% of overall fungal infections, significantly higher than the same region 15 years ago (2.5%).Citation30 This finding differs from the conclusion of Srinivasan’s study that the spectrum of fungal infection would not undergo a significant shift,Citation31 or, at least, that a significant shift in the proportion of some specific fungus could happen within a specific area.
The distribution of coinfections in our study (6.3%) was similar to those in previous studies (1.8–5.1%).Citation20,Citation32,Citation33 S. epidermidis and Fusarium sp. were the most common combination of pathogens. Ahn and colleagues reported the same result, that these two were the most common causative organisms in mixed bacterial and fungal keratitis.Citation34 The frequent involvement of CNS in dual infection suggests that the so-called “opportunistic pathogenic” CNS might play a role in the pathogenesis of coinfection keratitis. Mixed bacterial and fungal keratitis is generally more difficult to treat. Mixed infection should be considered when clinical features are atypical and antibiotics or an anti-fungal alone regimen fails.
A notable shift in pathogen distribution requires a corresponding change of pharmaceutical agents. We did not observe much change in the overall pathogen spectrum, except the significant decrease of S. saprophyticus and increase of Fusarium sp., as mentioned above. However, in Northern China, Sun and co-authors reported that gram-positive cocci increased from 25% in 1991 to 70.8% in 1997, while gram-negative bacilli decreased from 69% in 1990 to 23.4% in 1997.Citation2 Thus, more time might be needed to monitor the changing trend in Southern China.
Antibiotic resistance is a significant challenge.Citation22,Citation24,Citation35 In Southern China it has been observed that, to varying degrees, pathogens have become resistant to some commonly used antibiotics. An overall high resistance rate was found against chloromycetin, which has been used over decades. In contrast, aminoglycosides were still effective in suppressing all kinds of bacteria. It should be noted that the resistance pattern in Southern China was very different from the Preferred Practice Pattern guidelines for infectious keratitis.Citation18 Consequently, according to different types of pathogen, we propose the recommended initial antibiotic therapy regimen for empirical antibiotic treatment before identifying culture-proven pathogens (). However, since resistance might shift with time, these recommendations should be updated accordingly.
Table 7. Suggested initial antibiotic therapy for bacterial keratitis, Southern China.
Our study has several limitations. First, although patients treated in our center were mainly from Southern China, because patient demographic information was not registered in the database, we might have included a few patients from other regions of China. Second, for the same reason, we couldn’t analyze the correlation between demographic factors and distribution of pathogens and antibiotic susceptibility. Thus, it should be pointed out that what we tried to provide was only a rough picture of pathogen distribution and drug sensitivity in Southern China, where there is no existing data in the literature until now. Last but not least, given the medical hierarchical structure in China, our data might not necessarily be representative of the whole of Southern China. However, best effort has been made, through comparing results between studies conducted in a similar way in China, to minimize potential bias.
This study presents the epidemiological characteristics of bacterial and fungal keratitis in the largest eye center in Southern China. More than two-thirds of the culture-proven bacterial infections were found to be gram-positive cocci. S. epidermidis was most frequently detected, followed by P. aeruginosa, a distribution which is quite different from other regions in China. The most common fungus detected in our study was Fusarium sp., a similar finding to other regions in China and the world. Since most of the pathogens were found to exhibit some degree of antibiotic resistance, in clinical practice it is recommended that all corneal scrapings should be cultured for sensitivity.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Funding
This study is supported in part by a grant (81300739) from the National Natural Science Foundation of China, grants (2012B031800456, 2014B020226003) from the Technological Project Foundation of Guangdong Province, and a grant (201510010219) from the Technological Project Foundation of Guangzhou.
Additional information
Funding
References
- Limberg MB. A review of bacterial keratitis and bacterial conjunctivitis. Am J Ophthalmol 1991;112(4 Suppl):2S–9S.
- Sun X, Chen J, Sun X, et al. Distribution and shifting trends of bacterial keratitis in north China (1989–98). Br J Ophthalmol 2004;88:165–166.
- Hong J, Chen J, Sun X, et al. Paediatric bacterial keratitis cases in Shanghai: microbiological profile, antibiotic susceptibility and visual outcomes. Eye (Lond) 2012;26:1571–1578.
- Wang L, Sun S, Jing Y, et al. Spectrum of fungal keratitis in central China. Clin Experiment Ophthalmol 2009;37:763–771.
- Xie L, Zhong W, Shi W, et al. Spectrum of fungal keratitis in north China. Ophthalmology 2006;113:1943–1948.
- Zhang W, Pan Z, Wang Z, et al. [The variance of pathogenic organisms of purulent ulcerative keratitis]. Zhonghua Yan Ke Za Zhi 2002;38:8–12.
- Li X, Huang J, Zhang H. An analysis of hospital preparedness capacity for public health emergency in four regions of China: Beijing, Shandong, Guangxi, and Hainan. BMC Public Health 2008;8:319.
- NCCLS, C.a.L.S.I.F. Performance standards for antimicrobial susceptibility testing: 16th Informational supplement; 2006. Vol. 25.
- Lou B, Lin X, Luo L, et al. Residual lens cortex material: potential risk factor for endophthalmitis after phacoemulsification cataract surgery. J Cataract Refract Surg 2013;39:250–257.
- Csondes I, Kadlicsko S, Gaborjanyi R. Effect of different temperature and culture media on the growth of Macrophomina phaseolina. Commun Agric Appl Biol Sci 2007;72:839–848.
- Beasley TM, Schumacker RE. Multiple regression approach to analyzing contingency tables: post hoc and planned comparison procedures. J Exp Educat 1995;64:79–93.
- García-Pérez MA. Cellwise residual analysis in two-way contingency tables. Educat Psychol Measure 2003;63:825–839.
- Badiee P, Nejabat M, Alborzi A, et al. Comparative study of Gram stain, potassium hydroxide smear, culture and nested PCR in the diagnosis of fungal keratitis. Ophthalmic Res 2010;44:251–256.
- Thomas PA, Teresa PA, Theodore J, Geraldine P. PCR for the molecular diagnosis of mycotic keratitis. Expert Rev Mol Diagn 2012;12:703–718.
- Labbe A, Khammari C, Dupas B, et al. Contribution of in vivo confocal microscopy to the diagnosis and management of infectious keratitis. Ocul Surf 2009;7:41–52.
- Hau SC, Dart JK, Vesaluoma M, et al. Diagnostic accuracy of microbial keratitis with in vivo scanning laser confocal microscopy. Br J Ophthalmol 2010;94:982–987.
- Sharma S. Diagnosis of infectious diseases of the eye. Eye (Lond) 2012;26:177–184.
- American Academy of Ophthalmology. Preferred Practice Pattern® guidelines. Bacterial keratitis. San Francisco, CA: American Academy of Ophthalmology, 2013. Available at www.aao.org/ppp.
- Gopinathan U, Garg P, Fernandes M, et al. The epidemiological features and laboratory results of fungal keratitis: a 10-year review at a referral eye care center in South India. Cornea 2002;21:555–59.
- Bharathi MJ, Ramakrishnan R, Vasu S, et al. Aetiological diagnosis of microbial keratitis in South India – a study of 1618 cases. Indian J Med Microbiol 2002;20:19–24.
- Vajpayee RB, Angra SK, Sandramouli S, et al. Laboratory diagnosis of keratomycosis: comparative evaluation of direct microscopy and culture results. Ann Ophthalmol 1993;25:68–71.
- Alexandrakis G, Alfonso EC, Miller D. Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology 2000;107:1497–1502.
- Lichtinger A, Yeung SN, Kim P, et al. Shifting trends in bacterial keratitis in Toronto: an 11-year review. Ophthalmology 2012;119:1785–1790.
- Zhang C, Liang Y, Deng S, et al. Distribution of bacterial keratitis and emerging resistance to antibiotics in China from 2001 to 2004. Clin Ophthalmol 2008;2:575–579.
- Pan XJ, Jiang T, Zhu H, et al. Corneal infection in Shandong peninsula of China: a 10-year retrospective study on 578 cases. Int J Ophthalmol 2016;9:53–57.
- Li L, Liang YC, Zhang C, et al. Etiological study of suppurative keratitis. Rec Adv Ophthalmol 2008;28:749–753.
- Ibrahim YW, Boase DL, Cree IA. Epidemiological characteristics, predisposing factors and microbiological profiles of infectious corneal ulcers: the Portsmouth corneal ulcer study. Br J Ophthalmol 2009;93:1319–1324.
- Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea 2008;27:22–27.
- Jeng BH, Gritz DC, Kumar AB, et al. Epidemiology of ulcerative keratitis in Northern California. Arch Ophthalmol 2010;128:1022–1028.
- Lu JB, Chen JQ, Wang LY, et al. Mycotic keratitis in Guangzhou areas, during 1989–1997. Chin Ophthal Res 1998;16:289–291.
- Srinivasan M. Fungal keratitis. Curr Opin Ophthalmol 2004;15:321–327.
- Srinivasan M, Gonzales CA, George C, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, south India. Br J Ophthalmol 1997;81:965–971.
- Bharathi MJ, Ramakrishnan R, Meenakshi R, et al. Microbiological diagnosis of infective keratitis: comparative evaluation of direct microscopy and culture results. Br J Ophthalmol 2006;90:1271–1276.
- Ahn M, Yoon KC, Ryu SK, et al. Clinical aspects and prognosis of mixed microbial (bacterial and fungal) keratitis. Cornea 2011;30:409–413.
- Oldenburg CE, Lalitha P, Srinivasan M, et al. Emerging moxifloxacin resistance in Pseudomonas aeruginosa keratitis isolates in South India. Ophthalmic Epidemiol 2013;20:155–158.
