ABSTRACT
Purpose: Ethiopia is highly trachoma endemic. Baseline mapping was needed in Ethiopia’s Somali Region to guide elimination efforts.
Methods: Cross-sectional community-based surveys were conducted in 34 suspected trachoma-endemic woredas, grouped as 14 evaluation units (EUs), using a standardised mapping methodology developed for the Global Trachoma Mapping Project.
Results: In total, 53,467 individuals were enumerated. A total of 48,058 (89.9%) were present at the time of survey teams’ visits and consented to examination. The prevalence of trachomatous inflammation–follicular (TF) among children aged 1–9 years ranged from 4.1% in the EU covering Danot, Boh, and Geladin woredas in Doolo Subzone to 38.1% in the EU covering Kebribeyah and Hareshen woredas in Fafan Subzone (East). The trichiasis prevalence among adults aged over 15 years varied from 0.1% in the EU covering Afder, Bare, and Dolobay woredas in Afder Subzone (West) to 1.2% in the EU covering Awbere in Fafan Subzone (West).
Conclusion: Mass drug administration (MDA) with azithromycin is needed in 13 EUs (population 2,845,818). Two EUs (population 667,599) had TF prevalences in 1–9-year-olds of ≥30% and will require at least 5 years of MDA; 5 EUs (population 1,1193,032) had TF prevalences of 10–29.9% and need at least three years of MDA; 6 EUs (population 985,187) had TF prevalences of 5–9.9% and need at least one round of azithromycin distribution before re-survey. In all 13 of these EUs, implementation of facial cleanliness and environmental improvement measures is also needed. Surveys are still needed in the remaining 34 unmapped woredas of Somali Region.
Introduction
According to a published estimate, there were 32 million blind individuals and 191 million people with moderate-to-severe visual impairment worldwide in 2010.Citation1 Trachoma is the leading infectious cause of blindness, especially in populations that have poor environmental sanitation, inadequate water supply, and poor socio-economic status.Citation2,Citation3 More than 80% of the global burden of active trachoma is concentrated in 14 countries, all of which are in Africa.Citation4 Ethiopia is the second most populous country in Africa and has a very high burden of trachoma.Citation5
Worldwide, considerable progress has been made in trachoma elimination. The Ethiopian government has endorsed the World Health Assembly-supported goal of global elimination of trachoma as a public health problem by the year 2020 (GET2020), yet planning for and progress in elimination has been hampered by the low coverage of existing surveys.
The 2005–2006 Ethiopian National Survey on Blindness, Low Vision and Trachoma estimated region-level trachoma prevalences for Ethiopian Somali Region of 16.7% trachomatous inflammation–follicular (TF) in children aged 1–9 years, and 4.2% trachomatous trichiasis (TT) in adults aged 15 years and above.Citation6 However, according to WHO recommendations, district-level prevalence data are generally necessary for determining the need for implementation of the SAFE (surgery, antibiotics, facial cleanliness, environmental improvement) strategy for trachoma elimination.Citation7,Citation8 “Districts” in this recommendation are defined as “the administrative unit for health care management” which “for purposes of clarification consists of a population unit between 100,000 and 250,000 persons”.Citation9 However, where trachoma is expected to be highly and widely endemic, baseline surveys can frame larger populations, so that interventions can be provided to those most in need in a timely manner.
A true picture of trachoma prevalence will direct regional priorities for Somali Region’s blindness control programme, provide baseline data for monitoring and evaluation, and help guide those fighting trachoma to move swiftly towards meeting the GET2020 goal. In collaboration with the Global Trachoma Mapping Project (GTMP), the Ethiopian Somali Regional Health Bureau conducted population-based trachoma prevalence surveys throughout the region, using the standardised GTMP protocol.Citation10
Methods
Setting
Ethiopian Somali Region is one of nine regional states that make up the Federal Democratic Republic of Ethiopia. It occupies an area of about 350,000 kmCitation2 and is divided into nine zones (). These zones are further divided into 68 woredas (districts) and 4 city councils. According to the Central Statistical Authority’s 2007 Population and Housing Census, the total population of the region was 4.40 million in 2007.Citation11
Figure 1. Evaluation units for trachoma surveys, Global Trachoma Mapping Project, Somali Region, Ethiopia, 2013. Woredas and administrative zones are labelled.
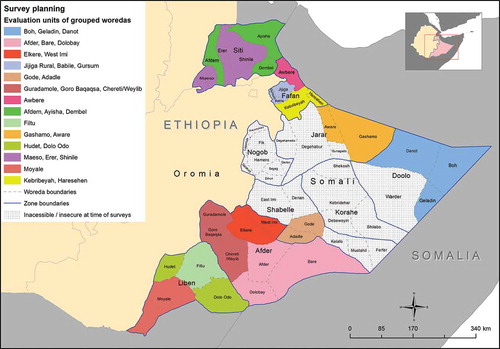
Thirty four of the region’s 68 woredas were identified as suspected-trachoma-endemic, secure and accessible to survey teams. Trachoma was expected to be highly and widely endemic, so the 34 woredas were grouped to form 14 evaluation units (EUs) of up to 500,000 inhabitants each, respecting existing administrative boundaries insofar as was possible ().
Survey design
Each survey followed a two-stage cluster-random sampling methodology designed to generate EU-level prevalence estimates for TF in children aged 1–9 years, and trichiasis in persons aged 15 years and above. In each EU, clusters were defined as villages, and a sampling frame of all villages in the EU was obtained from district head offices. In the first sampling stage, 26 villages were selected using probability proportional to size sampling. In the second stage, on the day of survey at each cluster, 30 households were selected using compact segment sampling. All residents of selected households aged 1 year or above were invited to participate. Full details of the sample size and the specifics of the survey design are outlined elsewhere.Citation10
Fieldwork
Prior to fieldwork, all 10 trachoma graders and all 10 data recorders completed the GTMP version 1 training course and passed their respective GTMP examinations. Full details of training and testing are outlined elsewhere.Citation10
Community sensitisation was carried out a few days in advance of the survey. Each selected cluster was visited by a survey coordinator, who briefed the village chief and community members and prepared the sampling frame for random segment selection by the survey team. On the day of the survey, at each selected household, consent to survey was obtained from the household head. All household members were enumerated, and present and consenting individuals were examined using sunlight and 2.5× magnifying loupes (Binomag Plastic, USA), for the presence or absence of TF, trachomatous inflammation–intense (TI) and trichiasis using the WHO simplified trachoma grading system.Citation12,Citation13 Global Positioning System (GPS) coordinates were recorded at each household for quality control purposes.
Two experienced ophthalmologists were employed as supervisors throughout the mapping, with field visits to each team at least once every 5 days. All field data were recorded in Android devices running the LINKS application (Task Force for Global Health, Decatur GA, USA).Citation10,Citation14 Upon completion of fieldwork each day, the recorder uploaded data from the phone over an encrypted connection to the GTMP secure server. Subsequent data processing and approval was undertaken as described elsewhere.Citation10
Data processing and analysis
All data analysis, which included replication of automated analyses undertaken centrally within the GTMP, was carried out using SPSS Version 20.0 statistical software (IBM Corp, Armonk, NY, USA, 2011). TF prevalences were adjusted for age in 1 year age groups, and trichiasis prevalences were adjusted for sex and age in 5 year age groupsCitation10, using the latest available census data.Citation11 EU-level prevalence estimates were calculated as the means of all cluster-level estimates of the same outcome. This accounted for unanticipated variability in the numbers examined at each cluster, but equally weighting all clusters. Prevalence estimates for all EUs combined (project-level estimates) were calculated as means of all EU-level outcome estimates.
To account for the clustered design of the survey, confidence intervals for EU-level estimates were calculated by bootstrapping adjusted cluster-level estimates. Confidence intervals for project-level estimates were calculated by bootstrapping adjusted cluster-level estimates with the inclusion of stratification of clusters by EU. Both EU- and project-level confidence intervals were estimated over 10,000 bootstrapping iterations, by taking the 2.5th and 97.5th centiles of these results. Logistic regression was used to investigate age and sex associations with trichiasis.
Ethical considerations
Ethical approval was obtained from the ethics committee of the London School of Hygiene & Tropical Medicine (Protocol no. 6319), and the Somali Regional State Health Bureau Ethics Committee (Protocol no. KS/we/01892/06). Informed verbal consent was obtained from all adult participants. For participants under 15 years of age, informed verbal consent was obtained from a parent or guardian. Individuals found to have active trachoma (TF ± TI) were offered treatment with 1% tetracycline eye ointment and individuals with trichiasis were referred for management.
Results
Training and fieldwork were conducted between February and July 2013. A total of 364 clusters were visited. From 10,955 selected households, 53,467 individuals were enumerated; 48,058 (89.9%) people were present and examined by the survey team, 5365 (10%) individuals were absent, and 42 (0.1%) refused to participate. 23,620 (44.2%) of those enumerated were children aged 1–9 years (). The median age of those enumerated was 12 years (25th centile: 5, 75th centile: 31). The sex distribution of those examined was 46.2% male and 53.8% female.
Table 1. Age and sex distribution of enumerated individuals, Global Trachoma Mapping Project, Somali Region, Ethiopia, 2013.
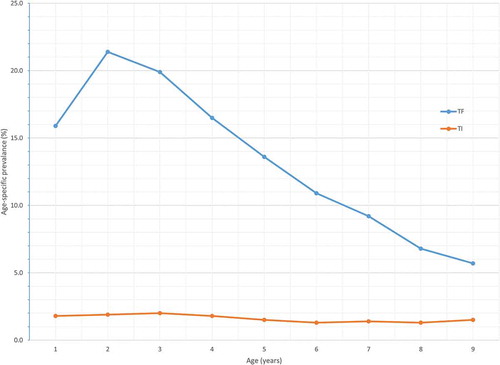
In 1–9-year-olds, the highest age-specific proportion of TF cases (21.4%) was noted in children aged 2 years, with the burden of TF decreasing with increasing age thereafter (χCitation2 for trend = 799.0, P < 0.0001). Similarly, the prevalence of TI decreased with increasing age, although this trend was not statistically significant (χCitation2 for trend = 3.6, P = 0.055) ().
shows the prevalence of TF and TI in children aged 1–9 years in each EU. The TF prevalence in children aged 1–9 years by EU is shown in . The overall age-adjusted TF prevalence in children aged 1–9 years for all project EUs combined was 13.7% (95% CI; 12.1–14.8). TF prevalence was particularly high in the EU covering Awbere woreda in Fafan Subzone (East) (38.1%) and in the EU covering Awbere woreda in Fafan Subzone (West) (33.1%). The overall project-level age-adjusted prevalence of TI in children aged 1–9 years was 1.7% (95% CI; 1.2–2.4). There was marked variation in the TI prevalence in this agegroup between EUs. The highest prevalences were in the EU covering Awbere woreda in Fafan Subzone (West) (7.0%), and the EU covering Kebribeyah and Hareshen woredas in Fafan Subzone (East) (6.8%).
Table 2. Prevalence of trachomatous inflammation–follicular (TF) and trachomatous inflammation–intense (TI) among children aged 1–9 years, Global Trachoma Mapping Project, Somali Region, Ethiopia, 2013.
Figure 2. Age-specific prevalences of trachomatous inflammation–follicular (TF) and trachomatous inflammation–intense (TI) in children aged 1–9 years, Global Trachoma Mapping Project, Somali Region, Ethiopia, 2013.
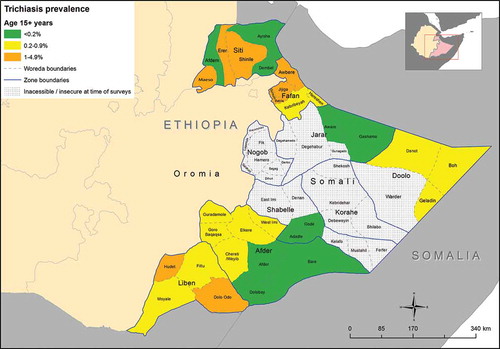
The project-level age- and sex-adjusted prevalence of trichiasis among those aged 15+ years was 0.5% (95% CI; 0.4–0.7). The estimated trichiasis prevalence was highest in the EU covering Awbere in Fafan Subzone (West) (1.2%) and the EU covering Jijiga Rural, Gursum, and Babile woredas in Fafan Subzone (Central) (1.1%) (, ).
Table 3. Prevalence of trichiasis among adults aged over 15 years, Global Trachoma Mapping Project, Somali Region, Ethiopia, 2013.
Figure 3. Prevalence of trachomatous inflammation–follicular (TF) in children aged 1–9 years, Global Trachoma Mapping Project, Somali Region, Ethiopia, 2013.
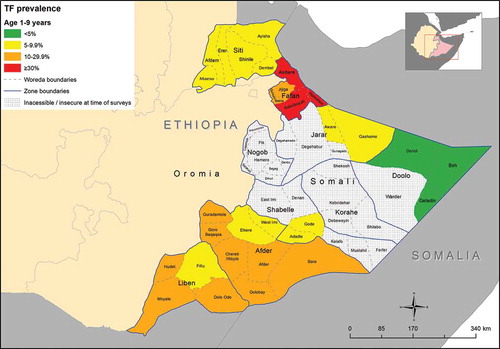
Figure 4. Prevalence of trichiasis among adults aged over 15 years, Global Trachoma Mapping Project, Somali Region, Ethiopia, 2013.
Overall, after adjusting for age, females had 3.4 times higher odds of having trichiasis than males (Likelihood ratio test; OR = 3.43, 95%CI [2.6 –4.5], p-value<0.0001).
Discussion
Our data have important implications for implementation of the SAFE strategy in the Ethiopian Somali Region. There was significant variation in the prevalence of TF, TI, and trichiasis across the 14 EUs studied. Among adults aged over 15 years, the Fafan Zone overall had the EUs with the highest trichiasis prevalences, and delivery of community-based trichiasis surgery services in these areas is a public health emergency. In other EUs, the overall need is less pressing, but every individual with trichiasis should be offered an operation to correct it as soon as possible, in order to limit the risk of irreversible visual impairment. In our surveys, trichiasis was far more likely to be found in women. This excess risk of trichiasis in women compared to men has been found in other surveysCitation15–Citation23 and is presumed to stem from more frequent infection of women with ocular C. trachomatis infection, due to their greater exposure to children, the main infection reservoir.Citation24 Specific measures to increase women’s access to trichiasis surgery are needed here.
Based on TF prevalences in 1–9 year-olds, and following WHO recommendations, Kebribeyah and Hareshen woredas in Fafan Subzone (East) and Awbere woreda in Fafan sub zone (West), need five years of A, F, and E implementation prior to an impact survey; 5 EUs require three years of A, F, and E implementation before impact surveys; and 6 EUs warrant a single round of azithromycin in the first instance, together with implementation of the F and E components of SAFE, before re-survey.
It will be noted that there is a large block of central Somali Region, comprising some 21 woredas, for which we currently have no data on trachoma prevalence ( and ). This is an important limitation to our work as a whole. Whether or not the current absence of data from these areas will affect prospects for trachoma elimination from Ethiopia will depend on whether trachoma is present there, on the extent of population movement between that area and other areas of the country, and the length of time that elapses before the necessary data are acquired, and interventions—if needed—can be commenced.
In conclusion, the prevalence of active trachoma in Ethiopian Somali region is high, with 13 of 14 EUs surveyed needing at least one round of MDA with azithromycin, plus implementation F and E. The trichiasis prevalence was high in four EUs and significant scale-up of efforts to train surgeons to address the backlog of trichiasis surgeries is needed urgently. To achieve the goal of GET2020, further baseline mapping will also be needed in the unmapped woredas of Somali Region, once security has improved.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Funding
This study was principally funded by the Global Trachoma Mapping Project (GTMP) grant from the United Kingdom’s Department for International Development (ARIES: 203145) to Sightsavers, which led a consortium of non-governmental organisations and academic institutions to support ministries of health to complete baseline trachoma mapping worldwide. The GTMP was also funded by the United States Agency for International Development (USAID), through the ENVISION project implemented by RTI International under cooperative agreement number AID-OAA-A-11-00048, and the END in Asia project implemented by FHI360 under cooperative agreement number OAA-A-10-00051. A committee established in March 2012 to examine issues surrounding completion of global trachoma mapping was initially funded by a grant from Pfizer to the International Trachoma Initiative. AWS was a Wellcome Trust Intermediate Clinical Fellow (098521) at the London School of Hygiene & Tropical Medicine. None of the funders had any role in project design, in project implementation or analysis or interpretation of data, in the decisions on where, how or when to publish in the peer reviewed press, or in preparation of the manuscript.
Additional information
Notes on contributors
Ahmed Badei Duale
ABD oversaw field work, interpreted the data, and drafted the manuscript. NNA and RW analysed the data. BK reviewed the data. RMF provided key geographical information system input. CKM, MD, and AWS designed the sampling and analysis and provided technical oversight; AWS is Chief Scientist to the GTMP. ABK, WA, and PAM trained the survey teams. All authors reviewed the draft manuscript critically for intellectual content, and read and approved the final version.
References
- Bourne RRA, Stevens GA, White RA, et al. Causes of vision loss worldwide 1990-2010: a systematic analysis. Lancet Glob Health. 2013; 1(6):e339–e349.
- Mariotti SP, Pascolini D, Rose-Nussbaumer J. Trachoma: global magnitude of a preventable cause of blindness. Br J Ophthalmol. 2009; 93(5):563–568.
- Burton MJ. Trachoma: an overview. Br Med Bull. 2007; 84: 99–116.
- Haddad D. Trachoma: the beginning of the end? Community Eye Health. 2012; 25(77): 18.
- Federal Ministry of Health of Ethiopia. National Trachoma Action Plan. Addis Ababa: Federal Democratic Republic of Ethiopia; 2012.
- Berhane Y, Alemayehu W, Bejiga A. National Survey on Blindness, Low Vision and Trachoma in Ethiopia. Addis Ababa: Federal Ministry of Health of Ethiopia; 2006.
- Bailey R, Lietman T. The SAFE strategy for the elimination of trachoma by 2020: Will it work? Bull World Health Organ. 2001; 79(3): 233–236.
- West SK. Blinding trachoma: prevention with the SAFE strategy. Am J Trop Med Hyg. 2003; 69(5 Suppl): 18–23.
- World Health Organization. Report of the 3rd global scientific meeting on trachoma, Johns Hopkins University, Baltimore, MA, 19-20 July 2010. Geneva: World Health Organization; 2010.
- Solomon AW, Pavluck A, Courtright P, et al. The Global Trachoma Mapping Project: Methodology of a 34-country population-based study. Ophthalmic Epidemiology. 2015; 22(3): 214–225.
- Central Statistical Agency of Ethiopia. Population and Housing Census – Somali Region. Addis Ababa: 2007.
- Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bull World Health Organ. 1987; 65(4): 477–483.
- Solomon AW, Peeling RW, Foster A, Mabey DCW. Diagnosis and assessment of trachoma. Clin Microbiol Rev. 2004;17(4): 982–1011.
- Pavluck A, Chu B, Mann Flueckiger R, Ottesen E. Electronic data capture tools for global health programs: evolution of LINKS, an android-, web-based system. PLoS Negl Trop Dis. 2014; 8(4):e2654.
- King JD, Jip N, Jugu YS, et al. Mapping trachoma in Nasarawa and Plateau States, central Nigeria. Br J Ophthalmol. 2010;94(1): 14–19.
- Kalua K, Singini I, Mukaka M, Senyonjo L. The epidemiology of trachoma in the lower shire valley of southern Malawi and implications for the SAFE strategy. Int J Trop Dis Heal. 2014; 4(5): 494–508.
- Faal H, Minassian D, Sowa S, Foster A. National survey of blindness and low vision in The Gambia: results. Br J Ophthalmol. 1989; 73(2): 82–87.
- Smith JL, Sivasubramaniam S, Rabiu MM, Kyari F, Solomon AW, Multilevel GC. Analysis of trachomatous trichiasis and corneal opacity in Nigeria: The role of environmental and climatic risk factors on the distribution of disease. PLoS Negl Trop Dis. 2015; 9: 7.
- Mpyet C, N M, Adamu MD, et al. Prevalence of trachoma in Katsina State, Nigeria: results of 34 district-level surveys. Ophthalmic Epidemiol. 2016; 23(sup1): 55–62.
- Bero B, Macleod C, Alemayehu W, et al. Prevalence of and risk factors for trachoma in Oromia regional State of Ethiopia: results of 79 population-based prevalence surveys conducted with the global trachoma mapping project. Ophthalmic Epidemiol. 2016; 23(6): 392–405.
- Mpyet C, Muhammad N, Adamu MD, et al. Prevalence of trachoma in Bauchi State, Nigeria: results of 20 local government area-level surveys. Ophthalmic Epidemiol. 2016; 23(sup1): 39–45.
- Elshafie BE, Osman KH, Macleod C, et al. The epidemiology of trachoma in Darfur states and Khartoum state, Sudan: results of 32 population-based prevalence surveys. Ophthalmic Epidemiol. 2016; 23(6): 381–391.
- Adamu Y, Macleod C, Adamu L, et al. Prevalence of trachoma in Benishangul Gumuz Region, Ethiopia: results of seven population-based surveys from the global trachoma mapping project. Ophthalmic Epidemiol. 2016; 23(sup1): 70–76.
- Solomon AW, Holland MJ, Burton MJ, et al. Strategies for control of trachoma: observational study with quantitative PCR. Lancet. 2003;362(9379): 198–204.
Appendix
The Global Trachoma Mapping Project Investigators are: Agatha Aboe (1,11), Liknaw Adamu (4), Wondu Alemayehu (4,5), Menbere Alemu (4), Neal D. E. Alexander (9), Berhanu Bero (4), Simon J. Brooker (1,6), Simon Bush (7,8), Brian K. Chu (2,9), Paul Courtright (1,3,4,7,11), Michael Dejene (3), Paul M. Emerson (1,6,7), Rebecca M. Flueckiger (2), Allen Foster (1,7), Solomon Gadisa (4), Katherine Gass (6,9), Teshome Gebre (4), Zelalem Habtamu (4), Danny Haddad Kalua (5), Amir B. Kello (4,5), Jonathan D. King (6,10,11), Richard Le Mesurier (4,7), Susan Lewallen (4,11), Thomas M. Lietman (10), Chad MacArthur (6,11), Colin Macleod (3,9), Silvio P. Mariotti (7,11), Anna Massey (8), Els Mathieu (6,11), Siobhain McCullagh (8), Addis Mekasha (4), Tom Millar (4,8), Caleb Mpyet (3,5), Beatriz Muñoz (6,9), Jeremiah Ngondi (1,3,6,11), Stephanie Ogden (6), Alex Pavluck (2,4,10), Joseph Pearce (10), Serge Resnikoff (1), Virginia Sarah (4), Boubacar Sarr (5), Alemayehu Sisay (4), Jennifer L. Smith (11), Anthony W. Solomon (1,2,3,4,5,6,7,8,9,10,11), Jo Thomson (4), Sheila K. West (1,10,11), Rebecca Willis (2,9).
Key: (1) Advisory Committee, (2) Information Technology, Geographical Information Systems, and Data Processing, (3) Epidemiological Support, (4) Ethiopia Pilot Team, (5) Master Grader Trainers, (6) Methodologies Working Group, (7) Prioritisation Working Group, (8) Proposal Development, Finances and Logistics, (9) Statistics and Data Analysis, (10) Tools Working Group, (11) Training Working Group.
