ABSTRACT
Purpose: To determine the prevalence of trachoma in four Local Government Areas (LGAs) of Kogi State, Nigeria.
Methods: In June 2014, we conducted population-based, cross-sectional surveys according to Global Trachoma Mapping Project (GTMP) protocols in selected LGAs of Kogi State. In each LGA, 25 clusters were selected with probability proportional to size. In each of these clusters, 25 households were enrolled for the survey. All residents of selected households aged ≥1 year were examined by GTMP-certified graders for trachomatous inflammation–follicular (TF) and trichiasis using the simplified trachoma grading scheme. Data on sources of household water and types of sanitation facilities were collected through questioning and direct observation.
Results: The age-adjusted TF prevalence in 1–9-year-olds ranged from 0.4% (95% CI 0.1–0.8%) in Bassa to 1.0% (95% CI 0.3–1.9%) in Omala. Across all four LGAs, only one case of trichiasis was found; this individual was in Omala, giving that LGA a trichiasis prevalence in individuals aged ≥15 years of 0.02% (95% CI 0.00–0.07%). Between 77 and 88% of households had access to water for hygiene purposes, while only 10–30% had access to improved sanitation facilities.
Conclusion: Trachoma is not a public health problem in any of the 4 LGAs surveyed. There is, however, the need to increase access to adequate water and sanitation services to contribute to the health and social and economic well-being of these communities.
Introduction
Trachoma is an infectious disease of the eye caused by particular strainsCitation1,Citation2 of the bacterium Chlamydia trachomatis. It is spread by direct contact with the ocular or nasal discharges of an infected person, by contact with shared clothing and towels carrying those discharges, or via eye-seeking flies (Musca sorbensCitation3) which act as mechanical vectors. Blindness from trachoma occurs after repeated cycles of infection and resolution over years to decades,Citation4,Citation5 approaching slowly and painfully.Citation6 It is the leading infectious cause of blindness worldwide.Citation7
Since 1993,Citation8 the World Health Organization has recommended implementation of the SAFE (surgery, antibiotics, facial cleanliness, environmental improvement) strategy in populations where trachoma presents a threat to public health. In order to justify use of the SAFE strategy despite competing public health priorities and limited resources, district-level data on the prevalence of trachoma are needed.Citation9 To this end, from 2012 to 2016, the Global Trachoma Mapping Project (GTMP)Citation10 supported countriesCitation11 to undertake population-based prevalence surveys in areas in which trachoma was suspected to be endemic.
Nigeria has a high trachoma burden.Citation12–Citation26 Kogi State, located in central Nigeria, has a population of 3.3 million peopleCitation27 and had not previously been formally surveyed for the disease. To its north, Kogi borders currently or previously trachoma-endemic districts (Local Government Areas, LGAs) of NasarawaCitation16 and NigerCitation24 States. The aim of this series of surveys was to determine the LGA-level prevalence of key signs of trachoma in selected northern LGAs of Kogi State bordering trachoma-endemic (or previously endemic) LGAs, and to generate data on household-level access to water and sanitation.Citation28 These data are needed to guide trachoma elimination efforts at national-, state- and LGA-levels, as required.
Methods
We undertook a population-based prevalence survey in each of four LGAs in Kogi State, as part of the GTMP.
Sample size
For each LGA, we calculated that selecting 25 households in each of 25 clusters would allow 1019 children aged 1–9 years to be examined. This sample size would be sufficient to allow an expected 10% prevalence of trachomatous inflammation–follicular (TF) to be estimated with absolute precision of 3%, assuming a design effect of 2.65 and inflating by a factor of 1.2 to adjust for non-response.Citation20–Citation26,Citation29
Team training
Each field team included a trachoma grader and a data recorder, who were recruited by the local Ministry of Health, were trained and certified according to GTMP protocols, as described in detail elsewhere.Citation29 Grader trainees were required to pass slide- and field-based tests of diagnostic accuracy and recorder trainees were required to pass an electronic examination on the accuracy of their data capture.
Household selection
In each surveyed LGA, we initially selected 25 villages using a systematic, probability-proportional-to-village-size methodology.Citation30 In selected villages, we chose 25 households using the random walk method, as practiced in other Nigerian States that undertook trachoma mapping with GTMP support.Citation20–Citation25
Data collection
At each selected household, global positioning system coordinates were recorded. Data on water and sanitation access were collected through interviews with household members and assessment of household sanitation facilities, where such facilities were present. All household residents aged ≥1 year were eligible to be included in the survey. Consenting individuals were examined by the grader for TF, trachomatous inflammation–intense (TI), and trichiasis, using the criteria set out in the WHO simplified grading scheme,Citation31 a 2.5× magnifying loupe and sunlight illumination. All data were recorded using LINKS softwareCitation29,Citation32 running on Android smartphones, and uploaded to a secure Cloud-based server once the phones were within range of a suitable network.Citation29
Ethical considerations
We adhered to the tenets of the Declaration of Helsinki.Citation33 Adults gave informed verbal consent for their own participation. Verbal consent for participation of those aged <15 years was provided by a parent or guardian. All consent was documented electronically by the recorder. Individuals with active trachoma were given two tubes of 1% tetracycline eye ointment and they (or a care-giver) were instructed on its application. Individuals with trichiasis were referred for surgery by a certified provider. The protocol was approved by the ethics committee of the London School of Hygiene & Tropical Medicine (reference 6319) and the National Health Research Ethics Committee of Nigeria (NHREC/01/01/2007).
Data analysis
Cluster-level proportions of children with TF were adjusted for age in 1-year age bands, and cluster-level proportions of ≥15-year-olds with trichiasis were adjusted for gender and age in 5-year age bands, using data from the 2006 census.Citation27 Confidence intervals (CIs) were calculated from adjusted cluster-level proportions by bootstrapping, with replacement, over 10,000 replications.
Univariable and multivariable analyses for associations of TF in 1–9-year-olds were performed using R 3.3.3 (2017; R Foundation for Statistical Computing, Vienna, Austria). For these analyses, we pooled data across all four LGAs, and used a multi-level hierarchical model to account for clustering at village- and LGA-level.
Prior to conducting multivariable analyses, we used Mantel–Haenszel tests of association to examine collinearity between explanatory variables, but this was not an absolute exclusion criterion. Age and sex were included in the multivariable model a priori, and a stepwise inclusion approach was used, adding variables that were statistically significant in the univariable analyses (Wald test), and retaining them in the final model if they were found to be statistically significant (LRT).
Results
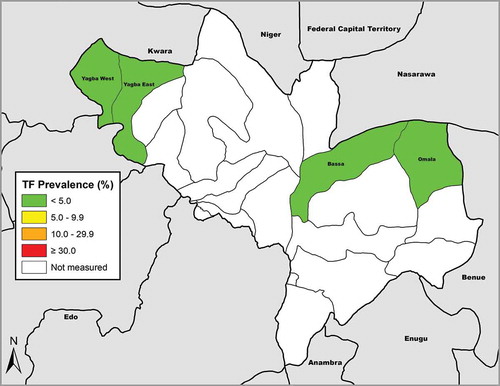
Fieldwork was undertaken in June 2014. For three of the four LGAs, fewer than 750 1–9-year-old children were examined during field team visits to 25 households in 25 villages, and so additional villages were selected, as shown in . In total, after recruitment of additional clusters, 3081 households were surveyed in 124 villages. There were 12,021 individuals resident in those households, of whom 6602 (55%) were female. Graders examined 11,404 (95%) residents; 326 (3%) were absent, and consent for examination was not given for 239 (2%, ). Of individuals examined, 6336 (56%) were female. A total of 3884 children aged 1–9 years (1974 boys) were examined. In Omala, one 58-year-old woman had bilateral trichiasis, giving an age- and gender-adjusted prevalence of trichiasis in individuals aged ≥15 years for that LGA of 0.02% (95% CI 0.00–0.07%). No cases of trichiasis were seen in Bassa, Yagba East or Yagba West (). The age-adjusted TF prevalence in 1–9-year-olds ranged from 0.4% (95% CI 0.1–0.8%) in Bassa to 1.0% (95% CI 0.3–1.9%) in Omala (, ).
Table 1. Number of 1–9-year-olds and number of ≥15-year-olds resident, examined, absent and refused, by Local Government Area (LGA), Global Trachoma Mapping Project, Kogi State, Nigeria, June 2014.
Table 2. Prevalence of trachomatous inflammation–follicular (TF) in 1–9-year-olds and prevalence of trichiasis in ≥15-year-olds, by Local Government Area, Global Trachoma Mapping Project, Kogi State, Nigeria, June 2014.
Figure 1. Prevalence of trachomatous inflammation-follicular (TF) in 1–9-year-old children, Global Trachoma Mapping Project, Kogi State, Nigeria, June 2014.
The proportion of households with access to an improved source of water for face-washing ranged from 30% in Bassa to 94% in Yagba West. Only 10% of households in Bassa LGA had an improved latrine compared to 33% in Yagba West. In all four LGAs, a majority of households had a source of water for face-washing within a 30 minute round trip ().
Table 3. Household-level access to water and sanitation, by Local Government Area (LGA), Global Trachoma Mapping Project, Kogi State, Nigeria, June 2014.
Associations of TF
We categorized age in 3-year intervals, and this grouping was shown to fit our final model better than using 1-year intervals (LRT, p = 0.0008) or 4-year intervals (LRT, p = 0.001).
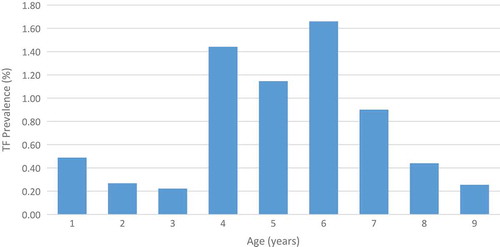
There was a clear difference in the prevalence of TF among children by age group, with higher disease prevalence in children between the ages of 4–6 years ().
Figure 2. Prevalence of trachomatous inflammation–follicular (TF) among 1–9-year-olds, by age, Global Trachoma Mapping Project, Kogi State, Nigeria, June 2014.
Results of the univariable analysis for associations of TF in children aged 1–9 years are shown in . Children living in households reporting open defaecation did not have significantly different odds of TF to those with access to a latrine facility. As a sensitivity analysis, we also compared the odds of TF for children living in households with access to improved sanitation versus those in households with unimproved sanitation (access to an unimproved latrine or use of open defaecation); the difference was not significant (p = 0.3). There was a similar lack of association of TF with access to private versus public or shared latrine. Results of the multivariable analysis are shown in . Compared to children aged 7–9 years, children aged 4–6 years had 2.6 (95% CI 1.1–6.3) times greater odds of having TF. Children living in households with four or more children had 2.4 (95% CI 1.1–5.3) times greater odds of having TF than children in households with fewer than four children.
Table 4. Univariable analysis of associations of trachomatous inflammation–follicular in 1–9-year-olds, Global Trachoma Mapping Project, Kogi State, Nigeria, June 2014.
Table 5. Multivariable analysis of risk factors related to trachomatous inflammation–follicular in 1–9-year-olds, Global Trachoma Mapping Project, Kogi State, Nigeria, June 2014.
Discussion
Trachoma is not a public health problem in these Kogi State LGAs. The TF prevalence in 1–9-year-olds was well below 5% in each LGA surveyed, and the prevalence of trichiasis in individuals aged ≥15 years was <0.2% in each LGA, indicating that none of the components of the SAFE strategy need be deployed at public health level for the purposes of trachoma elimination.Citation34 Data from Nigeria’s 2007 National Blindness and Visual Impairment SurveyCitation35 indicate that risk of trichiasis and corneal opacity is concentrated towards the country’s north,Citation18 and we take the present set of results as confirmation that trachoma mapping south of the LGAs surveyed here is not indicated. Trachoma elimination efforts should be directed to the north of Nigeria, where trachoma has been shown to be of public health importance.Citation18–Citation25
Although a majority of households in each of these LGAs had access to a source of water for face-washing within a 30 minute round trip of the household, less than one-third of households in each LGA had access to an improved sanitation facility. It could therefore be hypothesized that despite low sanitation coverage, relatively good access to water may have facilitated maintenance of clean faces, contributing to low levels of active disease.Citation36,Citation37 However, in our analyses, neither access to water nor access to latrines was significantly associated with the presence of TF in children in these LGAs. Characteristics independently associated with TF were being aged 4–6 years, and living in a household with four or more children. Children aged 4–6 years may be less likely to practice facial hygiene than children aged 7–9 years. Children in households with more children may have more frequent or more sustained contact with other children than those living in households with fewer children. However, the importance of the associations that we observed here may be questionable, given the very low prevalence of active trachoma in this population.Citation38
Our work had some limitations. First, the random walk was used in GTMP sub-projects in northern Nigeria because of local insecurity and our consequent desire to use a household-sampling strategy that was familiar to local residents.Citation20–Citation25 We used it in Kogi State by extension, even though we know it to be epidemiologically sub-optimal.Citation39–Citation41 Second, in three of the four LGAs, we added extra clusters mid-survey, due to lighter-than-expected recruitment of examinees in the first 25 selected villages. We recognize that adding clusters to a set of previously-chosen, systematically sampled clusters compromises the principle of equal-probability sampling. It is somewhat reassuring to note that prevalence estimates were below WHO-defined thresholdsCitation34 for elimination as a public health problem whether generated with or without the additional clusters. Third, our surveys were under-powered for precisely estimating the LGA-level prevalence of trichiasis, but the fact that only one individual with trichiasis was found amongst 6381 adults examined across four LGAs reinforces the impression that end-stage trachoma is rare here. Fourth, we did not examine eyes with trichiasis for conjunctival scar. Recognition of the importance of doing so arose in late 2015,Citation42 after these surveys were complete, but again, only one trichiasis case was seen, so the lack of conjunctival scarring data is perhaps inconsequential when assessing the public health relevance of the Kogi findings.
All four LGAs meet the elimination target for TF (prevalence <5% in 1–9-year-olds) and trichiasis (prevalence <0.2% in ≥15-year-olds). However, in order to achieve the United Nations Sustainable Development Goals for water and sanitation, safeguard health, improve quality of life, and fulfil the human right to water and sanitation, there is a need to improve access to adequate water and sanitation services in these LGAs.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the writing and content of this article.
Funding
This study was principally funded by the Global Trachoma Mapping Project (GTMP) grant from the United Kingdom’s Department for International Development (DFID; ARIES: 203145) to Sightsavers, which led a consortium of non-governmental organizations and academic institutions to support health ministries to complete baseline trachoma mapping worldwide. The GTMP was also funded by the United States Agency for International Development (USAID), through the ENVISION project implemented by RTI International under cooperative agreement number AID-OAA-A-11-00048, and the END in Asia project implemented by FHI360 under cooperative agreement number OAA-A-10-00051. A committee established in March 2012 to examine issues surrounding completion of global trachoma mapping was initially funded by a grant from Pfizer to the International Trachoma Initiative. AWS was a Wellcome Trust Intermediate Clinical Fellow (098521) at the London School of Hygiene & Tropical Medicine, and is now, like SB, a staff member of the World Health Organization. VVF contributed to this study as part of his internship at WHO. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated. None of the funders had any role in project design, in project implementation or analysis or interpretation of data, in the decisions on where, how or when to publish in the peer reviewed press, or in preparation of the manuscript.
References
- Caldwell HD, Wood H, Crane D, et al. Polymorphisms in Chlamydia trachomatis tryptophan synthase genes differentiate between genital and ocular isolates. J Clin Invest. 2003;111(11):1757–1769.
- Hadfield J, Harris SR, Seth-Smith HMB, et al. Comprehensive global genome dynamics of Chlamydia trachomatis show ancient diversification followed by contemporary mixing and recent lineage expansion. Genome Res. 2017;27(7):1220–1229.
- Emerson PM, Bailey RL, Mahdi OS, Walraven GE, Lindsay SW. Transmission ecology of the fly Musca sorbens, a putative vector of trachoma. Trans R Soc Trop Med Hyg. 2000;94(1):28–32.
- Grayston JT, Wang SP, Yeh LJ, Kuo CC. Importance of reinfection in the pathogenesis of trachoma. Rev Infect Dis. 1985;7(6):717–725.
- Gambhir M, Basanez MG, Burton MJ, et al. The development of an age-structured model for trachoma transmission dynamics, pathogenesis and control. PLoS Negl Trop Dis. 2009;3(6):e462.
- Palmer SL, Winskell K, Patterson AE, et al. ‘A living death’: a qualitative assessment of quality of life among women with trichiasis in rural Niger. Int Health. 2014;6(4):291–297.
- Bourne RR, Stevens GA, White RA, et al. Causes of vision loss worldwide, 1990-2010: a systematic analysis. Lancet Global Health. 2013;1(6):e339–49.
- Francis V, Turner V. Achieving Community Support for Trachoma Control (WHO/PBL/93.36). Geneva: World Health Organization; 1993.
- Smith JL, Haddad D, Polack S, et al. Mapping the global distribution of trachoma: why an updated atlas is needed. PLoS Negl Trop Dis. 2011;5(6):e973.
- Solomon AW, Kurylo E. The global trachoma mapping project. Community Eye Health. 2014;27(85):18.
- Heggen AE, Solomon AW, Courtright P. Perspectives of National Coordinators and Partners on the Work of the Global Trachoma Mapping Project. Ophthalmic Epidemiol. 2016;23(6):366–372.
- Smith JL, Flueckiger RM, Hooper PJ, et al. The geographical distribution and burden of trachoma in Africa. PLoS Negl Trop Dis. 2013;7(8):e2359.
- Rabiu MM, Abiose A. Magnitude of trachoma and barriers to uptake of lid surgery in a rural community of northern Nigeria. Ophthalmic Epidemiol. 2001;8(2–3):181–190.
- Mpyet C, Ogoshi C, Goyol M. Prevalence of trachoma in Yobe State, north-eastern Nigeria. Ophthalmic Epidemiol. 2008;15(5):303–307.
- Jip NF, King JD, Diallo MO, et al. Blinding trachoma in Katsina state, Nigeria: population-based prevalence survey in ten local government areas. Ophthalmic Epidemiol. 2008;15(5):294–302.
- King JD, Jip N, Jugu YS, et al. Mapping trachoma in Nasarawa and Plateau States, central Nigeria. Br J Ophthalmol. 2010;94(1):14–19.
- Ramyil A, Wade P, Ogoshi C, et al. Prevalence of trachoma in Jigawa State, north-western Nigeria. Ophthalmic Epidemiol. 2015;22(3):184–189.
- Smith JL, Sivasubramaniam S, Rabiu MM, Kyari F, Solomon AW, Gilbert C. Multilevel analysis of trachomatous trichiasis and corneal opacity in Nigeria: the role of environmental and climatic risk factors on the distribution of disease. PLoS Negl Trop Dis. 2015;9(7):e0003826.
- Mpyet C, Lass BD, Yahaya HB, Solomon AW. Prevalence of and risk factors for trachoma in Kano state, Nigeria. PloS One. 2012;7(7):e40421.
- Mpyet C, Muhammad N, Adamu MD, et al. Trachoma Mapping in Gombe State, Nigeria: results of 11 Local Government Area Surveys. Ophthalmic Epidemiol. 2016;23(6):406–411.
- Mpyet C, Muhammad N, Adamu MD, et al. Prevalence of Trachoma in Katsina State, Nigeria: results of 34 district-level surveys. Ophthalmic Epidemiol. 2016;23(sup1):55–62.
- Mpyet C, Muhammad N, Adamu MD, et al. Prevalence of Trachoma in Bauchi State, Nigeria: results of 20 Local Government Area-Level Surveys. Ophthalmic Epidemiol. 2016;23(sup1):39–45.
- Muhammad N, Mpyet C, Adamu MD, et al. Mapping Trachoma in Kaduna State, Nigeria: results of 23 Local Government Area-Level, Population-Based Prevalence Surveys. Ophthalmic Epidemiol. 2016;23(sup1):46–54.
- Adamu MD, Mpyet C, Muhammad N, et al. Prevalence of Trachoma in Niger State, North Central Nigeria: results of 25 Population-Based Prevalence Surveys Carried Out with the Global Trachoma Mapping Project. Ophthalmic Epidemiol. 2016;23(sup1):63–69.
- Mpyet C, Muhammad N, Mohammed AD, et al. Prevalence of trachoma in Kano state, Nigeria: results of 44 Local Government Area-level surveys. Ophthalmic Epidemiol. 2017;24(3):195–203.
- Muhammad N, Mohammed A, Isiyaku S, Adamu MD, Gwom A, Rabiu MM. Mapping trachoma in 25 local government areas of Sokoto and Kebbi states, northwestern Nigeria. Br J Ophthalmol. 2014;98(4):432–437.
- National Population Commission. 2006 Population and Housing Census of the Federal Republic of Nigeria: National and State Population and Housing Tables, Priority Tables (Volume 1). Abuja: National Population Commission; 2009.
- Boisson S, Engels D, Gordon BA, et al. Water, sanitation and hygiene for accelerating and sustaining progress on neglected tropical diseases: a new Global Strategy 2015-20. Int Health. 2016;8(Suppl 1):i19–i21.
- Solomon AW, Pavluck A, Courtright P, et al. The Global Trachoma Mapping Project: methodology of a 34-country population-based study. Ophthalmic Epidemiol. 2015;22(3):214–225.
- Solomon AW, Zondervan M, Kuper H, Buchan JC, Mabey DCW, Foster A. Trachoma Control: A Guide for Programme Managers. Geneva: World Health Organization; 2006.
- Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bull World Health Organ. 1987;65(4):477–483.
- Pavluck A, Chu B, Mann Flueckiger R, Ottesen E. Electronic data capture tools for global health programs: evolution of LINKS, an Android-, web-based system. PLoS Negl Trop Dis. 2014;8(4):e2654.
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama. 2013;310(20):2191–2194.
- World Health Organization. Validation of Elimination of Trachoma as a Public Health Problem (WHO/HTM/NTD/2016.8). Geneva: World Health Organization; 2016.
- Dineen B, Gilbert CE, Rabiu M, et al. The Nigerian national blindness and visual impairment survey: rationale, objectives and detailed methodology. BMC Ophthalmol. 2008;8:17.
- West S, Munoz B, Lynch M, et al. Impact of face-washing on trachoma in Kongwa, Tanzania. Lancet. 1995;345(8943):155–158.
- Pinsent A, Burton MJ, Gambhir M. Enhanced antibiotic distribution strategies and the potential impact of facial cleanliness and environmental improvements for the sustained control of trachoma: A modelling study. BMC Medicine. 2016;14(1):1–10.
- Solomon AW, Foster A, Mabey DC. Clinical examination versus Chlamydia trachomatis assays to guide antibiotic use in trachoma control programmes. Lancet Infect Dis. 2006;6(1):5–6. author reply 7-8.
- Brogan D, Flagg EW, Deming M, Waldman R. Increasing the accuracy of the Expanded Programme on Immunization’s cluster survey design. Ann Epidemiol. 1994;4(4):302–311.
- Turner AG, Magnani RJ, Shuaib M. A not quite as quick but much cleaner alternative to the Expanded Programme on Immunization (EPI) Cluster Survey design. Int J Epidemiol. 1996;25(1):198–203.
- Grais RF, Rose AM, Guthmann JP. Don’t spin the pen: two alternative methods for second-stage sampling in urban cluster surveys. Emerg Themes Epidemiol. 2007;4:8.
- World Health Organization Alliance for the Global Elimination of Trachoma by 2020. Second Global Scientific Meeting on Trachomatous Trichiasis. Cape Town, 4-6 November 2015 (WHO/HTM/NTD/2016.5). Geneva: World Health Organization; 2016.
Appendix
The Global Trachoma Mapping Project Investigators are as follows: Agatha Aboe (1,11), Liknaw Adamu (4), Wondu Alemayehu (4,5), Menbere Alemu (4), Neal D. E. Alexander (9), Ana Bakhtiari (2,9), Berhanu Bero (4), Sarah Bovill (8), Simon J. Brooker (1,6), Simon Bush (7,8), Brian K. Chu (2,9), Paul Courtright (1,3,4,7,11), Michael Dejene (3), Paul M. Emerson (1,6,7), Rebecca M. Flueckiger (2), Allen Foster (1,7), Solomon Gadisa (4), Katherine Gass (6,9), Teshome Gebre (4), Zelalem Habtamu (4), Danny Haddad (1,6,7,8), Erik Harvey (1,6,10), Dominic Haslam (8), Khumbo Kalua (5), Amir B. Kello (4,5), Jonathan D. King (6,10,11), Richard Le Mesurier (4,7), Susan Lewallen (4,11), Thomas M. Lietman (10), Chad MacArthur (6,11), Colin Macleod (3,9), Silvio P. Mariotti (7,11), Anna Massey (8), Els Mathieu (6,11), Siobhain McCullagh (8), Addis Mekasha (4), Tom Millar (4,8), Caleb Mpyet (3,5), Beatriz Muñoz (6,9), Jeremiah Ngondi (1,3,6,11), Stephanie Ogden (6), Alex Pavluck (2,4,10), Joseph Pearce (10), Serge Resnikoff (1), Virginia Sarah (4), Boubacar Sarr (5), Alemayehu Sisay (4), Jennifer L. Smith (11), Anthony W. Solomon (1,2,3,4,5,6,7,8,9,10,11), Jo Thomson (4), and Sheila K. West (1,10,11), Rebecca Willis (2,9).
Key: (1) Advisory Committee, (2) Information Technology, Geographical Information Systems, and Data Processing, (3) Epidemiological Support, (4) Ethiopia Pilot Team, (5) Master Grader Trainers, (6) Methodologies Working Group, (7) Prioritisation Working Group, (8) Proposal Development, Finances and Logistics, (9) Statistics and Data Analysis, (10) Tools Working Group, (11) Training Working Group.
