ABSTRACT
Purpose: Senegal is endemic for trachoma, an infectious and potentially blinding eye disease. To complete the country’s district-level baseline map of trachoma, we conducted population-based surveys in 17 health districts that were suspected-endemic but had yet to be surveyed.
Methods: We randomly selected 30 clusters (villages) per district and 30 households per village, and estimated the district-level prevalences of trachomatous inflammation—follicular (TF) in children aged 1–9 years, and trichiasis in persons aged ≥15 years. Data on household-level water, sanitation, and hygiene variables were also collected. Global Trachoma Mapping Project methods were followed in training, fieldwork, and data handling.
Results: 25,704 children aged 1–9 years and 30,345 adults aged 15 years and above were examined. In children aged 1–9 years, the prevalence of TF was <5% in all 17 districts, with the exception of Saint-Louis (5.1%, 95% CI 3.2–7.5). Trichiasis prevalence in participants aged 15 years and above ranged by district from 0%–1.1% (95% CI 0.7–1.5), with 9 districts having trichiasis prevalences above the elimination threshold of 0.2%. Trichiasis was seen to be significantly less frequent in males than in females (0.17% [95% CI 0.12–0.24] versus 0.49% [95% CI 0.38–0.61], p < 0.001). The prevalence of trichiasis rose steeply with age; 62% of cases were observed in people aged 55 years or above.
Conclusions: Active trachoma is not a public health problem in 16 of the 17 surveyed districts, and implementation of the full Surgery (S) – Antibiotics (A) – Facial cleanliness (F) – Environmental improvement (E) strategy is not a programmatic priority. Increased provision of trichiasis surgery is warranted in nine districts.
Introduction
Trachoma is a chronic keratoconjunctivitis caused by four serovars of Chlamydia trachomatis: A, B, Ba, and C.Citation1 Active trachoma, which is most commonly present in young children, is characterized by chronic, follicular conjunctivitis.Citation2,Citation3 Reinfection occurs in situations where individual and group hygiene are poor, and water and sanitation are lacking. Repeated infections result in conjunctival scarring causing entropion (in-turning of the eyelids) and trichiasis (eyelashes touching the eyeball). Corneal opacification resulting from mechanical trauma from in-turned lashes, a dry ocular surface and secondary infection, can ultimately lead to blindness.Citation1,Citation4
Trachoma is responsible for a significant disease burden in low-income countries. Current estimates suggest that 200 million people are at risk of blindness from trachoma, 1.9 million are already blind or severely visually impaired, and 3.2 million have trichiasis.Citation5,Citation6
Efforts to eliminate trachoma as a public health problem, led by the World Health Organization (WHO) Alliance for the Global Elimination of Trachoma by 2020 (GET2020), rely on a multifaceted approach: SAFE (Surgery for trichiasis, Antibiotics to treat C. trachomatis infection, Facial cleanliness and Environmental improvement to reduce transmission). The thresholds for elimination of trachoma as a public health problem are a “trachomatous inflammation—follicular” (TF) prevalence <5% in children aged 1–9 years, and a “trachomatous trichiasis” (TT) prevalence unknown to the health system of <0.2% in adults aged 15 years and above.Citation7 To plan interventions at baseline, to assess their impact, and to demonstrate achievement of elimination goals, reliable prevalence data at the district level are required.
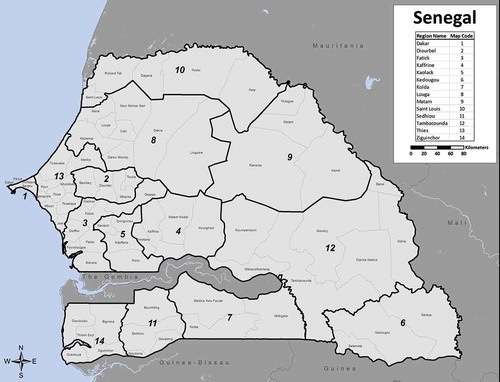
In 1997, it was suggested that trachoma was the second-leading cause of blindness in Senegal, with nearly 25,000 people blind as a result of the diseaseCitation8; its control has therefore been a major focus of the national blindness prevention policy. In line with GET2020, Senegal has set the goal of eliminating trachoma as a public health problem by 2020.Citation9 In 2000, prevalence surveys were conducted in eight of the country’s then-10 medical regions (i.e., Dakar, Diourbel, Fatick, Kaolack, Louga, Saint Louis, Tambacounda, and Thiès), to determine the prevalence of trachoma. Kolda and Ziguinchor regions were not included in that round of surveys, for security reasons. These surveys were not designed to provide data at the health district level.Citation10
Four of the country’s medical regions were subdivided in 2008, resulting in 14 regions overall and the formation of new health districts.Citation11 Between 2004 and 2013, district-level baseline surveys were completed in 37 districts (), using WHO-recommended methods and sampling 30 clusters per district.Citation12,Citation13 These surveys were conducted incrementally as funds became available, with priority given to zones that were expected to be at greater risk of trachoma based on the findings of the region-level prevalence surveys.
Table 1. District-level estimates of baseline trachoma prevalence in Senegal generated prior to 2014.
Based on these findings, and in line with WHO guidance then in effect,Citation13 antibiotic mass drug administration (MDA) was progressively introduced from 2005–2014 in 17 of the 18 districts where baseline district (or sub-district) TF prevalence was ≥10% among children aged 1–9 years. The eighteenth district, a highly populous and urbanized area with a seasonally fluctuating population, was resurveyed in 2016 to better target MDA requirements,Citation14 and initiated MDA in 2017. Facial cleanliness and environmental improvement measures have been implemented periodically in certain districts, but have not been consistently documented. Surgery for trichiasis was initiated in 2006 and has principally been conducted in regions that required MDA.
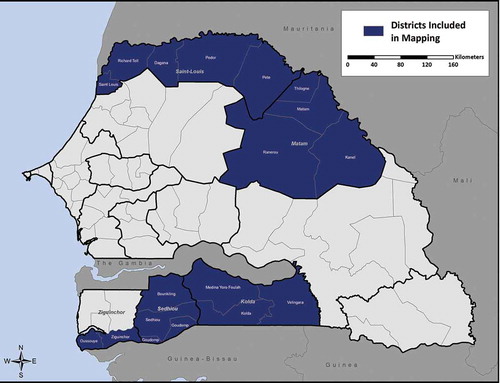
In order to complete the country’s district-level baseline map of trachoma and determine the need for trachoma elimination interventions in those districts suspected to be endemic but not yet mapped, in 2014, we conducted surveys in 17 health districts in collaboration with the Global Trachoma Mapping Project (GTMP).Citation15
Methods
Ethics statement
The surveys were led by the Ministry of Health and Social Work (MSAS)’s National Eye Health Promotion Program (PNPSO). Ethical clearance was obtained from the MSAS’s Planning of Research and Statistics Directorate (Protocole SEN13/66 - Nº044 MSAS/DPRS/DR, 21 January 2014), based on the review of the National Ethics Committee for Health Research; and from the ethics committee of the London School of Hygiene & Tropical Medicine (reference 6319).
In each village, the survey team paid a courtesy visit to the village head to explain the purpose of the survey. General information about trachoma was given prior to starting surveys. Verbal informed consent was given by the head of the household for children aged <15 years, and by participants themselves when aged 15 years or above. Consent for trachoma examination and interviews was documented by the field team on the electronic data form in the GTMP-LINKS application.Citation15 Children found to have TF were given tetracycline 1% ointment to apply twice daily for 6 weeks. Trichiasis cases were referred for surgery.
Evaluation units
Senegal is divided into 14 medical regions, which are further divided into 76 health districts (). The 17 health districts mapped in 2014 were drawn from five medical regions. The two northern regions (Saint Louis and Matam) are bordered by Mauritania, and the three southern regions (Ziguinchor, Sédhiou, Kolda) are bordered by Gambia to the north and by Guinea-Bissau and/or Republic of Guinea to the south (). The north is characterized by a hot desert climate, and the south by a tropical wet and dry climate.
All districts in Saint Louis, Matam, Séhiou, and Kolda were surveyed. In Ziguinchor Region, two districts were surveyed; the region’s other three districts completed their surveys in 2010 ().
The population of the districts surveyed ranged from 50,944 to 303,659.
Survey design and sampling
Our work here consisted of a series of community-based cross-sectional surveys designed to assess district-level prevalence of TF in children aged 1–9 years, and trichiasis in adults aged 15 years and above. This involved examination of participants for clinical signs of trachoma using the WHO simplified trachoma grading system,Citation16 recording each of TT, TF, and trachomatous inflammation-intense (TI) as absent or present; the other signs of the simplified system (trachomatous conjunctival scarring (TS) and corneal opacity (CO)) were not recorded.Citation17 This was the first time that trachoma baseline surveys in Senegal consistently assessed both females and males for trichiasis; most previous surveys of trichiasis conducted in the country had included women only. Inclusion of males as well as females is in line with the WHO protocol,Citation13 and is an important methodological consideration as it avoids the overestimation of prevalence in the overall population resulting from examining females only.Citation18
A two-stage cluster random sampled design with probability proportional to size was used to select the sample in each district, as recommended by WHO.Citation13 Thirty clusters (villages) per district were randomly selected in the first stage. The second-stage selection, witnessed by the head of each selected village, took place in the field. Thirty households per village were randomly drawn from a list of households provided by the village head.Citation15 If the village had fewer than 30 households, the nearest village was also visited and random selection performed to complete 30 households.
As outlined elsewhere,Citation15 the sample size for the survey was calculated to estimate with 95% confidence an expected 10% TF prevalence in children aged 1–9 years, with absolute precision of 3% and a design effect of 2.65. With inflation by a factor of 1.2 to allow for non-response, this gave rise to a sample size of 1,222 children in selected households per district, with the expectation that about 1,019 children would be examined.
Training
Eight ophthalmic clinical officers (Technicien supérieur opthalmologique or TSO) working in the Senegalese health system (prospective trachoma graders), and five people with information technology backgrounds (prospective data recorders), underwent a 5-day training program led by a GTMP-certified master graderCitation15 (Boubacar Sarr) and a GTMP-certified grader trainerCitation15 (Mactar Sissoko), who also provided field supervision during the survey. Version 2 of the training system was used.Citation17 Prospective trachoma graders were trained on examination using the WHO simplified grading system and on standardized procedures for selection of households.Citation17 A practical test was conducted in a non-survey cluster in Thiès district, with the help of children attending three traditional Koranic schools (daraas).
To participate in the survey, prospective trachoma graders had to achieve a kappa score of at least 0.8 in inter-grader agreement with the grader trainer, in identification of TF in children. Data recorders were trained to conduct household interviews and to capture data using Android smartphones (Motorola Atrix 2, Motorola Inc., Schaumburg, IL), and the LINKS application from the Task Force for Global Health, following the GTMP protocol. After training, 4 trachoma graders and 4 data recorders passed their respective tests and were selected to conduct the survey.
Household survey
Each village has a Health Post Head Nurse (Infirmier Chef de Poste) whose main responsibilities are to manage the health post (center) and to supervise all health-related activities at village level. Health Post Head Nurses were briefed about the surveys by the Health District Head Doctor (Médecin-Chef de District) prior to teams visiting the selected villages. They facilitated contact with the village heads and assisted the survey team in developing a calendar for visits, as well as designating one village resident to serve as a guide. There were four survey teams, each consisting of one grader, one data recorder, one driver, and one guide.
Upon arrival in the village and after meeting with the village head, consent was obtained from the household head of each selected household. Global positioning system (GPS) data were collected at each household. All eligible participants were examined for trachoma signs using a 2.5× magnifying binocular loupe and a torch (flashlight) or sunlight. Household interview questions included, inter alia, the source of drinking water, time needed to fetch water, main source of water for face washing, defecation habits, and sanitation facilities. Some information was collected by direct observation, as described elsewhere.Citation15
Data management and analysis
Field data collection was performed using the LINKS app on Android smartphones. Data forms were uploaded each day using 256-bit SSL encryption to the GTMP secure server, housed on the Amazon Elastic Compute Cloud.Citation19 Prevalences were calculated with gender- and age-adjustment using R 3.0.2 (The R Foundation for Statistical Computing, Vienna, Austria) and Structured Query Language, as described elsewhere,Citation15 using 2013 Senegal census data as the reference population set.Citation20 The district-level adjusted prevalence was determined by mean of adjusted cluster-level proportions of children with TF. The same method was used to calculate the district-level adjusted trichiasis prevalence, but with adjustment for both gender and age in 5-year age groups. Confidence intervals around prevalence estimates were calculated by bootstrapping the cluster means over 10,000 iterations and taking the 2.5th and 97.5th centiles of all ordered results.
Prevalence was calculated for trichiasis, which can be due to multiple causes,Citation21 rather than for TT, because the presence or absence of TS was not recorded in eyes with trichiasis; consensus for recording TS was established after these surveys were conducted.Citation22
Risk factors associated with active trachoma were investigated using STATA 14 (StataCorp, College Station, TX). Variables were examined in univariate analyses as well as through multiple regression. Risk factor means were generated without sample weights, and standard errors were adjusted for clustering using Taylor linearization.
Results
Study population
Between March and October 2014, a total of 71,493 (25,704 children aged 1–9 years, 36,633 adults aged 15 years and above) individuals were enumerated and 63,716 (25,144 children aged 1–9 years, 30,345 adults aged 15 years and above) were examined for trachoma (response rate of 89.1%) in the 17 districts. Of the 7,777 people not examined, 98.7% were absent and 59.0% were male aged 15 years and above. The mean age of participants included in the study was 21.1 years (sd = 19.8); 54.7% were female ().
Table 3. Prevalence of trachomatous inflammation–follicular (TF) and trichiasis by district, Senegal, 2014.
Table 2. Characteristics of the surveyed population by district, Senegal, 2014.
Prevalence of TF and trichiasis, and risk factors
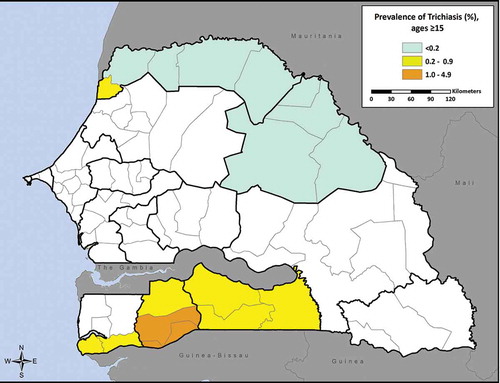
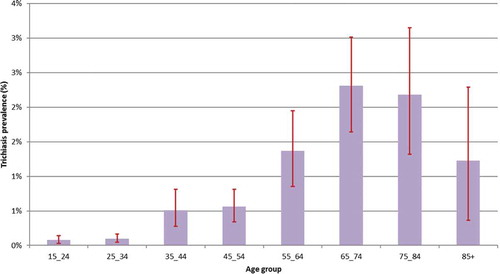
summarizes the prevalence of TF and trichiasis in each district. All districts had a TF prevalence below 5% with the exception of Saint-Louis (5.1%, 95% CI 3.2–7.5) (). Among children aged 1–9 years sampled, the proportion of males with TF was not significantly higher than that of females (2.9% [375/12,554] versus 2.6% [334/12,441], p = 0.17) (). The prevalence of trichiasis in individuals aged 15 years and above ranged by district from 0% to 1.1% (95% CI 0.7–1.5) (). We identified no cases of trichiasis in three districts (Matam, Ranérou, Pété). Of the 203 cases identified in the other 11 districts, the proportion of males with trichiasis was significantly lower than that of females (0.3% [58/15,125] versus 0.7% [145/21,305], p < 0.001). This difference between prevalence of trichiasis in male and female adults remained significant after adjusting both estimates for age (0.17% [95% CI 0.12–0.24] versus 0.49% [95% CI 0.38–0.61], p < 0.001). The prevalence of trichiasis rose steeply with age; 62% of cases were in people aged 55 years or above ().
Figure 3. Prevalence of trachomatous inflammation–follicular (TF) in the 17 districts mapped for trachoma, Senegal, 2014.
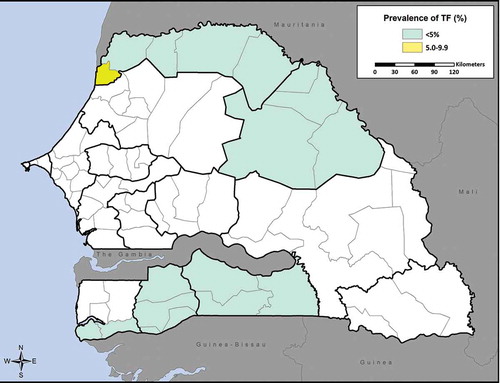
Figure 5. Trachomatous inflammation–follicular (TF) prevalence by age amongst children aged 1–9 years across 17 surveyed districts, Senegal, 2014. Whiskers indicate 95% confidence intervals.
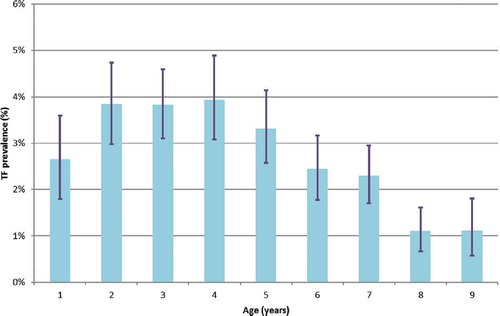
Figure 6. Trichiasis prevalence by age amongst individuals aged ≥15 years across 17 surveyed districts, Senegal, 2014. Whiskers indicate 95% confidence intervals.
Household-level access to improved water source for face washing ranged by district from 16.6% to 87.0% (). “Improved” sources of water included piped water into dwelling; piped water to yard/plot; public tap or standpipe; tubewell or borehole; protected dug well; protected spring; and rainwater.Citation17,Citation23 Respondents from nearly half the households (47.5%) reported using an unprotected dug well as the main source of water for washing faces. Household-level access to improved latrines ranged by district from 7.3% to 93.1% (). “Improved” sanitation included flush toilet; piped sewer system; septic tank; flush/pour flush to pit latrine; ventilated improved pit latrine (VIP); pit latrine with slab; composting toilet; and flush/pour flush to unknown place.Citation17,Citation23 67.0% of respondents reported having a round trip to collect water of less than 30 minutes (). It should be noted that within a given household, responses about availability and use of latrines could be provided by several different respondents.
Univariate analysis identified water retrieval time as the only risk factor among those investigated to have a significant (p ≤ 0.05) relationship to TF in children aged 1–9 years. Specifically, a round-trip of less than 30 minutes to collect water for face washing was associated with lower odds of TF (OR 0.54, 95% CI 0.4–0.7, p < 0.001). No significant relationship was observed between TF and self-reported location of defecation by adults aged 15 years and above (using latrines vs. no structure) (OR 1.11, 95% CI 0.8–1.5, p = 0.504), having an improved latrine type in the household (OR 1.05, 95% CI 0.8–1.4, p = 0.723), or having an improved water source for face washing (OR 1.27, 95% CI 0.98–1.64, p = 0.065). Similarly, no significant relationship was found between TF and having a functional handwashing station nearby, specified as one with soap and water within 15 meters (OR 1.67, 95% CI 0.96–2.89, p = 0.068).
The relationship between water retrieval time and TF remained significant when considered in multivariate analysis alongside the other risk factor covariates mentioned in the above paragraph (OR 0.50, 95% CI 0.4–0.7, p < 0.001). As in the univariate analysis, none of the other risk factors showed relationships to TF prevalence, though the low prevalence of TF detected in this survey makes the analysis of risk factors more difficult.
When analyses included TF in all age groups, similar results were found, showing a significant, negative relationship between a round-trip of less than 30 minutes to collect water for face washing and TF prevalence (OR 0.53, 95% CI 0.4–0.7, p < 0.001). No other investigated risk factor was found to have a significant relationship with TF.
Discussion
Obtaining prevalence data at the district level is a prerequisite for planning and implementing coherent and efficient interventions for trachoma elimination. The present study, which used methods recommended by WHO for sampling of populations and examination for trachoma, was designed to determine trachoma prevalence in 17 districts in Senegal which had had no prior district-level trachoma surveys.
Active trachoma
Based on TF prevalence estimates, implementation of the full SAFE strategy is not warranted in 16 of the 17 districts surveyed. In Saint Louis district, which registered a TF prevalence of 5.1%, a one-year implementation of the SAFE strategy’s A, F, and E components, followed by an impact survey, could be considered.Citation24 These prevalences, which are generally lower than those for other districts of Senegal presented in , are in line with expectations of risk based on the earlier region-level prevalence surveys.
Trichiasis
According to WHO guidelines, TT prevalence ≥0.2% in persons aged 15 years or above constitutes a public health problem: the TT threshold for elimination is a TT prevalence of <1 case per 1,000 total population, with an assumption that 50% of the population is aged 15 years or above.Citation25,Citation26 Our results show that trichiasis is a public health problem in 9 of the sampled districts (i.e., Kolda, Médina Yoro Foulah, Vélingara, Saint Louis, Bounkiling, Goudomp, Sédhiou, Oussouye, and Ziguinchor); consequently, increased provision of surgical interventions for trichiasis is required in these districts. The prevalences recorded are generally lower than those presented in , a result which is in line with expectations of risk based on the earlier region-level prevalence surveys. The inability to distinguish between TT and trichiasis not caused by trachoma was a limitation to our surveys; it is possible that the actual prevalence of TT was lower than surveyed.
The observation, in certain districts, of a high trichiasis prevalence and low TF prevalence likely reflects higher endemicity of active trachoma several decades prior to these surveys. If active trachoma has since become rare, this is likely due to improvement in living conditions, as evidenced by the relatively good access to water and availability of latrines that we demonstrated (). The finding that trichiasis prevalence was higher among females than males adds weight to the observation made in other contexts that women are more likely to have trichiasis than men, perhaps as a result of their increased contact with young children.Citation18 However, with nearly 17% (5,417/32,392) of adult males absent compared to 5.8% of adult females (2,257/39,101), caution must be applied, as these absences among males could bias upward the estimated prevalence of trichiasis overall. Our process of age- and gender-standardization of trichiasis prevalence estimates will have partially counterbalanced this potential bias, but it is not possible to estimate the residual magnitude of the effect.
There are approximately 120–130 TSOs in Senegal, and all of them are trained to perform trichiasis surgery. The quality of surgery delivered depends largely on how frequently the TSOs have the opportunity to practice this skill – which, in turn, depends partly on patients’ access to and demand for surgery. To increase access, the MSAS has tried different strategies, such as offering surgery free of charge (with the service paid for by a partner organization). More aggressive strategies may be warranted to increase demand for surgery, particularly among people who may currently be practicing epilation as an alternative to surgery. One such strategy is known locally as “commando,” whereby TSOs rotate through and focus on performing surgeries in different regions of the country over the course of the year. This strategy would have the added advantage of giving the selected TSOs the opportunity to conduct the procedure more frequently, thereby improving their surgical skills which would help to increase demand for the service. Such a strategy will not be optimal after elimination as a public health problem, when services will need to be integrated into usual health care delivery platforms. Strengthening of the health care system is vital to ensure that this process is not followed by complete loss of capacity to detect trichiasis and deliver trichiasis surgery.
Risk factors
We examined selected potential risk factors for trachoma. The most obvious finding to emerge from our analysis was a significant negative correlation between having active trachoma and requiring less than 30 minutes to retrieve water for face washing. It has been argued that better access to water may reduce the transmission of C. trachomatis infection.Citation3 However, previous studies evaluating the association between increased distance to water and prevalence of active disease have delivered inconsistent results.Citation27–Citation35
Others have suggested that the quantity of water brought into the household might be more important than the distance.Citation36 Bailey et al., working in The Gambia, found that after controlling for distance to water and other factors, families with trachoma used less water for washing children than those without trachoma, irrespective of the amount of available water in the household.Citation37 In the current study, the quantity of water brought was not measured and use of water was not observed, but a round trip of less than 30 minutes to collect water was associated with lower TF prevalence. It would be of interest to study facial cleanliness as a follow-up. Unlike previous studies,Citation34,Citation35,Citation38,Citation39 we did not find an association between TF prevalence and reported use of unimproved sanitation by household adults.
Implications for program planning
For the districts surveyed here, the main priorities should be implementation of the S component of SAFE in the nine districts with trichiasis prevalences ≥0.2% in adults aged 15 years and above, to prevent worsening of vision in those who are already going blind from trachoma; and a one-year implementation of the SAFE strategy’s A, F, and E components in the single district with TF prevalence 5.1% in children aged 1–9 years, followed by an impact survey. MDA in this district is scheduled for 2017.
In 2015, the International Trachoma Initiative, which supplies donated azithromycin (Zithromax®, Pfizer) for MDA against trachoma, notified national programs that it would welcome azithromycin applications for districts with baseline TF prevalence of 5–9.9% among children aged 1–9 years, to be distributed alongside F and E interventions, in an effort to ensure that all districts achieve TF prevalences below 5%.Citation24 A total of nine districts which have not yet initiated MDA for trachoma fall into this category. Of these, one district completed mapping in 2004, three in 2005, one in 2010, two in 2011, one in 2013, and one (which will conduct MDA in 2017, as noted above) as part of the 2014 round of surveys reported here. In collaboration with its partners, Senegal’s MSAS is resurveying the eight districts in this category that conducted mapping prior to 2014, and will determine appropriate strategies based on the findings.
Declaration of interest
None of the authors have any proprietary interests or conflicts of interest related to this submission.
Acknowledgments
We gratefully thank the populations and leadership of the communities where the surveys were conducted. These surveys would not have been possible without the field workers, the health post head nurses and the health district head doctors who facilitated the work with the communities. We also thank John Kraemer of Georgetown University for his assistance in analyzing the survey data, and Rebecca M. Flueckiger, Alexandre Pavluck, and Brian K. Chu of the Task Force for Global Health for their work on the GTMP.
Additional information
Funding
References
- Baneke A. Review: targeting trachoma: strategies to reduce the leading infectious cause of blindness. Travel Med Infect Dis. 2012;10(2):92–96. doi:10.1016/j.tmaid.2012.01.005.
- Solomon AW, Holland MJ, Burton MJ, et al. Strategies for control of trachoma: observational study with quantitative PCR. Lancet. 2003;362(9379):198–204. doi:10.1016/S0140-6736(03)13909-8.
- Hu VH, Harding-Esch EM, Burton MJ, Bailey RL, Kadimpeul J, Mabey DCW. Epidemiology and control of trachoma: systematic review. Trop Med Int Heal. 2010;15(6):673–691. doi:10.1111/j.1365-3156.2010.02521.x.
- Taylor HR, Burton MJ, Haddad D, West S, Wright H. Trachoma. Lancet. doi:10.1016/S0140-6736(13)62182-0.
- Bourne RRA, Stevens GA, White RA, et al. Causes of vision loss worldwide, 1990–2010: A systematic analysis. Lancet Glob Health. 2013;1(6):339–349. doi:10.1016/S2214-109X(13)70113-X.
- World Health Organization Alliance for the Global Elimination of Trachoma by 2020. Eliminating Trachoma: Accelerating Towards 2020. London: International Coalition for Trachoma Control. 2016.
- World Health Organization. Validation of Elimination of Trachoma as a Public Health Problem (WHO/HTM/NTD/2016.8). Geneva: World Health Organization 2016.
- Ministère de la Santé Publique et de l’Action Sociale. Programme National De Lutte Contre La Cécité. Plan d’Action 1998-2007. Dakar: Ministère de la Santé Publique et de l'Action Sociale. 1997.
- Ministère de la Santé de l’Hygiène Publique et de la Prévention. Plan Directeur De Lutte Intégrée Contre Les Maladies Tropicales Négligées. Dakar: Ministère de la Santé, de l'Hygiène Publique et de la Prévention; 2011–2015. 2011.
- Saal MB, Schemann JF, Saar B, et al. Trachoma in Senegal: results of a national survey. Médecine Trop. 2003;63(1):53–59.
- République du Sénégal. Décret N° 2008-1025 Du 10 Septembre 2008. Senegal: Journal Officiel N° 6446 du mercredi 31 décembre 2008 N - S; 2008. http://www.jo.gouv.sn/spip.php?article7213.
- Harding-Esch EM, Kadimpeul J, Sarr B, et al. Population-based prevalence survey of follicular trachoma and trachomatous trichiasis in the Casamance region of Senegal. BMC Public Health. 2018;18(1):62. doi:10.1186/s12889-017-4605-0.
- Solomon AW, Zondervan M, Kuper H, Buchan J, Mabey DCW, Foster A. Trachoma Control: A Guide for Programme Managers. Geneva: World Health Organization. 2006.
- Sarr B, Sissoko M, Fall M, Cohn D, Solomon AW, Bailey RL. Segmented mapping of trachoma in Touba District, Senegal. In: Trachoma Scientific Informal Workshop. Geneva; 2017.
- Solomon AW, Pavluck AL, Courtright P, et al. The global trachoma mapping project: methodology of a 34-country population-based study. Ophthalmic Epidemiol. 2015;22(3):214–225. doi:10.3109/09286586.2015.1037401.
- Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bull World Health Organ. 1987;65(4):477–483.
- Courtright P, Gass K, Lewallen S, et al. Global Trachoma Mapping Project: Training for Mapping of Trachoma (Version 2). London: International Coalition for Trachoma Control; 2013. http://www.trachomacoalition.org/node/357.
- Cromwell EA, Courtright P, King JD, Rotondo LA, Ngondi J, Emerson PM. The excess burden of trachomatous trichiasis in women: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2009;103(10):985–992. doi:10.1016/j.trstmh.2009.03.012.
- Pavluck A, Chu B, Mann Flueckiger R, Ottesen E. Electronic data capture tools for global health programs: evolution of LINKS, an Android-, Web-Based System. PLoS Negl Trop Dis. 2014;8(4):1–19. doi:10.1371/journal.pntd.0002654.
- Agence Nationale de la Statistique et de la Démographie. Recensement Général de La Population et de l’Habitat, de l’Agriculture et de l’Elevage (RGPHAE) 2013. Rapport Provisoire; 2014.
- World Health Organization Strategic and Technical Advisory Group on Neglected Tropical Diseases. Technical Consultation on Trachoma Surveillance. September 11−12, 2014, Task Force for Global Health, Decatur, USA (WHO/HTM/NTD/2015.02). Geneva: World Health Organization. 2015.
- World Health Organization Alliance for the Global Elimination of Trachoma by 2020. Second Global Scientific Meeting on Trachomatous Trichiasis. Cape Town, 4-6 November 2015 (WHO/HTM/NTD/2016.5).. Geneva: World Health Organization. 2016.
- http://www.wssinfo.org/definitions-methods/watsan-categories/(accessed 4 Nov 2016).
- International Trachoma Initiative. Diagram on Decision Making for Antibiotic Treatment of Trachoma, version 9.
- World Health Organization. Report of the 2nd Global Scientific Meeting on Trachoma, Geneva, 25-27 August, 2003. Geneva: World Health Organization. 2003.
- World Health Organization. Report of the 3rd Global Scientific Meeting on Trachoma, Johns Hopkins University, Baltimore, MD, 19-20 July 2010. Geneva: World Health Organization. 2010.
- West S, Lynch M, Turner V, et al. Water availability and trachoma. Bull World Health Organ. 1989;67(1):71–75.
- Taylor HR, West SK, Mmbaga BB, et al. Hygiene factors and increased risk of trachoma in central Tanzania. Arch Ophthalmol. 1989;107(12):1821–1825. doi:10.1001/archopht.1989.01070020903037.
- Zerihun N. Trachoma in Jimma zone, south western Ethiopia. Trop Med Int Health. 1997;2(12):1115–1121.
- J-F S, Sacko D, Malvy D, et al. Risk factors for trachoma in Mali. Int J Epidemiol. 2002;31(1):194–201.
- Kuper H, Solomon AW, Buchan J, Zondervan M, Foster A, Mabey D. A critical review of the SAFE strategy for the prevention of blinding trachoma. Lancet Infect Dis. 2003;3(6):372–381. doi:10.1016/S1473-3099(03)00659-5.
- Stocks ME, Ogden S, Haddad D, Addiss DG, McGuire C, Freeman MC. Effect of water, sanitation, and hygiene on the prevention of trachoma: a systematic review and meta-analysis. PLoS Med. 2014;11(2):e1001605. doi:10.1371/journal.pmed.1001605.
- Bero B, Macleod C, Alemayehu W, et al. Prevalence of and risk factors for trachoma in Oromia Regional State of Ethiopia: results of 79 population-based prevalence surveys conducted with the Global Trachoma Mapping Project. Ophthalmic Epidemiol. 2016;23(6):392–405. doi:10.1080/09286586.2016.1243717.
- Elshafie BE, Osman KH, Macleod C, et al. The epidemiology of trachoma in Darfur States and Khartoum State, Sudan: results of 32 population-based prevalence surveys. Ophthalmic Epidemiol. 2016;23(6):381–391. doi:10.1080/09286586.2016.1243718.
- Adera TH, Macleod C, Endriyas M, et al. Prevalence of and risk factors for trachoma in Southern Nations, Nationalities, and Peoples’ Region, Ethiopia: results of 40 population-based prevalence surveys carried out with the Global Trachoma Mapping Project. Ophthalmic Epidemiol. 2016;23(sup1). doi:10.1080/09286586.2016.1247876.
- Kupka K, Nizetic B, Reinhards J. Sampling studies on the epidemiology and control of trachoma in southern Morocco. Bull World Health Organ. 1968;39(4):547–566.
- Bailey R, Downes B, Downes R, Mabey D. Trachoma and water use; a case control study in a Gambian village. Trans R Soc Trop Med Hyg. 1991;85(6):824–828. doi:10.1016/0035-9203(91)90470-J.
- Ko R, Macleod C, Pahau D, et al. Population-based trachoma mapping in six evaluation units of Papua New Guinea. Ophthalmic Epidemiol. 2016;23(sup1):22–31. doi:10.1080/09286586.2016.1235715.
- Mpyet C, Muhammad N, Adamu MD, et al. Prevalence of trachoma in Kano State, Nigeria: results of 44 district local government area-level surveys. Ophthalmic Epidemiol. 2017;24(3):195–203. doi:10.1080/09286586.2016.1265657.
Appendix
The Global Trachoma Mapping Project Investigators are: Agatha Aboe (1,11), Liknaw Adamu (4), Wondu Alemayehu (4,5), Menbere Alemu (4), Neal D. E. Alexander (9), Berhanu Bero (4), Simon J. Brooker (1,6), Simon Bush (7,8), Brian K. Chu (2,9), Paul Courtright (1,3,4,7,11), Michael Dejene (3), Paul M. Emerson (1,6,7), Rebecca M. Flueckiger (2), Allen Foster (1,7), Solomon Gadisa (4), Katherine Gass (6,9), Teshome Gebre (4), Zelalem Habtamu (4), Danny Haddad (1,6,7,8), Erik Harvey (1,6,10), Dominic Haslam (8), Khumbo Kalua (5), Amir B. Kello (4,5), Jonathan D. King (6,10,11), Richard Le Mesurier (4,7), Susan Lewallen (4,11), Thomas M. Lietman (10), Chad MacArthur (6,11), Colin Macleod (3,9), Silvio P. Mariotti (7,11), Anna Massey (8), Els Mathieu (6,11), Siobhain McCullagh (8), Addis Mekasha (4), Tom Millar (4,8), Caleb Mpyet (3,5), Beatriz Muñoz (6,9), Jeremiah Ngondi (1,3,6,11), Stephanie Ogden (6), Alex Pavluck (2,4,10), Joseph Pearce (10), Serge Resnikoff (1), Virginia Sarah (4), Boubacar Sarr (5), Alemayehu Sisay (4), Jennifer L. Smith (11), Anthony W. Solomon (1,2,3,4,5,6,7,8,9,10,11), Jo Thomson (4), Sheila K. West (1,10,11), Rebecca Willis (2,9).
Key: (1) Advisory Committee, (2) Information Technology, Geographical Information Systems, and Data Processing, (3) Epidemiological Support, (4) Ethiopia Pilot Team, (5) Master Grader Trainers, (6) Methodologies Working Group, (7) Prioritisation Working Group, (8) Proposal Development, Finances and Logistics, (9) Statistics and Data Analysis, (10) Tools Working Group, (11) Training Working Group.