ABSTRACT
Purpose: To determine prevalence of trachoma after interventions in 15 local government areas (LGAs) of Kebbi, Sokoto and Zamfara States, Nigeria.
Methods: A population-based impact survey was conducted in each LGA using Global Trachoma Mapping Project (GTMP) protocols. In each LGA, 25 villages were selected, except in Arewa LGA, where we selected 25 villages from each of four subunits to obtain finer-resolution prevalence information. Villages were selected with probability proportional to size. In each village, 25 households were enrolled and all consenting residents aged ≥1 year were examined by GTMP-certified graders for trachomatous inflammation—follicular (TF) and trachomatous trichiasis (TT). Information on sources of household water and types of sanitation facilities used was collected through questioning and direct observation.
Results: The number of households enrolled per LGA ranged from 623 (Kware and Tangaza) to 2488 (Arewa). There have been marked reductions in the prevalence of TF and TT since baseline surveys were conducted in all 15 LGAs. Eight of the 15 LGAs have attained TF prevalences <5% in children, while 10 LGAs have attained TT prevalences <0.2% in persons aged ≥15 years. Between 49% and 96% of households had access to water for hygiene purposes within 1 km of the household, while only 10–59% had access to improved sanitation facilities.
Conclusion: Progress towards elimination of trachoma has been made in these 15 LGAs. Collaboration with water and sanitation agencies and community-based trichiasis surgery are still needed in order to eliminate trachoma by the year 2020.
Introduction
For the elimination of trachoma as a public health problem, the World Health Organization (WHO) recommends implementation of the SAFE strategy (Surgery for trachomatous trichiasis (TT); Antibiotics to clear ocular Chlamydia trachomatis infection; Facial cleanliness and Environmental improvement to reduce C. trachomatis transmission).Citation1,Citation2 The district-level elimination targets set by WHO are a prevalence of trachomatous inflammation—follicular (TF) of <5% in children aged 1–9 years, and a prevalence of TT unknown to the health system of <0.2% in persons aged ≥15 years.Citation3 ExperimentalCitation4,Citation5 and operationalCitation6,Citation7 data suggest that the SAFE strategy works.
Figure 2. Local government areas for trachoma impact surveys, Kebbi, Sokoto and Zamfara States, Nigeria, 2014–2016.
Countries are now scaling up or are already fully implementing SAFE at scale,Citation8,Citation9 in an effort to achieve elimination by the year 2020. The decision to continue or discontinue elements of the SAFE strategy depends on data from impact surveys, which are carried out after specified periods of programme implementation.Citation10–Citation12
In Nigeria, State governments partner with non-governmental organisations to deploy trachoma elimination activities at the district (local government area, LGA) level. As elsewhere, the duration of intervention varies between LGAs, depending on prevalence estimates of TF and TT. In Kebbi, Sokoto and Zamfara States of north-western Nigeria, trachoma programmes were gradually rolled out from 2003. Baseline surveys were undertaken. Local systems and capacities were developed to deliver community-based trichiasis surgery, with operations performed by trained ophthalmic nurses. Tetracycline eye ointment and azithromycin were distributed, facial cleanliness was promoted, and interventions launched to improve access to water and sanitation.
To assess the collective impact of these interventions in reducing the prevalence of disease towards elimination targets, we carried out impact surveys in the LGAs of Kebbi, Sokoto and Zamfara States. The systems and processes of the Global Trachoma Mapping ProjectCitation13 (GTMP) were used. This paper reports the results of those surveys, comparing the prevalence estimates obtained with those generated at baseline.
Methods
Setting
Baseline population-based prevalence surveys for trachoma were conducted in 2004 in six LGAs of Kebbi,Citation14 six LGAs of Sokoto [unpublished data], and six LGAs of ZamfaraCitation15 (). In 2006, six further baseline surveys were conducted in Sokoto State [unpublished data]. In 2011 and 2012, baseline surveys were conducted in 25 LGAs of Sokoto and Kebbi.Citation16 In total, baseline surveys were conducted in 49 LGAs of Kebbi, Sokoto and Zamfara during the period 2004–2012. Based on LGA-level prevalence estimates of TF in children aged 1–9 years, 20 LGAs required 3 years of azithromycin mass drug administration (MDA, TF prevalence 10–29.9%) and 7 other LGAs required 5 years of azithromycin MDA (TF prevalence ≥30.0%), together with appropriate F & E interventions. The remaining 22 LGAs had TF prevalence estimates <10% and (according to then-current WHO guidanceCitation12) did not qualify for district-wide antibiotic MDA. Based on the prevalence of trichiasis in persons aged ≥15 years, 40 LGAs surveyed required community-based trichiasis surgery to reach the trichiasis elimination threshold. The decision was made to implement various aspects of the SAFE strategy in 49 LGAs found to have trachoma of public health significance (27 LGAs with TF prevalence ≥10% and 40 LGAs with trichiasis prevalence ≥0.2%, of which 22 LGAs required public-health-level trichiasis interventions [S], 9 LGAs required A, F and E, and 18 LGAs required S, A, F and E). Health ministries in the three states and relevant LGA offices partnered with the non-governmental organisation Sightsavers to intervene against trachoma.
Table 1. Results of 15 selected baseline trachoma prevalence surveys conducted from 2004 to 2006, and consequent public health-level actions, Kebbi, Sokoto and Zamfara States, NigeriaCitation14,Citation15,unpublished data.
Interventions commenced in 2003 with provision of trichiasis surgery in six LGAs (including Arewa, Argungu and Augie) of Kebbi, five LGAs (including Goronyo, Illela, Isa and Rabah) of Sokoto and four LGAs (including Birnin Magaji, Shinkafi and Zurmi) of Zamfara. By the end of 2016, in the 15 LGAs under review here, a total of 19,860 trichiasis surgeries had been carried out ().
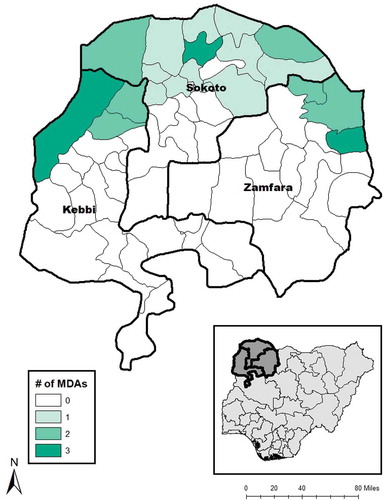
MDA of antibiotics commenced in 2004, using oral azithromycin (Zithromax®, Pfizer, New York, NY, USA) and/or 1% tetracycline eye ointment, each of which was purchased on the open market. Funding limitations meant that quantities of antibiotics available prior to 2010 were very limited, and as a consequence, MDA was somewhat patchy and irregular. Donated azithromycin (Zithromax®, Pfizer, supplied through the International Trachoma InitiativeCitation9) became available in 2010 in Arewa (Kebbi), Gwadabawa (Sokoto) and Birnin Magaji (Zamfara) and was expanded in 2011 to include two further LGAs in Kebbi and 14 further LGAs in Sokoto ().
Figure 1. Number of rounds of antibiotic mass drug administration (MDA) for trachoma, Kebbi, Sokoto and Zamfara States, Nigeria, 2010–2013.
The facial cleanliness and environmental improvement aspects of the SAFE strategy were principally implemented through health education and community sensitisation. Front-line heath workers (government employees) provided basic health education alongside other primary health care duties at community health centres on a daily basis. Community drug distributors displayed and discussed posters about transmission, prevention and treatment of trachoma during community meetings and MDA events, with the aim of encouraging safer hygiene practices and facial cleanliness. Trachoma-endemic communities were also provided with sanitation hardware (e.g., wheelbarrows, shovels) to be used during monthly general sanitation exercises for ensuring that faeces was disposed of in ways that kept it away from human contact. Community members were encouraged to build toilets. Trained ophthalmic nurses also carried out community mobilisation and health education about trachoma during MDA and trichiasis surgery campaign activities, and encouraged, through inspection, the use of sanitation facilities.
Impact survey rationale and design
Beginning in 2014, in each of the 15 LGAs (), we conducted cross-sectional population-based prevalence surveys of persons aged ≥1 year, selected using multistage cluster random sampling. These LGAs had not necessarily completed the WHO-recommended numbers of MDA rounds indicated by their baseline TF prevalence,Citation12 but given the chequered history of intervention and the long time period elapsed since the previous round of surveys, re-focussing the programme by undertaking fresh estimates was felt to be a good idea.
We determined the required sample size for each LGA based on the requirements to estimate a TF prevalence around the elimination threshold, using the single-population proportion-for-precision formula: n = 1.2 × (design effect) × p(1 − p)/(2 × d/((1.96 × 2)2)). We used the following assumptionsCitation11,Citation17: expected TF prevalence in 1–9-year-olds (p) = 4%, required absolute precision (d) = 2%, risk of α error = 5% and design effect = 2.65. The calculated minimal sample size was 978 children.
In each LGA (with the exception of Arewa), we selected 25 clusters (villages) from a list of all villages in the LGA, using a probability-proportional-to-village-size methodology.Citation12 We then segmented each selected village (using pre-existing administrative units of approximately equal size) and selected one of the units at random, by drawing lots. From the selected administrative unit, 25 households were selected using the random walk method.Citation18–Citation23 Based on there being a mean of two children aged 1–9 years per household, this was expected to allow recruitment from a resident population of 1250 1–9-year-olds in each LGA, which would allow for partial non-response. In Arewa, we subdivided the LGA into four subunits and selected 25 clusters from each, in order to investigate how much additional information sub-district-level surveys would provide in this environment at the impact survey stage; thus a total of 100 clusters were selected in Arewa. All residents aged ≥1 year, living in selected households, were invited to participate.
Pre-survey field team training, certification and data collection techniques followed standard GTMP protocols.Citation17 We used version 3 of the GTMP training system.Citation24
Ethics
Protocols were approved by the Ethics Committees of the Ministries of Health of Kebbi and Sokoto States, the National Health Research Ethics Committee of Nigeria (NHREC/01/01/2007) and the London School of Hygiene & Tropical Medicine (6319 and 8355), while Zamfara State Ministry of Health gave permission for the survey in the absence of a State Ethics Committee. After field teams explained the examination protocol to each adult in a language they understood, verbal consent for enrolment and examination was obtained. (Most survey participants could neither read nor write.) Heads of households gave consent for the participation of minors, while adults gave consent for their own participation. Consent was documented in an Open Data Kit-based Android smartphone application (LINKS).Citation25 Individuals with active trachoma were given two tubes of 1% tetracycline eye ointment and instructed on its use; persons with trichiasis were referred for lid surgery at the nearest facility at which certified trichiasis surgeons could be accessed. Examiners cleaned their hands with an alcohol-based skin cleaning agent after examination of each participant.
Data collection and definitions
We used the WHO simplified grading scheme to grade trachoma.Citation26 We recorded the presence or absence of TF, trachomatous inflammation—intense, and trichiasis, on the basis of the assessments of GTMP-certified trachoma graders, one of whom examined each subject using ×2.5 magnifying loupes. In any eye with trichiasis, the grader noted the presence or otherwise of trachomatous conjunctival scarring.Citation27 For the purposes of this paper, we defined TT as the presence of trichiasis plus trachomatous conjunctival scarring (or the grader finding the upper eyelid impossible to evert) in the same eye. GPS coordinates for each household were recorded, and data on household-level access to water and sanitation were collected through interviews and inspection (where relevant) of household sanitation facilities. Sanitation facilities were categorised as improved or unimproved, as per the WHO/UNICEF Joint Monitoring Program (JMP) for Water Supply and Sanitation definitions used for monitoring progress towards the Millennium Development Goals.Citation28 Water sources referred to those used for washing purposes, and not specifically for drinking. Types of water sources were categorised as improved and unimproved as per the JMP definitions.Citation28 A household was defined as a compound head together with all individuals normally resident in the compound and eating from the same pot.
Data analysis
Data cleaning was undertaken by an objective (non-programme-linked) data manager (RW). Data analysis was performed using R (R Foundation for Statistical Computing, Vienna, Austria) and Structured Query Language. We controlled for age and gender of those examined, and the total number of people examined per cluster, using algorithms applied across all constituent projects of the GTMP.Citation18–Citation23,Citation29–Citation49 For TF, the proportion of children aged 1–9 years who had that sign in each cluster was adjusted for age in 1-year age bands. For trichiasis and TT, the proportion of adults aged ≥15 years who had that sign in each cluster was adjusted for age and gender in 5-year age bands. Arithmetic means of the adjusted cluster-level proportions gave the LGA prevalence for each sign. Confidence intervals were generated by bootstrapping, with replacement, the adjusted cluster-level proportions, over 10,000 iterations.
Results
Surveys were conducted between 2014 and 2016. The number of households enrolled per LGA ranged from 623 (Kware and Tangaza) to 2488 (Arewa). The number of children aged 1–9 years examined exceeded the sample size estimate in every LGA except Kware, where 921 children (94% of the target) were examined. At least as many persons aged ≥15 years as children aged 1–9 years were included in most LGAs – a reflection of local demographics rather than refusal to participate, which was rare ().
Table 2. Enumeration and examination characteristics of sampled individuals in 15 local government areas, impact surveys for trachoma, Kebbi, Sokoto and Zamfara States, Nigeria, 2014–2016.
Prevalence of trachoma
Prevalence estimates for TT in ≥15-year-olds and TF in 1–9-year-olds years old are shown in . Following a 2015 impact survey (using the same methodologies described above) in which the TF prevalence in 1–9-year-olds years old was estimated to be 5.4%, Birnin Magaji was given one further round of azithromycin MDA, in 2016. An impact survey was conducted 8 months later; the TT and TF prevalence estimates reported in (and the data provided in and ) are those generated in the second impact survey, which followed a total of four rounds of azithromycin MDA.
Table 3. Prevalence of trachomatous inflammation—follicular (TF) and trichiasis or trachomatous trichiasis (TT) at baseline and impact surveys, 15 local government areas of Kebbi, Sokoto and Zamfara States, Nigeria.
Table 4. Prevalence of trachomatous inflammation—follicular (TF) and trachomatous trichiasis (TT), impact surveys, four sub-districts of Arewa local government area, Kebbi State, Nigeria, 2014.
Table 5. Number of individuals with trichiasis, number of individuals with trichiasis + trachomatous conjunctival scarring (a combination referred to here as trachomatous trichiasis, TT: see text) and prevalence of each of these things, impact surveys, 15 local government areas (LGAs) of Kebbi, Sokoto and Zamfara States, Nigeria, 2014–2016.
Marked reductions compared to baseline estimates, in both the TT and TF indices, were noted in all 15 LGAs. In the various sub-districts in Arewa, the prevalence of TF in 1–9-year-olds ranged from 2.2% to 5.7%, while the prevalence of TT in persons ≥15-year-olds ranged from 0.5% to 1.1% ().
If the trichiasis prevalence at baseline is compared to the all-trichiasis prevalence at impact survey (ignoring the presence or absence of trachomatous conjunctival scarring), prevalence reductions from baseline to impact are still noted ().
Access to water and sanitation
Across LGAs, access to a water source within 1 km of households ranged from 49% to 96%, while access to an improved water source ranged from 2% (Tangaza) to 79% (Birnin Magaji). Across all LGAs surveyed, access to improved sanitation facilities was generally low, with Birnin Magaji (59%) having the highest levels of access ().
Table 6. Household access to water and improved sanitation facilities, 15 local government areas of Kebbi, Sokoto and Zamfara States, Nigeria, 2014–2016.
Discussion
In the LGAs for which we report trachoma impact survey data here, there has been implementation of some or all aspects of the SAFE strategy to varying extents. Eight of the 15 LGAs (Arewa, Argungu, Gwadabawa, Kware, Rabah, Birnin Magaji, Shinkafi and Zurmi) have now attained the elimination target for TF (<5% prevalence in 1–9-year-olds) and so enter the 2-year surveillance phase.Citation50 Implementation of azithromycin MDA, even though irregular, combined with education on the need for personal and environmental hygiene, likely contributed to declines in TF prevalence. Reduced TF prevalence is associated with a reduction in the reservoir of ocular C. trachomatis, so this is expected to be accompanied by decreased community transmission intensityCitation51 and, ultimately, a fall in the incidence of TT.Citation52
We are, however, unable to confidently credit apparent falls in TF prevalence () to implementation of the A, F and E components of the SAFE strategy. We cannot even say with certainty that prevalence estimates at impact survey were significantly lower than at baseline: scrutiny of the baseline survey methodologies and data could not be undertaken because the original datasets (and for some, even survey reports) could not be located, despite intensive search. Baseline surveys conducted in these LGAs used methodologies that almost certainly diverged in important ways from the standards that were set up some years later by the GTMP, including the fact that the graders were not internationally standardised. Furthermore, the present study was not a randomised trial, but a series of post-intervention surveys, and intervention delivery was intermittent, incomplete and hard to quantify for each of the A, F and E components. Though our survey methodology is considered best-in-class,Citation53 we acknowledge residual weaknesses, including the use of random walk to select households in Nigeria; reliance on self-report of access to water and use of sanitation; exclusive focus in questions about sanitation use on where household adults defecate (ignoring sites of disposal of children’s faeces); suboptimal recruitment (); and relatively low sample sizes of adults.
Notwithstanding these limitations, our data reveal a need to improve access to water and sanitation in these LGAs. From the perspective of sustaining progress against active trachoma, if water and sanitationCitation54 and behaviour change effortsCitation55 do in fact help to reduce C. trachomatis transmission, as many believe,Citation56,Citation57 there is a particular imperative to undertake these activities in the LGAs that have entered the surveillance phase on the pathway towards elimination. In these populations, antibiotics will no longer be used to suppress the prevalence of infection, and keeping transmission intensity low presumably depends on how successful F and E have been. While >80% of households we sampled in Arewa and Birnin Magaji had access to washing water within 1 km of the residence, the other four LGAs in which TF was <5% fell short of this mark. Of particular concern in all LGAs is the very low access to improved sanitation facilities, which may presage high muscid fly densities and a greater chance of transmission of residual infection.Citation58–Citation61 In rural areas of Nigeria as a whole, in the interval between 2000 and 2015, access to improved sanitation reportedly decreased from 35% to 27% – a trend that, if continuing, requires urgent correction – while access to improved drinking water sources increased from 36% to 62%.Citation62
Although Arewa as a whole achieved the TF elimination prevalence target, two of its sub-districts had TF prevalences above that mark, estimated in fully fledged independent surveys with more-than-adequate sample sizes (). TF prevalences, however, were not significantly different between the sub-districts. This observation, though limited in scope, probably supports the recent revisionCitation50 to WHO guidance on impact surveys, in which previous recommendationsCitation63 to frame impact surveys at sub-district level were changed in favour of districts being the standard evaluation unit at baseline, impact and surveillance phases of the programme. We will, however, keep a close watch for recrudescence of disease in the communities of Arewa.
Six surveyed LGAs (Augie, Gada, Binji, Goronyo, Isa and Tangaza) require one round of azithromycin MDA before impact surveys are repeated. During this period, education on personal and environmental hygiene must be ongoing. However, for education to be accepted and implemented by communities, access to water and sanitation needs to be improved: all these LGAs had poor household access to improved sanitation, and two of them (Gada and Goronyo) also had <80% household access to washing water within 1 km of the household. One LGA (Illela) had a TF prevalence indicating a need for three further rounds of azithromycin MDA together with F and E.
The estimated TT prevalence was lower at impact survey than at baseline survey in all LGAs. In Arewa, the TT prevalence in sub-district 1 in 2014 (1.1, 95% CI 0.6–1.6) was, at face value, higher than the whole-LGA trichiasis prevalence in 2004 (1.0, 95% CI 0.1–1.9), despite more than double the estimated 2004 backlog of Arewa trichiasis patients having been managed in the intervening period ( and ). This result should be more realistically interpreted in the light of the imprecision of each estimate, but can also be taken as a cautionary note against comparing prevalence estimates generated at different administrative levels at different times. Looking solely at LGA-level data, there is also a lesson here about the continuing incidence of TT even as TF prevalence falls, and the pitfalls inherent in using surgical output data to predict the success or otherwise of meeting the TT elimination threshold prevalence.Citation27
Ten LGAs recorded TT prevalences of <0.2% in ≥15-year-olds, the elimination threshold. In these LGAs, there may be a reduced need for community-based TT surgery, but structures to identify and treat individuals who develop TT should be maintained.Citation3 Robust follow-up mechanisms should also be continued, in order to detect post-surgical recurrence in those previously operated, and to ensure patients who elected for epilation over surgery but eventually want more formal intervention are able to access it. In LGAs with TT prevalence estimates ≥0.2%, progress (shown by reductions in trichiasis prevalence, and ) should also be celebrated, with an eye to encouraging local teams to redouble their efforts towards achieving elimination targets. Health ministries will review the number, distribution, and motivation of surgeons, and the extent to which they are adequately equipped with the materials necessary to do their jobs.
Much progress has been made towards the elimination of trachoma in Kebbi, Sokoto and Zamfara States. While some LGAs still require antibiotic MDA, greater emphasis needs to be placed on collaboration with agencies involved in water and sanitation to improve access to these services. In addition, community-based trichiasis surgery needs to be reinforced in four LGAs to finish the task of elimination of trachoma as a public health problem.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- World Health Assembly. Global Elimination of Blinding Trachoma. 51st World Health Assembly, Geneva, 16 May 1998, Resolution WHA51.11. Geneva: World Health Organization; 1998.
- Francis V, Turner V. Achieving Community Support for Trachoma Control (WHO/PBL/93.36). Geneva: World Health Organization; 1993.
- World Health Organization. Validation of Elimination of Trachoma as a Public Health Problem (WHO/HTM/NTD/2016.8). Geneva: World Health Organization; 2016.
- Evans JR, Solomon AW. Antibiotics for trachoma. Cochrane Database sysT Rev. 2011;3:CD001860.
- Burton M, Habtamu E, Ho D, Gower EW. Interventions for trachoma trichiasis. Cochrane Database sysT Rev. 2015;11:CD004008.
- Ngondi J, Onsarigo A, Matthews F, et al. Effect of 3 years of SAFE (surgery, antibiotics, facial cleanliness, and environmental change) strategy for trachoma control in southern Sudan: a cross-sectional study. Lancet. 2006;368(9535):589–595. doi:10.1016/S0140-6736(06)69202-7.
- Hammou J, El Ajaroumi H, Hasbi H, Nakhlaoui A, Hmadna A, El Maaroufi A. In Morocco, the elimination of trachoma as a public health problem becomes a reality. Lancet Global Health. 2017;5(3):e250–e1. doi:10.1016/S2214-109X(17)30023-2.
- World Health Organization Alliance for the Global Elimination of Trachoma by 2020. Eliminating Trachoma: Accelerating Towards 2020. London: International Coalition for Trachoma Control; 2016.
- Emerson PM, Hooper PJ, Sarah V. Progress and projections in the program to eliminate trachoma. PLoS Negl Trop Dis. 2017;11(4):e0005402. doi:10.1371/journal.pntd.0005402.
- World Health Organization. Report of the 2nd Global Scientific Meeting on Trachoma, Geneva, 25-27 August, 2003 (WHO/PBD/GET 03.1). Geneva: World Health Organization; 2003.
- World Health Organization. Report of the 3rd Global Scientific Meeting on Trachoma, Johns Hopkins University, Baltimore, MA, 19-20 July 2010 (WHO/PBD/2.10). Geneva: World Health Organization; 2010.
- Solomon AW, Zondervan M, Kuper H, Buchan JC, Mabey DCW, Foster A. Trachoma Control: A Guide for Programme Managers. Geneva: World Health Organization; 2006.
- Solomon AW, Kurylo E. The global trachoma mapping project. Community Eye Health. 2014;27:18.
- Muhammad N, Rabiu MM. Prevalence of Trachoma in 6 Local Government Areas of Kebbi State [Paper Presented at the Annual Conference of the Ophthalmological Society of Nigeria]. Kaduna: National Eye Centre; 2006.
- Rabiu MM, Muhammad N. Report of Trachoma Population Based Survey in 6 Local Government Areas of Zamfara State. Kaduna: Sightsavers International; 2005.
- Muhammad N, Mohammed A, Isiyaku S, Adamu MD, Gwom A, Rabiu MM. Mapping trachoma in 25 local government areas of Sokoto and Kebbi states, northwestern Nigeria. Br J Ophthalmol. 2014;98(4):432–437. doi:10.1136/bjophthalmol-2013-303703.
- Solomon AW, Pavluck A, Courtright P, et al. The global trachoma mapping project: methodology of a 34-country population-based study. Ophthalmic Epidemiol. 2015;22(3):214–225. doi:10.3109/09286586.2015.1037401.
- Mpyet C, Muhammad N, Adamu MD, et al. Trachoma mapping in Gombe State, Nigeria: results of 11 local government area surveys. Ophthalmic Epidemiol. 2016;23(6):406–411. doi:10.1080/09286586.2016.1230633.
- Mpyet C, Muhammad N, Adamu MD, et al. Prevalence of trachoma in Katsina State, Nigeria: results of 34 district-level surveys. Ophthalmic Epidemiol. 2016;23(Sup 1):55–62. doi:10.1080/09286586.2016.1236975.
- Mpyet C, Muhammad N, Adamu MD, et al. Prevalence of trachoma in Bauchi State, Nigeria: results of 20 local government area-level surveys. Ophthalmic Epidemiol. 2016;23(Sup 1):39–45. doi:10.1080/09286586.2016.1238945.
- Muhammad N, Mpyet C, Adamu MD, et al. Mapping trachoma in Kaduna State, Nigeria: results of 23 local government area-level, population-based prevalence surveys. Ophthalmic Epidemiol. 2016;23(Sup 1):46–54. doi:10.1080/09286586.2016.1250918.
- Adamu MD, Mpyet C, Muhammad N, et al. Prevalence of trachoma in Niger State, North Central Nigeria: results of 25 population-based prevalence surveys carried out with the global trachoma mapping project. Ophthalmic Epidemiol. 2016;23(Sup 1):63–69. doi:10.1080/09286586.2016.1242757.
- Mpyet C, Muhammad N, Mohammed AD, et al. Prevalence of trachoma in Kano State, Nigeria: results of 44 local government area-level surveys. Ophthalmic Epidemiol. 2017;24(3):195–203. doi:10.1080/09286586.2016.1265657.
- Courtright P, Gass K, Lewallen S, et al. Global trachoma mapping project: training for mapping of trachoma (version 3) http://www.trachomacoalition.org/node/122]. London: International Coalition for Trachoma Control; 2014.
- Pavluck A, Chu B, Mann Flueckiger R, Ottesen E. Electronic data capture tools for global health programs: evolution of LINKS, an Android-, web-based system. PLoS Negl Trop Dis. 2014;8(4):e2654. doi:10.1371/journal.pntd.0002654.
- Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bull World Health Organ. 1987;65:477–483.
- World Health Organization Alliance for the Global Elimination of Trachoma by 2020. Second Global Scientific Meeting on Trachomatous Trichiasis. Cape Town, 4-6 November 2015 (WHO/HTM/NTD/2016.5). Geneva: World Health Organization; 2016.
- World Health Organization and UNICEF. Core questions on drinking-water and sanitation for household surveys. Geneva: World Health Organization/UNICEF; 2006. http://www.wssinfo.org/fileadmin/user_upload/resources/1268174016-JMP_Core_Questions.pdf. Accessed September 1, 2013.
- Kalua K, Phiri M, Kumwenda I, et al. Baseline trachoma mapping in Malawi with the global trachoma mapping project (GTMP). Ophthalmic Epidemiol. 2015;22(3):176–183. doi:10.3109/09286586.2015.1035793.
- Meng N, Seiha D, Thorn P, et al. Assessment of Trachoma in Cambodia: Trachoma Is Not a Public Health Problem. Ophthalmic Epidemiol. 2016;23(Sup 1):3–7. doi:10.1080/09286586.2016.1230223.
- Kalua K, Chisambi A, Chinyanya D, et al. Completion of Baseline Trachoma Mapping in Malawi: Results of Eight Population-Based Prevalence Surveys Conducted with the Global Trachoma Mapping Project. Ophthalmic Epidemiol. 2016;23(Sup 1):32–8. doi:10.1080/09286586.2016.1230224.
- Mwingira UJ, Kabona G, Kamugisha M, et al. Progress of Trachoma Mapping in Mainland Tanzania: Results of Baseline Surveys from 2012 to 2014. Ophthalmic Epidemiol. 2016;23(6):373–80. doi:10.1080/09286586.2016.1236974.
- Omar FJ, Kabona G, Abdalla KM, et al. Baseline Trachoma Surveys in Kaskazini A and Micheweni Districts of Zanzibar: Results of Two Population-Based Prevalence Surveys Conducted with the Global Trachoma Mapping Project. Ophthalmic Epidemiol. 2016;23(6):412–7. doi:10.1080/09286586.2016.1235206.
- Bero B, Macleod C, Alemayehu W, et al. Prevalence of and risk factors for trachoma in Oromia Regional State of Ethiopia: results of 79 population-based prevalence surveys conducted with the global trachoma mapping project. Ophthalmic Epidemiol. 2016;23(6):392–405. doi:10.1080/09286586.2016.1243717.
- Southisombath K, Sisalermsak S, Chansan P, et al. National Trachoma Assessment in the Lao People's Democratic Republic in 2013-2014. Ophthalmic Epidemiol. 2016;23(Sup 1):8–14. doi:10.1080/09286586.2016.1236973.
- Elshafie BE, Osman KH, Macleod C, et al. The epidemiology of trachoma in Darfur States and Khartoum State, Sudan: results of 32 population-based prevalence surveys. Ophthalmic Epidemiol. 2016;23(6):381–391. doi:10.1080/09286586.2016.1243718.
- Ko R, Macleod C, Pahau D, et al. Population-based trachoma mapping in six evaluation units of Papua New Guinea. Ophthalmic Epidemiol. 2016;23(Sup 1):22–31. doi:10.1080/09286586.2016.1235715.
- Adamu Y, Macleod C, Adamu L, et al. Prevalence of trachoma in Benishangul Gumuz Region, Ethiopia: results of seven population-based surveys from the global trachoma mapping project. Ophthalmic Epidemiol. 2016;23(Sup 1):70–76. doi:10.1080/09286586.2016.1247877.
- Sherief ST, Macleod C, Gigar G, et al. The prevalence of trachoma in Tigray Region, Northern Ethiopia: results of 11 population-based prevalence surveys completed as part of the global trachoma mapping project. Ophthalmic Epidemiol. 2016;23(Sup 1):94–99. doi:10.1080/09286586.2016.1250917.
- Adera TH, Macleod C, Endriyas M, et al. Prevalence of and risk factors for trachoma in Southern nations, nationalities, and peoples’ region, Ethiopia: results of 40 population-based prevalence surveys carried out with the global trachoma mapping project. Ophthalmic Epidemiol. 2016;23(Sup 1):84–93. doi:10.1080/09286586.2016.1247876.
- Abashawl A, Macleod C, Riang J, et al. Prevalence of trachoma in Gambella Region, Ethiopia: results of three population-based prevalence surveys conducted with the global trachoma mapping project. Ophthalmic Epidemiol. 2016;23(Sup 1):77–83. doi:10.1080/09286586.2016.1247875.
- Sokana O, Macleod C, Jack K, et al. Mapping Trachoma in the Solomon Islands: Results of Three Baseline Population-Based Prevalence Surveys Conducted with the Global Trachoma Mapping Project. Ophthalmic Epidemiol. 2016;23(Sup 1):15–21. doi:10.1080/09286586.2016.1238946.
- Macleod CK, Butcher R, Mudaliar U, et al. Low prevalence of ocular Chlamydia trachomatis infection and active trachoma in the Western Division of Fiji. PLoS Negl Trop Dis. 2016;10(7):e0004798. doi:10.1371/journal.pntd.0004798.
- Bio AA, Boko PM, Dossou YA, et al. Prevalence of trachoma in northern Benin: results from 11 population-based prevalence surveys covering 26 districts. Ophthalmic Epidemiol. 2017;24(4):265–273. doi:10.1080/09286586.2017.1279337.
- Taleo F, Macleod CK, Marks M, et al. Integrated mapping of yaws and trachoma in the five northern-most provinces of Vanuatu. PLoS Negl Trop Dis. 2017;11(1):e0005267. doi:10.1371/journal.pntd.0005267.
- Abdala M, Singano CC, Willis R, et al. The epidemiology of trachoma in Mozambique: results of 96 population-based prevalence surveys. Ophthalmic Epidemiol. 25(Sup 1):201–210. doi:10.1080/09286586.2017.1351996.
- Kilangalanga J, Ndjemba JM, Uvon PA, et al. Trachoma in the democratic republic of the Congo: results of 46 baseline prevalence surveys conducted with the global trachoma mapping project. Ophthalmic Epidemiol. 2018;25(Sup 1):192–200. doi:10.1080/09286586.2017.1306869.
- Duale AB, Ayele NN, Macleod CK, et al. Epidemiology of trachoma and its implications for implementing the “SAFE” strategy in Somali Region, Ethiopia: results of 14 population-based prevalence surveys. Ophthalmic Epidemiol. 2018;25(Sup 1):25–32. doi:10.1080/09286586.2017.1409358.
- Phiri I, Manangazira P, Macleod CK, et al. The burden of and risk factors for trachoma in selected districts of Zimbabwe: results of 16 population-based prevalence surveys. Ophthalmic Epidemiol. 2018;25(Sup 1):181–191. doi:10.1080/09286586.2017.1298823.
- World Health Organization Strategic and Technical Advisory Group on Neglected Tropical Diseases. Technical Consultation on Trachoma Surveillance. September 11−12, 2014, Task Force for Global Health, Decatur, USA (WHO/HTM/NTD/2015.02). Geneva: World Health Organization; 2015.
- Migchelsen SJ, Sepulveda N, Martin DL, et al. Serology reflects a decline in the prevalence of trachoma in two regions of The Gambia. Sci Rep. 2017;7(1):15040. doi:10.1038/s41598-017-15056-7.
- Gambhir M, Basanez MG, Burton MJ, et al. The development of an age-structured model for trachoma transmission dynamics, pathogenesis and control. PLoS Negl Trop Dis. 2009;3(6):e462. doi:10.1371/journal.pntd.0000462.
- Engels D. The global trachoma mapping project: a catalyst for progress against neglected tropical diseases. Ophthalmic Epidemiol. 2016;23(sup1):1–2. doi:10.1080/09286586.2016.1257139.
- Garn JV, Boisson S, Willis R, Bakhtiari A, Al-Khatib T, Amer K, et al. Sanitation and water supply coverage thresholds associated with active trachoma: modeling cross-sectional data from 13 countries. PLoS Negl Trop Dis. 2018;12(1):e0006110. doi:10.1371/journal.pntd.0006110.
- Delea MG, Solomon H, Solomon AW, Freeman MC. Interventions to maximize facial cleanliness and achieve environmental improvement for trachoma elimination: a review of the grey literature. PLoS Negl Trop Dis. 2018. doi:10.1371/journal.pntd.0006178.
- Ejere HO, Alhassan MB, Rabiu M. Face washing promotion for preventing active trachoma. Cochrane Database Syst Rev. 2015;2:CD003659.
- Rabiu M, Alhassan MB, Ejere HO, Evans JR. Environmental sanitary interventions for preventing active trachoma. Cochrane Database Syst Rev. 2012;(2):CD004003.
- Emerson PM, Bailey RL, Walraven GE, Lindsay SW. Human and other faeces as breeding media of the trachoma vector Musca sorbens. Med Vet Entomol. 2001;15(3):314–320. doi:10.1046/j.0269-283x.2001.00318.x.
- Emerson PM, Lindsay SW, Alexander N, et al. Role of flies and provision of latrines in trachoma control: cluster-randomised controlled trial. Lancet. 2004;363(9415):1093–1098. doi:10.1016/S0140-6736(04)15891-1.
- Abdou A, Nassirou B, Kadri B, et al. Prevalence and risk factors for trachoma and ocular Chlamydia trachomatis infection in Niger. Br J Ophthalmol. 2007;91(1):13–17. doi:10.1136/bjo.2006.099507.
- Courtright P, Sheppard J, Lane S, Sadek A, Schachter J, Dawson CR. Latrine ownership as a protective factor in inflammatory trachoma in Egypt. Br J Ophthalmol. 1991;75(6):322–325. doi:10.1136/bjo.75.6.322.
- World Health Organization and UNICEF. Drinking water, sanitation and hygiene service levels, Nigeria. 2017. https://washdata.org/data#!/nga. Accessed August 09, 2017.
- World Health Organization. Report on the Meeting on Post-Endemic Surveillance for Blinding Trachoma. Geneva: World Health Organization; 2008.
Appendix
The Global Trachoma Mapping Project Investigators are Agatha Aboe (1,11), Liknaw Adamu (4), Wondu Alemayehu (4,5), Menbere Alemu (4), Neal D. E. Alexander (9), Ana Bakhtiari (9), Berhanu Bero (4), Sarah Bovill (8), Simon J. Brooker (1,6), Simon Bush (7,8), Brian K. Chu (2,9), Paul Courtright (1,3,4,7,11), Michael Dejene (3), Paul M. Emerson (1,6,7), Rebecca M. Flueckiger (2), Allen Foster (1,7), Solomon Gadisa (4), Katherine Gass (6,9), Teshome Gebre (4), Zelalem Habtamu (4), Danny Haddad (1,6,7,8), Erik Harvey (1,6,10), Dominic Haslam (8), Khumbo Kalua (5), Amir B. Kello (4,5), Jonathan D. King (6,10,11), Richard Le Mesurier (4,7), Susan Lewallen (4,11), Thomas M. Lietman (10), Chad MacArthur (6,11), Colin Macleod (3,9), Silvio P. Mariotti (7,11), Anna Massey (8), Els Mathieu (6,11), Siobhain McCullagh (8), Addis Mekasha (4), Tom Millar (4,8), Caleb Mpyet (3,5), Beatriz Muñoz (6,9), Jeremiah Ngondi (1,3,6,11), Stephanie Ogden (6), Alex Pavluck (2,4,10), Joseph Pearce (10), Serge Resnikoff (1), Virginia Sarah (4), Boubacar Sarr (5), Alemayehu Sisay (4), Jennifer L. Smith (11), Anthony W. Solomon (1,2,3,4,5,6,7,8,9,10,11), Jo Thomson (4); Sheila K. West (1,10,11), Rebecca Willis (2,9).
Key: (1) Advisory Committee, (2) Information Technology, Geographical Information Systems, and Data Processing, (3) Epidemiological Support, (4) Ethiopia Pilot Team,(5) Master Grader Trainers, (6) Methodologies Working Group, (7) Prioritisation Working Group, (8) Proposal Development, Finances and Logistics, (9) Statistics andData Analysis, (10) Tools Working Group, (11) Training Working Group
