ABSTRACT
Purpose: To assess Eritrea’s progress towards elimination of trachoma as a public health problem, we reviewed and compiled current knowledge on the distribution and burden of trachoma in Eritrea, then undertook further population-based surveys where indicated, with support from the Global Trachoma Mapping Project (GTMP).
Methods: For the systematic review, undertaken in March 2014, we searched (1) PubMed, using the terms ((blind* or trachoma or trichiasis) AND Eritrea); (2) the online database of rapid assessments of avoidable blindness; (3) our own grey literature collections; and (4) the Global Atlas of Trachoma database. In June and July 2014, we conducted nine population-based prevalence surveys, for each of which 30 villages were systematically selected with probability proportional to population size; in each village, 30 households were systematically selected. All consenting residents of selected households aged ≥1 year were examined by GTMP-certified graders for signs of trachoma. Data on household-level access to water and sanitation were also collected.
Results: One previous rapid assessment of avoidable blindness, three peer-reviewed publications, and two grey literature reports detailing sets of trachoma prevalence surveys conducted in 2006 and 2011, respectively, were located. Post-intervention impact surveys were needed in seven evaluation units (EUs, framed at sub-Zoba-level: population range 40,000–120,000) of Debub and Northern Red Sea, while baseline surveys were needed in two EUs of Anseba. Four of the seven impact survey EUs and both baseline survey EUs returned trachomatous inflammation—follicular prevalences in 1–9-year-olds of ≥5%; six of the seven impact survey EUs and one of the two baseline survey EUs returned trichiasis prevalences in ≥15-year-olds of ≥0.2%. The prevalence of access to water and sanitation varied widely between EUs.
Conclusion: Interventions are still required in Eritrea to eliminate trachoma as a public health problem. Data from these surveys will guide the Ministry of Health to undertake programme planning using a sound evidence base.
Introduction
Trachoma is an eye disease caused by particular strainsCitation1,Citation2 of the bacterium Chlamydia trachomatis. It is strongly associated with poverty.Citation3 In 2016, approximately 190 million people were thought to live in endemic areas globally,Citation4 where transmission of C. trachomatis from eye to eye is believed to occur via eye-seeking flies, fingers and fomites.Citation5 Recurrent episodes of active (inflammatory) trachoma in children can produce trachoma’s blinding complication, trichiasis, in later life.Citation6–Citation8
The World Health Organization (WHO) defines “elimination of trachoma as a public health problem” as a prevalence of the active trachoma sign “trachomatous inflammation—follicular” (TF)Citation9 among 1–9-year-olds of <5%, and a prevalence of trichiasis unknown to the health systemCitation10 among ≥15-year-olds of <0.2%, in every previously-endemic district.Citation11 Achieving this worldwide would constitute the Global Elimination of Trachoma as a public health problem, which is targeted for completion by the end of the year 2020 (GET2020).Citation12,Citation13
Trachoma is recognized to be an endemic disease in Eritrea.Citation14 Located in the Horn of Africa, the country is bordered by the Red Sea to the east, Djibouti to the south-east, EthiopiaCitation15,Citation16 to the south, and SudanCitation17 to the north and west. It is divided into six regions (Zobas) and has an estimated total population of 3.8 million people. Zobas are divided into a total of 58 districts (sub-Zobas), each of which has a population of 40,000–120,000 people.
WHO recommends that surveys to determine whether or not public-health-level interventions are needed against trachoma normally be conducted in evaluation units (EUs) of 100,000–250,000 people. (This usually corresponds to the unit of administration for healthcare.Citation10) WHO further recommends that baseline surveys may be done in larger EUs than this, in order to get programmes started.Citation10 Prior to 2014, some baseline trachoma surveys had already been completed in Eritrea, and interventions had been ongoing since 2011 in eight sub-Zobas.
The purpose of this work, undertaken in 2014, was to review and compile current knowledge on the distribution and burden of trachoma in Eritrea, then to conduct population-based surveys where indicated, with support from the Global Trachoma Mapping Project.Citation18–Citation21 In each EU that needed to be surveyed, we used internationally-standardized approaches to determine the prevalence of trichiasis in adults aged ≥15 years, the prevalence of TF in children aged 1–9 years, and prevalence of access to improved water and sanitation.Citation22
Methods
We undertook work in two phases. First, we carried out a systematic review to consolidate what was already known about the epidemiology of trachoma in Eritrea. A PubMed (https://www.ncbi.nlm.nih.gov/pubmed/) search was conducted for articles containing information on trachoma epidemiology in the country, published in any language prior to the search date of 7 March 2014. We used search terms ((blind* or trachoma or trichiasis) AND Eritrea). We supplemented this with a search of the online database of rapid assessments of avoidable blindness (http://raabdata.info/repository/), review of relevant grey literature held by the authors, and review of the data from Eritrea held by the Global Atlas of Trachoma (www.trachomaatlas.orgCitation23,Citation24) as of 7 March 2014.
Second, guided by the results of the first phase, we implemented a series of cross-sectional surveys using two-stage cluster sampling in a total of nine EUs: seven sub-Zobas of Debub and Northern Red Sea (where impact surveys were due) and two EUs in Anseba Zoba (where sufficiently detailed baseline data were not available: see below).
Sample size
Sample-size calculations were determined based on TF considerations, as recommended by WHO.Citation25 The EU-level sample size for baseline surveys is based on an expected TF prevalence in 1–9-year-olds of 10% and desired absolute precision of 3%. Using the single-population-proportion-for-precision formulaCitation26 with a design effect of 2.65, to have 95% confidence of estimating a prevalence of 10% ± 3%, 1019 children are needed.Citation19 The EU-level sample size for impact surveys is based on an expected TF prevalence in 1–9-year-olds of 4% and desired absolute precision of 2%.Citation10 Using the same formula and a design effect of 2.65, to have 95% confidence of estimating a prevalence of 4% ± 2%, 978 children are needed. Inflating these figures by a factor of 1.2 to account for non-response, we aimed to sample enough households to be able to enumerate 1222 resident children (baseline EUs) or 1174 resident children (impact survey EUs).
Sampling plan
The first-stage sampling units were villages (population range 100–1000 people). The sampling frame was the list of all villages obtained from Eritrea’s Ministry of Local Government; 30 were systematically selected, with probability that was proportional to village population size.Citation25 In each selected village, 30 households were selected, as follows. In small villages, the list of households was obtained from village administrators and households were systematically selected. In large villages, four zones were created, one zone was drawn at random, then the small-village selection method was undertaken. Although children aged 1–9 years and adults aged ≥15 years were the age groups of interest, to enhance community acceptability, reduce the amount of explanation that field teams had to provide, and avoid creating incentives for age misrepresentation, in selected households, we included all residents aged ≥1 year.
Team training and data collection
Survey teams comprised one ophthalmic clinical officer, one recorder and one driver. Team preparation was undertaken in accordance with version 2 of the standard GTMP training system.Citation27
All residents aged ≥1 year in sampled households were enumerated and asked to agree to be examined for signs of trachoma. Household-level data on location (using GPS coordinates) and access to water and sanitation were also recorded. Teams moved from one selected household to the next. Findings were recorded in LINKS-GTMPCitation19,Citation28 on Android smartphones.
Data management
Data were encrypted and uploaded to a cloud-based server, and managed according to standard GTMP protocols, as previously described.Citation19 However, because available census data provided age breakdowns only in 5-year age bands, we initially undertook age standardization of TF prevalence estimates using age bins of 1–4 and 5–9 years, and the assumption that 1–4-year-olds comprise 80% of the 0–5-year-old population. For the purposes of this publication, we have also undertaken age standardization of TF prevalence estimates using 1-year age bands, as employed elsewhere.Citation29,Citation30 In each case, the proportion of 1–9 year-olds with TF in each village was adjusted using the relevant age bands. Similarly, the proportion of ≥15-year-olds with trichiasis was adjusted in 5-year age and gender bands. These adjustments were intended to partially compensate for incomplete examination recruitment in different age and gender groups.Citation31 EU-level prevalence estimates of TF and trichiasis were calculated as the arithmetic means of adjusted village-level proportions of TF and trichiasis, respectively.
Ethical considerations
Informed written consent was obtained from the head of the household to enter and work in each compound. Owing to low levels of literacy and in accordance with local norms, informed verbal consent was obtained for examination. Examiners cleaned their hands with alcohol-based hand gel between each examination, to avoid transferring pathogens between examinees. Individuals with conjunctivitis were treated with 1% tetracycline eye ointment. Individuals needing trichiasis surgery were referred to the Zoba or sub-Zoba hospital where they were offered management by the resident ophthalmologist, ophthalmic officer or trichiasis surgeon. Other medical problems were managed in the field or referred, as appropriate. Each work was conducted in accordance with the principles of the Declaration of Helsinki. Approval was obtained from the ethics committee of the London School of Hygiene & Tropical Medicine (6319 and 8355) and the Eritrea Ministry of Health National Ethics Committee.
Results
Systematic review
Our PubMed search returned 14 papers, only one of whichCitation14 provided data on prevalence of trachoma generated since the 1987 publicationCitation9 of the WHO simplified trachoma grading system. (The same study was also found in the database of rapid assessments of avoidable blindness, as the only Eritrea-related work stored there.) In 2008, Müller et alCitation14 deployed survey teams to 66 villages systematically selected with probability proportional to village size from a list of all villages in Eritrea. Compact segment sampling was used to select 50 people aged ≥50 years in each village. Amongst 3163 people examined, 386 (12.3%) were bilaterally blind or severely visually impaired; 4 (1.0%) of these had their loss of vision attributed to trachoma.Citation14
Two earlier published papers, published in 1963Citation32 and 1964,Citation33 held some data on trachoma that may now be of only limited value, by virtue of their epidemiological power and/or vintage. In 1963, Renna reported results of investigations in the Eastern lowlands of Eritrea in an area surrounding Massaua; the MacCallan trachoma grading systemCitation34 was used to evaluate 485 1–4-year-olds and 1098 5–14-year-olds, finding signs of active trachoma in 194 (40%) and 411 (37%) of those groups, respectively.Citation32 Vozza et al. completed a survey in 1960 in 10 villages of Serayé, a former province of Eritrea that in the 1996 reclassification was divided by the border created between the Zobas of Debub and Gash-Barka. They found one or more signs of trachoma in 97% of 5015 persons examined; the prevalence of trichiasis in the all-ages population was estimated to be 7%.Citation33
Our own grey literature collections included reports of two tranches of trachoma prevalence surveys. In 2006, the Eritrea Ministry of Health undertook trachoma surveys in Debub, Gash-Barka, and Northern Red Sea. A total of three EUs were surveyed, with each one framing the entirety of a Zoba. In each EU, 30 households were enrolled from each of 20 villages.Citation35 Results are shown in and . These surveys were adequately powered for making decisions regarding intervention at EU level; further division of the data (to calculate 12, 14 and nine sub-Zoba-level prevalence estimates for Debub, Gash-Barka and Northern Red Sea, respectively) probably introduces excessive imprecision, and those estimates are therefore not reported here. It is important to note, however, that on the basis of those calculations, one sub-Zoba of Debub (May-Mine, bordering Tigray Region of EthiopiaCitation15 to the south) was deemed to have a TF prevalence in 1–9-year-olds of >30%.Citation35
Table 1. Sampling details and prevalence of trachomatous inflammation—follicular (TF) in 1–9-year-olds and trichiasis in ≥15-year-olds, Ministry of Health trachoma surveys, Eritrea, 2006.Citation35
Figure 1. Prevalence of trachomatous inflammation—follicular (TF) in 1–9-year-olds, trachoma prevalence surveys, Eritrea, 2006 (Debub, Gash-Barka, and Northern Red Sea [NRS], each surveyed as a separate evaluation unit) and 2011 (Anseba, Maekel, and Southern Red Sea [SRS], surveyed as a single [discontinuous] evaluation unit composed of all three zobas).
![Figure 1. Prevalence of trachomatous inflammation—follicular (TF) in 1–9-year-olds, trachoma prevalence surveys, Eritrea, 2006 (Debub, Gash-Barka, and Northern Red Sea [NRS], each surveyed as a separate evaluation unit) and 2011 (Anseba, Maekel, and Southern Red Sea [SRS], surveyed as a single [discontinuous] evaluation unit composed of all three zobas).](/cms/asset/6fff27a1-05a6-4342-b0a5-e4bb3aec225a/iope_a_1545036_f0001_oc.jpg)
Interventions against trachoma, including mass distribution of the antibiotic azithromycin, were undertaken from 2011 to 2013 in all eight sub-Zobas of Debub and Northern Red Sea thought to have TF prevalence ≥10%, based on the 2006 survey data.Citation35 By 2014, impact surveys were needed in seven of the eight; the other (May-Mine) was already programmed for two further years of intervention before an impact survey was due.
The second tranche of surveys was conducted by the Ministry of Health in 2011 in the remaining three Zobas (Anseba, Maekel and Southern Red Sea) of Eritrea. Surveys were powered to determine prevalence of TF across the three Zobas as a single EU, with a total of 1135 1–9-year-olds examined. Results are shown in and .Citation36 Though the TF prevalence in 1–9-year-olds was <5%, it is difficult to have confidence that active trachoma was rare or absent in any given sub-Zoba, since the surveys were not powered to estimate prevalence at that level. However, neither Maekel (which contains Asmara and its surrounds) nor Southern Red Sea was considered by the Ministry of Health prior to the surveys as being likely to have trachoma as a public health problem. Individuals aged 10–39 years were not examined, and trichiasis prevalence was determined in ≥40-year-olds (). It has been estimated that a general conversion factor for translating a trichiasis prevalence in ≥15-year-olds to one for ≥40-year-olds is ×2.5,Citation37 meaning that the equivalent trichiasis prevalence estimate in ≥15-year-olds for this EU would be 0.6%. It is noted that the proportion of children examined who had TF, and the proportion of adults examined who had trichiasis, were both considerably higher in Anseba than in the other two Zobas.Citation36 We therefore planned further baseline surveys in Anseba, as part of our second phase of work.
Table 2. Sampling details and prevalence of trachomatous inflammation—follicular (TF) in 1–9-year-olds and trichiasis in ≥40-year-olds, Ministry of Health trachoma surveys, Eritrea, 2011.Citation36
Global Trachoma Mapping Project surveys
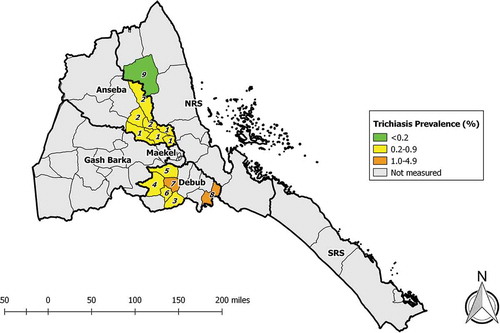
We undertook survey fieldwork in June–July 2014 in the nine sub-zobas identified to need trachoma prevalence surveys: two repeat baseline surveys in Anseba, six impact surveys in Debub, and one impact survey in Northern Red Sea. Across the nine EUs, 8059 households were visited, and a total of 10,338 1–9-year-olds and 17,056 ≥15-year-olds were enumerated. Of these, 9956 (96%) and 13,926 (82%), respectively, were examined (). The EU-level prevalence of TF in 1–9-year-olds ranged from 1.6% (95%CI 0.8–2.6) to 9.2% (95%CI 6.6–12.2) (, ). The EU-level prevalence of trichiasis in ≥15-year-olds ranged from 0.10% (95% CI 0.00–0.26) to 1.15% (95%CI 0.73–1.66) (, ).
Table 3. Number of 1–9-year-olds and number of ≥ 15-year-olds resident, examined, absent and refused; prevalence of trachomatous inflammation—follicular (TF); and prevalence of trichiasis; trachoma prevalence surveys, Eritrea, June and July, 2014.
Figure 2. Prevalence of trachomatous inflammation—follicular (TF) in 1–9-year-olds, trachoma prevalence surveys, June and July, 2014. Sub-Zobas are labelled with numbers; the key is found in both and . (NRS, Northern Red Sea; SRS, Southern Red Sea).
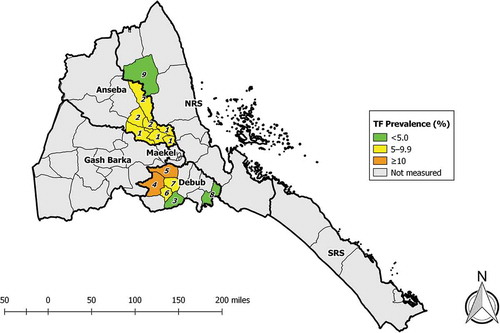
Figure 3. Prevalence of trichiasis in ≥15-year-olds, trachoma prevalence surveys, Eritrea, June and July, 2014. Sub-Zobas are labelled with numbers; the key is found in both and . (NRS, Northern Red Sea; SRS, Southern Red Sea).
There was considerable variation between EUs in the prevalence of household-level access to water and sanitation ().
Table 4. Household-level access to improved water and sanitation facilities by evaluation unit, trachoma prevalence surveys, Eritrea, June and July, 2014.
Discussion
Trachoma is still a public health problem in parts of Eritrea. Both EUs of Anseba (a Zoba previously surveyed in 2011 as part of a large, three-zoba EU) returned 2014 baseline TF prevalence estimates in 1–9-year-olds indicating a need for intervention with the A, F and E components of the SAFE strategy.Citation38,Citation39 One of those two Anseba EUs also had a trichiasis prevalence in adults above the 0.2% elimination threshold, and will therefore need public-health-level implementation of the S component of SAFE.Citation11 Of the seven EUs in which we completed impact surveys, three had TF prevalences <5% and now enter the two-year active trachoma surveillance period,Citation11,Citation40 while the other four require ongoing A, F and E interventions. Only one of the seven impact survey EUs (Nafka sub-Zoba of Northern Red Sea) had a trichiasis prevalence in adults <0.2%.
Because available Eritrea census publications report age-specific data for the bands 1–4 years and 5–9 years, we initially undertook age standardization of TF estimates against those two broad divisions. Elsewhere in similar circumstances, the GTMP developed a practice of evenly sub-dividing such totals into one-year age bands and age-standardizing against the result, using the rationale that ignoring mortality from 12 to 59 months and from 60 to 119 months would be preferable to ignoring the (often substantial) differences in age-specific TF prevalence within these age brackets. We therefore undertook repeat analysis using one-year age band standardization for the purposes of compiling this manuscript. For Areza and Dubarwa sub-Zobas in Debub, standardizing against broader and finer age bands gave programmatically different TF prevalence estimates. For both EUs, the TF prevalence was determined to be within the 5.0–9.9% range using the finer age bands, and just greater than 10% using the broader ones. Although the confidence intervals for these paired alternate estimates had considerable overlap, the distinctions are functionally important, because EUs in which TF prevalence is 10.0–29.9% qualify for three years of annual mass antibiotic treatment, whereas WHO recommends only that targeted antibiotic treatment be considered in EUs in which TF prevalence is 5.0–9.9%.Citation25 (Recent data suggest that a single round of mass treatment may be effective for EUs in that prevalence range.Citation41) Further work to determine the most appropriate way to undertake age standardization of TF prevalence estimates may be needed.
The data reported here have other deficiencies. We did not examine for the presence or absence of trachomatous scarringCitation9 in tarsal conjunctivae of eyes with trichiasis.Citation42 The extremely high proportion of resident children apparently examined () suggests that field teams either enumerated only those present at the time of the visit or were assiduous in returning at the end of the day to examine children who were initially absent; our field supervisors believe the latter explanation is correct. (This belief is supported by the fact that many absentee adults were enumerated.) In Sena’fe sub-Zoba, only 858 1–9-year-olds were examined: 88% of the 978 targeted, though availability of data from 30 (rather than fewer) clusters and the relatively tight confidence intervals suggest that the TF prevalence estimate here would be reproducible. There were large differences in the numbers of absentee adults recorded between EUs, an observation for which we also lack a definitive explanation. Finally, GPS satellite fixesCitation43 were frequently elusive, and many houses therefore lacked GPS coordinate information, limiting our ability to quality-assure household selection.Citation44
These were not easy surveys. First, selected villages were often very hard to reach, with terrain that did not allow vehicle access. Field teams were forced to start work early and travel on foot for several hours to reach the villages of interest. Second, delays occasioned by difficulties in transferring financial resources into the country meant that fieldwork commenced at the onset of the rainy season. Potential examinees were therefore busy with time-sensitive planting activities, and our graders and recorders had to find them in nearby fields. These timing issues made making return visits to households (to find people absent at the time of the first visit) a challenge. Third, crippling power shortages and limited bandwidth complicated the process of data upload-to-cloud in Asmara. We are proud of the commitment of our teams in having successfully completed the work despite these hurdles.
There remain a few sub-Zobas from the 2006 baseline surveys in which TF or trichiasis prevalence estimates were calculated (with wide confidence intervals, based on relatively few sampled clusters) to be above elimination thresholds. Eventually, to facilitate completion of a dossier claiming elimination of trachoma as a public health problem from Eritrea,Citation11 it may be necessary to re-survey these sub-Zobas. Repeat surveys will also be necessary to ensure that trachoma has been eliminated from EUs in which more precisely estimated prevalence estimates suggest that trachoma remains a public health problem. The priority for this round of surveys was to undertake baseline mapping in Anseba and to carry out the impact surveys in Debub and Northern Red Sea. Completion of the systematic review and nine population-based prevalence surveys described here provides a check-in point for ongoing efforts to eliminate trachoma from Eritrea. Such efforts are an important part of the global programme, which is now, following decades of work, starting to meet with success.Citation45
Disclosure statement
None of the authors have any proprietary or conflict of interest with this submission. The authors alone are responsible for the writing and content of this article.
Additional information
Funding
References
- Caldwell HD, Wood H, Crane D, et al. Polymorphisms in Chlamydia trachomatis tryptophan synthase genes differentiate between genital and ocular isolates. J Clin Invest. 2003;111(11):1757–1769. doi:10.1172/JCI17993.
- Hadfield J, Harris SR, Hmb S-S, et al. Comprehensive global genome dynamics of Chlamydia trachomatis show ancient diversification followed by contemporary mixing and recent lineage expansion. Genome Res. 2017;27(7):1220–1229. doi:10.1101/gr.212647.116.
- Habtamu E, Wondie T, Aweke S, et al. Trachoma and relative poverty: a case-control study. PLoS Negl Trop Dis. 2015;9(11):e0004228. doi:10.1371/journal.pntd.0004228.
- World Health Organization. WHO alliance for the global elimination of trachoma by 2020: progress report on elimination of trachoma, 2014–2016. Wkly Epidemiol Rec. 2017;92(26): 359–368.
- Mabey DC, Solomon AW, A. F. Trachoma. Lancet. 2003;362(9379):223–229. doi:10.1016/S0140-6736(03)13914-1.
- Grayston JT, Wang SP, Yeh LJ, Kuo CC. Importance of reinfection in the pathogenesis of trachoma. Rev Infect Dis. 1985;7:717–725.
- Gambhir M, Basanez MG, Burton MJ, et al. The development of an age-structured model for trachoma transmission dynamics, pathogenesis and control. PLoS Negl Trop Dis. 2009;3(6):e462. doi:10.1371/journal.pntd.0000462.
- West SK, Munoz B, Mkocha H, Hsieh YH, Lynch MC. Progression of active trachoma to scarring in a cohort of Tanzanian children. Ophthalmic Epidemiol. 2001;8:137–144.
- Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bull World Health Organ. 1987;65:477–483.
- World Health Organization. Report of the 3rd global scientific meeting on trachoma, Johns Hopkins University, Baltimore, MA, 19–20 July 2010 (WHO/PBD/2.10). Geneva: World Health Organization; 2010.
- World Health Organization. Validation of elimination of trachoma as a public health problem (WHO/HTM/NTD/2016.8). Geneva: World Health Organization; 2016.
- World Health Organization. Future approaches to trachoma control: report of a global scientific meeting, Geneva, 17–20 June 1996 (WHO/PBL/96.56). Geneva: World Health Organization, 1997.
- World Health Assembly. Global elimination of blinding trachoma. 51st World Health Assembly, Geneva, 16 May 1998, Resolution WHA51.11. Geneva: World Health Organization; 1998.
- Müller A, Zerom M, Limburg H, et al. Results of a rapid assessment of avoidable blindness (RAAB) in Eritrea. Ophthalmic Epidemiol. 2011;18(3):103–108. doi:10.3109/09286586.2010.545932.
- Sherief ST, Macleod C, Gigar G, et al. The prevalence of trachoma in Tigray region, Northern Ethiopia: results of 11 population-based prevalence surveys completed as part of the Global Trachoma Mapping Project. Ophthalmic Epidemiol. 2016;23(Suppl. 1):94–99. doi:10.1080/09286586.2016.1250917.
- Negash K, Macleod C, Adamu A, et al. Prevalence of trachoma in the Afar Region of Ethiopia: results of seven population-based surveys from the Global Trachoma Mapping Project. Ophthalmic Epidemiol. 2018;25(Sup 1):3–10. doi:10.1080/09286586.2017.1362008.
- Hassan A, Ngondi JM, King JD, et al. The prevalence of blinding trachoma in northern states of Sudan. PLoS Negl Trop Dis. 2011;5(5):e1027. doi:10.1371/journal.pntd.0001370.
- Solomon AW, Kurylo E. The global Trachoma Mapping Project. Community Eye Health. 2014;27:18.
- Solomon AW, Pavluck A, Courtright P, et al. The Global Trachoma Mapping Project: methodology of a 34-country population-based study. Ophthalmic Epidemiol. 2015;22(3):214–225. doi:10.3109/09286586.2015.1037401.
- Heggen AE, Solomon AW, Courtright P. Perspectives of national coordinators and partners on the work of the Global Trachoma Mapping Project. Ophthalmic Epidemiol. 2016;23(6):366–372. doi:10.1080/09286586.2016.1229795.
- Engels D. The Global Trachoma Mapping Project: a catalyst for progress against neglected tropical diseases. Ophthalmic Epidemiol. 2016;23(sup1):1–2. doi:10.1080/09286586.2016.1257139.
- Boisson S, Engels D, Gordon BA, et al. Water, sanitation and hygiene for accelerating and sustaining progress on neglected tropical diseases: a new Global Strategy 2015–20. Int Health. 2016;8(Suppl 1:):i19–i21. doi:10.1093/inthealth/ihv073.
- Polack S, Brooker S, Kuper H, Mariotti S, Mabey D, Foster A. Mapping the global distribution of trachoma. Bull World Health Organ. 2005;83(12):913–919. doi:10.1371/journal.pntd.0000973.
- Smith JL, Haddad D, Polack S, et al. Mapping the global distribution of trachoma: why an updated atlas is needed. PLoS Negl Trop Dis. 2011;5(6):e973. doi:10.1371/journal.pntd.0001370.
- Solomon AW, Zondervan M, Kuper H, Buchan JC, Mabey DCW, Foster A Trachoma control: a guide for programme managers. Geneva: World Health Organization; 2006.
- Kirkwood BR. Essentials of Medical Statistics. Oxford: Blackwell Science; 1988.
- Courtright P, Gass K, Lewallen S, et al. Global Trachoma Mapping Project: training for mapping of trachoma (version 2) [Available at: http://www.trachomacoalition.org/node/357]. London: International Coalition for Trachoma Control; 2013.
- Pavluck A, Chu B, Mann Flueckiger R, Ottesen E. Electronic data capture tools for global health programs: evolution of LINKS, an Android-, web-based system. PLoS Negl Trop Dis. 2014;8(4):e2654. doi:10.1371/journal.pntd.0002654.
- Bero B, Macleod C, Alemayehu W, et al. Prevalence of and risk factors for trachoma in oromia regional state of ethiopia: results of 79 population-based prevalence surveys conducted with the Global Trachoma Mapping Project. Ophthalmic Epidemiol. 2016;23(6):392–405. doi:10.1080/09286586.2016.1243717.
- Mpyet C, Muhammad N, Adamu MD, et al. Prevalence of trachoma in bauchi state, nigeria: results of 20 local government area-level surveys. Ophthalmic Epidemiol. 2016;23(Sup 1):39–45. doi:10.1080/09286586.2016.1238945.
- Solomon AW, Bella AL, Negussu N, Willis R, Taylor HR. How much trachomatous trichiasis is there? A guide to calculating district-level estimates. Community Eye Health. 2018. In press.
- Renna V. [Ocular diseases and the causes of blindness in Eritrea. I. Investigation of the diffusion and gravity of trachoma in the eastern lowlands]. Boll Ocul. 1963;42:677–684.
- Vozza R, Renna V, Felici A. Epidemiological survey on trachoma in eritrea. Rev Int Trach. 1964;41:303–314.
- MacCallan AF. The epidemiology of trachoma. Br J Ophthalmol. 1931;15:369–411.
- Ministry of Health of the State of Eritrea. Report of the national trachoma prevalece survey. Asmara: Ministry of Health; 2006.
- Ministry of Health of the State of Eritrea. Trachoma Prevalence Survey Report of Three Zobas (Anseba, Maekel and Southern Red Sea Zobas). Asmara: Ministry of Health; 2012.
- World Health Organization Strategic and Technical Advisory Group on Neglected Tropical Diseases. Design and validation of a trachomatous trichiasis-only survey (WHO/HTM/NTD/PCT/2017.08). Geneva: World Health Organization; 2018.
- Kuper H, Solomon AW, Buchan J, Zondervan M, Foster A, Mabey D. A critical review of the SAFE strategy for the prevention of blinding trachoma. Lancet Infect Dis. 2003;3:372–381.
- Francis V, Turner V Achieving community support for trachoma control (WHO/PBL/93.36). Geneva: World Health Organization; 1993.
- World Health Organization Strategic and Technical Advisory Group on Neglected Tropical Diseases. Technical consultation on trachoma surveillance. September 11−12, 2014, Task Force for Global Health, Decatur, USA (WHO/HTM/NTD/2015.02). Geneva: World Health Organization; 2015.
- Kalua K, Chisambi A, Chainyanya D, et al. One round of azithromycin MDA adequate to interrupt transmission in districts with prevalence of trachomatous inflammation-follicular of 5.0–9.9%: evidence from Malawi. PLoS Negl Trop Dis. 2018;12(6):e0006543. doi:10.1371/journal.pntd.0006543.
- World Health Organization Alliance for the Global Elimination of Trachoma by 2020. Second global scientific meeting on trachomatous trichiasis. Cape Town, 4–6 November 2015 (WHO/HTM/NTD/2016.5). Geneva: World Health Organization; 2016.
- Polack SR, Solomon AW, Alexander ND, et al. The household distribution of trachoma in a Tanzanian village: an application of GIS to the study of trachoma. Trans R Soc Trop Med Hyg. 2005;99(3):218–225. doi:10.1016/j.trstmh.2004.06.010.
- Solomon AW, Willis R, Pavluck AL, et al. Quality assurance and quality control in the Global Trachoma Mapping Project. Am J Trop Med Hyg. 2018;99(4):858-863. doi:10.4269/ajtmh.18-0082.
- Solomon AW, Emerson PM, Resnikoff S. Trachoma then and now: update on mapping and control. Community Eye Health. 2017;30:90–91.
Appendix
The authors are grateful to the following for their diligent contributions in the field for the 2014 surveys: Ghebre Hailemicael, Mehari Tekle, Mehari Semereab, Bisrat Woldeghiorgis, Yohannes Zehaye, Hussien Abdela, Samuel Kebede, and Yebio Ghebreab (Graders); Fessha Zerai, Tesfagabir Akale, Semir Abdu Talke, Fesha Teame, Aman Goitom, Hailemicael Hagos, Goitom Habtemariam, and Ghebrehiwet Arefaine (Recorders); and Micael Teklai, Feshaye Ghergis, and Ghebreselasie Ghebremariam (Facilitators).
The Global Trachoma Mapping Project Investigators are: Agatha Aboe (1,11), Liknaw Adamu (4), Tawfik Al-Khatib (5), Wondu Alemayehu (4,5), Menbere Alemu (4), Neal D. E. Alexander (9), Ana Bakhtiari (2,9), Berhanu Bero (4), Sophie Boisson (3), Sarah Bovill (8), Simon J. Brooker (1,6), Simon Bush (7,8), Brian K. Chu (2,9), Paul Courtright (1,3,4,7,11), Michael Dejene (3), Paul M. Emerson (1,6,7), Rebecca M. Flueckiger (2), Allen Foster (1,7), Solomon Gadisa (4), Katherine Gass (6,9), Teshome Gebre (4), Zelalem Habtamu (4), Danny Haddad (1,6,7,8), Erik Harvey (1,6,10), Dominic Haslam (8), Khumbo Kalua (5), Amir B. Kello (4,5), Jonathan D. King (6,10,11), Richard Le Mesurier (4,7), Susan Lewallen (4,11), Thomas M. Lietman (10), Chad MacArthur (6,11), Colin Macleod (3,9), Silvio P. Mariotti (7,11), Patrick A. Massae (5), Anna Massey (8), Els Mathieu (6,11), Siobhain McCullagh (8), Addis Mekasha (4), Tom Millar (4,8), Caleb Mpyet (3,5), Beatriz Muñoz (6,9), Jeremiah Ngondi (1,3,6,11), Stephanie Ogden (6), Alex Pavluck (2,4,10), Joseph Pearce (10), Serge Resnikoff (1), Virginia Sarah (4), Boubacar Sarr (5), Alemayehu Sisay (4), Jennifer L. Smith (11), Anthony W. Solomon (1,2,3,4,5,6,7,8,9,10,11), Jo Thomson (4); Sheila K. West (1,10,11), Rebecca Willis (2,9).
1. Advisory Committee, 2. Information Technology, Geographical Information Systems, and Data Processing, 3. Epidemiological Support, 4. Ethiopia Pilot Team, 5. Master Grader Trainers, 6. Methodologies Working Group, 7. Prioritisation Working Group, 8. Proposal Development, Finances and Logistics, 9. Statistics and Data Analysis, 10. Tools Working Group, 11. Training Working Group.
