ABSTRACT
Purpose: To estimate the prevalence of trachoma in suspected-endemic areas of Chad, and thereby determine whether trachoma is a public health problem requiring intervention.
Methods: We divided the suspected-endemic population living in secure districts into 46 evaluation units (EUs), and used the standardized methodologies of the Global Trachoma Mapping Project. A two-stage cluster-sampling procedure was adopted. In each EU, the goal was to examine at least 1019 children aged 1–9 years by recruiting 649 households; all consenting residents aged ≥ 1 year living in those households were examined. Each participant was examined for trachomatous inflammation—follicular (TF), trachomatous inflammation—intense (TI), and trichiasis.
Results: Two EUs had data that could not be validated, and were excluded from the analysis. GPS data for three other pairs of EUs suggested that EU divisions were inaccurate; data for each pair were combined within the pair. In the 41 resulting EUs, 29,924 households in 967 clusters were visited, and 104,584 people were examined. The age-adjusted EU-level prevalence of TF in 1–9-year-olds ranged from 0.0% to 23.3%, and the age- and gender-adjusted EU-level prevalence of trichiasis in ≥ 15-year-olds ranged from 0.02% to 1.3%. TF was above the WHO elimination threshold in 16 EUs (39%) and trichiasis was above the WHO elimination threshold in 29 EUs (71%). Women had a higher prevalence of trichiasis than did men in 31 EUs (76%). A higher ratio of trichiasis prevalence in women to trichiasis prevalence in men was associated (p = 0.03) with a higher prevalence of trichiasis at EU level.
Conclusion: Public health-level interventions against trachoma are needed in Chad. Over 10,000 people need management of their trichiasis; women account for about two-thirds of this total. The association between a higher ratio of trichiasis prevalence in women to that in men with higher overall trichiasis prevalence needs further investigation.
Background
Trachoma, the leading infectious cause of blindness worldwide,Citation1 is a chronic kerato-conjunctivitis caused by the bacterium Chlamydia trachomatis.Citation2 Infections, most commonly occurring in children,Citation3 may lead to sub-epithelial follicles or more pronounced inflammation.Citation4 Repeated infectionCitation5,Citation6 can lead to scarring of the conjunctivaeCitation7 which, when severe enough, can deform the eyelid and cause eyelashes to touch the globe (trichiasis).Citation4 Uncorrected trichiasis can result in corneal abrasion, ulceration, opacification, and potentially, vision loss and blindness.
In 1993, the World Health Organization (WHO) endorsed the SAFE strategy,Citation8 a comprehensive management plan for elimination of trachoma as a public health problem. SAFE refers to surgery (S) to correct trichiasis, mass distribution of antibiotics (A) to clear infection, and facial cleanliness (F) and environmental improvement (E) to reduce C. trachomatis transmission.Citation9 To determine whether public health-level interventions are required, population-based surveys to generate prevalence estimates of trachomatous inflammation—follicular (TF) and trichiasis are recommended.Citation10,Citation11
Chad is a central African country of approximately 12 million people spread across three distinct ecologic zones: the Sahara Desert, the Sahel, and the Savanna. Currently the country has a total of 33 ophthalmic nurses; 35 ophthalmic technicians; and nine ophthalmologists (approximately one for every 1.5 million people) of whom 5 are in the capital N’Djamena. Though absolute numbers of eye-care personnel are low, Chad is fortunate that 90% of them work in the public sector – specifically in five departments of ophthalmology (within two secondary and three tertiary hospitals) and 21 secondary eye care units.
A number of population-based trachoma prevalence surveys were undertaken in Chad in 1984,Citation12 1985,Citation13 2001Citation14 and 2004Citation15 (); however, due to financial constraints, SAFE strategy implementation was not commenced until 2015. The 1984–2004 surveys occurred prior to the recent growth in interest in trachoma elimination,Citation17 and were conducted at region level, covering large populations and wide geographical areas (). Because of the age and relatively low resolution of existing data, in order to inform programmatic action, baseline mapping or re-mapping was felt to be required.Citation18 We set out to estimate the prevalence of TF in 1–9-year-olds and the prevalence of trichiasis in adults in population units of 100,000–250,000 people in suspected-trachoma-endemic areas of rural Chad.
Table 1. Findings from trachoma prevalence surveys in Chad, 1984–2004.
Materials and methods
Administratively, Chad is divided into 23 regions. Each region (other than the capital, N’Djamena, which has a different internal administrative structure) is divided into two to six health districts, the level at which trachoma elimination activities are implemented.Citation19 There are 61 health districts in total, of which 45 were suspected to have trachoma as a public health problem and therefore to qualify for mapping, based on criteria published elsewhere.Citation20 Surveys were conducted in 2014 and 2015. Due to insecurity prevailing at that time, five suspected-trachoma-endemic health districts (Bol and Ngouri in Lac Region, Nokou in Kanem Region, Mandelia in Chari Baguirmi Region and Bardaï in Tibesti Region) could not be surveyed.
Survey design, field team training and certification, fieldwork, and data handling were conducted according to the systems and methodologies of the Global Trachoma Mapping Project (GTMP).Citation20–Citation23 Each of the 40 secure health districts was generally surveyed as a single evaluation unit (EU), though six health districts with populations (estimated using 2009 population census dataCitation24 and a mean annual population growth rate of 3.6%) significantly larger than the standard 100,000–250,000-person EU were divided into two EUs each, resulting in a total of 46 independent EUs.
Village-level population estimates were provided by the Division of Health & Information Systems. According to the 2009 census,Citation24 the proportion of the population aged 1–9 years was 36% and rural households had a mean of 5.3 residents. The estimated sample size requirement per EU was based on an expected TF prevalence of 10% in children aged 1–9 years, a design effect of 2.65, and a desire to be 95% confident of estimating the TF prevalence with ± 3% absolute precision.Citation22 The resulting sample size (n = 1019) was increased by 20% to account for non-response; this resulted in a total of 649 households being required per EU.
Using a two-stage cluster-sampling design, 22 clusters (villages or neighbourhoods) were systematically selected in each EU using probability-proportional-to-population-size sampling. In each cluster, compact segment samplingCitation25,Citation26 was then used to select 30 households. All residents over the age of 12 months who had resided for at least six months in selected households were eligible for enrolment.
A survey team consisted of a grader (ophthalmic technician), a recorder (high school graduate at ease with Android smart phones and fluent in major local languages), a local facilitator and a driver. Members of the survey team underwent standardized GTMP training, using version 2 of the system.Citation27 Candidate graders were assessed after training, and only those obtaining a kappa of ≥ 0.7 for diagnosis of TF in an inter-grader agreement test with a GTMP-certified grader trainer were accepted as survey graders. Trachoma grading was done according to the WHO simplified grading system.Citation4 Graders used 2.5× magnifying binocular loupes and sunlight illumination to examine consenting residents. In eyes diagnosed as having trichiasis, the presence or absence of trachomatous conjunctival scarringCitation28,Citation29 was not recorded, so we are unable to confirm that trichiasis cases detected were due to trachoma; consequently, we refer here to the prevalence of trichiasis instead of the prevalence of trachomatous trichiasis. Each survey team was trained to ask questions relating to access to water and sanitation at each selected household.Citation27
All data were captured electronically, through the Open Data Kit-based Android phone application purpose-built for the GTMP. Once saved, data were sent to and stored on the GTMP Cloud-based secure server, then cleaned and analyzed.Citation22 For each survey cluster, the proportion of 1–9-year-old children with TF was adjusted by age in one-year age bands, while the proportion of ≥ 15-year-olds with trichiasis was adjusted by age and gender in five-year age bands; age and gender data from the 2009 Chad census were used as the reference population for this purpose.Citation24 For each EU, the primary outcome of interest was the age-adjusted prevalence of TF in 1–9-year-olds; intended secondary outcomes were the age- and gender-adjusted prevalence of trichiasis in ≥ 15-year-olds, and household-level access to water and sanitation. Confidence intervals for TF and trichiasis prevalence estimates were calculated by bootstrapping sets of 22 adjusted cluster-level proportions for each sign, with replacement, over 10,000 replicates, and taking the 2.5th and 97.5th centiles of the ordered results. Additional gender-specific age-adjusted estimates of trichiasis prevalence, with 95% confidence intervals, were calculated in analogous fashion. The ratio of trichiasis prevalence in females to that in males in each EU was also determined, and linear regression modelling (Stata 11, College Station TX, USA) used to generate an intra-class correlation coefficient, to assess the association between EU-level trichiasis prevalence and ratio of gender-specific prevalences.
Ethical clearance was obtained from the Chadian Ethical Committee for Applied Research, led by the Ministry of Higher Education; and from the London School of Hygiene & Tropical Medicine (6319). The examination procedure was explained to each eligible adult in the local language and verbal consent for enrolment and examination was obtained. For eligible children, verbal consent was obtained from a parent or guardian. Individuals with active trachoma were offered 1% tetracycline ointment for application into the conjunctival sac twice-daily for six weeks. Individuals with trichiasis were offered management by a surgeon.
Results
Fieldwork was undertaken from May 2014 to November 2015. At a subsequent field team meeting, it emerged that fieldworkers had considered their main task to be the acquisition of information on TF and trichiasis. Contrary to the survey protocol and the standard GTMP training package, in some villages, questions about access to water and sanitation had not been systematically asked in each selected household. Rather, information had been collected from the head of the village at the beginning of the day and those answers used for each household visited. Although this shortcut was not employed by all teams, it cast doubt on the accuracy of our water and sanitation data, and we do not include those data in this manuscript. Data cleaning revealed inconsistency in definitions of EU and district boundaries. In particular, in three health districts (Pala, Béré & Kélo, and Donomanga & Laï) that had each been split into two EUs, GPS data revealed considerable overlap between clusters that had ostensibly been drawn from separate EUs. Data from these pairs of within-health-district EUs were therefore combined to re-constitute health-district-level EUs; the numbers of clusters finally included in each EU are shown in . GPS dataCitation30 were not received at all from a high proportion of households mapped in both of Moundou’s two EUs; those that had GPS data were geolocated in a pattern inconsistent with known administrative divisions. For that reason, the data from Moundou did not pass GTMP quality control, and the outputs were, as expected, rejected by the health ministry. We therefore present data here from what became a total of 41 surveys.
Table 2. Numbers of children and adults enumerated and examined, by evaluation unit, Global Trachoma Mapping Project, Chad, 2014–2015.
Figure 1. Prevalence of trachomatous inflammation—follicular (TF) in 1–9-year-olds, Global Trachoma Mapping Project, Chad, 2014–2015. Evaluation units are labelled with numbers; the key is found in , and .
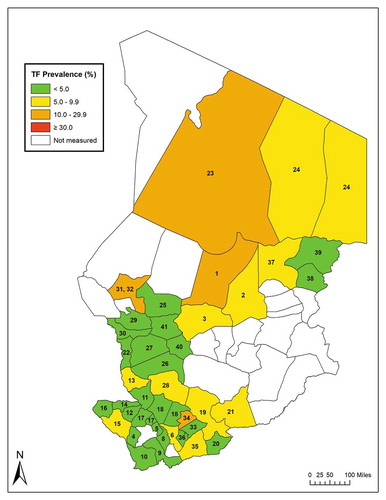
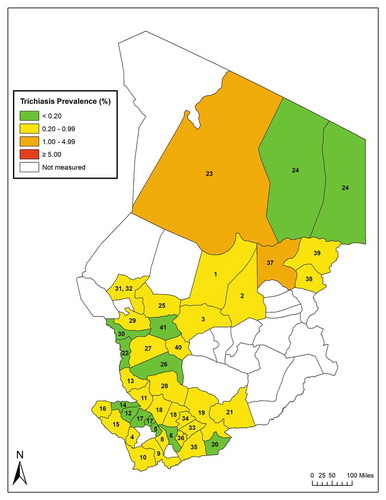
Figure 2. Prevalence of trichiasis in ≥ 15-year-olds, Global Trachoma Mapping Project, Chad, 2014–2015. Evaluation units are labelled with numbers; the key is found in , and .
In total, 104,705 people were enrolled and 104,584 (99%) examined in 29,239 households recruited from 967 clusters (). There were almost equal numbers of 1–9-year-olds (n = 55,885) and ≥ 15-year-olds (n = 48,699) examined. While the sampling process was designed to facilitate examination of at least 1,019 children in each EU, the survey teams in five EUs did not reach this target, with the lowest number of 1–9-year-olds examined in an EU being 956; reports from the field indicated that in many locations, households had fewer resident children than expected.
The adjusted TF prevalence in 1–9-year-old children was ≥ 5% (above the WHO threshold for eliminationCitation31) in 16 (39%) of the 41 EUs. Five EUs (12%) had TF prevalence estimates of 10–29.9%, and 11 EUs (27%) had TF prevalence estimates of 5–9.9% (, ).
Table 3. Prevalence of trachomatous inflammation—follicular (TF) in 1–9-year-olds, prevalence of trichiasis in ≥ 15-year-olds, backlog of trichiasis cases and number of trichiasis cases needing management to reach the WHO elimination threshold, by evaluation unit, Global Trachoma Mapping Project, Chad, 2014–2015.
In 12 EUs (29%), the age- and gender-adjusted trichiasis prevalence was below the WHO elimination thresholdCitation31 of 0.2% in ≥ 15-year-olds (, ). In the remaining 29 EUs, trichiasis prevalence was ≥ 0.2%. Two EUs had trichiasis prevalence estimates of > 1%. The estimated number of trichiasis patients requiring management to achieve elimination of trichiasis as a public health problem at the time of conclusion of the surveys was 10,562 ().
Analysis of gender-specific age-adjusted trichiasis prevalence estimates revealed mean EU-level prevalences of 0.55% in women and 0.28% in men; in 31 (76%) of the 41 EUs, the prevalence of trichiasis was higher in women than men (). There were five EUs in which none of the men examined had trichiasis. The mean ratio of prevalence in women to that in men (excluding the five EUs in which prevalence in men was 0) was 1.70 (SE = 0.53) in the EUs below the WHO elimination threshold, and 2.31 (SE = 0.48) in the EUs above the WHO elimination threshold. A higher prevalence of trichiasis was associated with a greater excess of disease in women (correlation coefficient = 250, SE = 114, p = 0.03).
Table 4. Prevalence of trichiasis in males and females aged ≥ 15 years, by evaluation unit, Global Trachoma Mapping Project, Chad, 2014–2015.
Discussion
The results of these and previous surveys demonstrate that trachoma is a public health problem in Chad. To move towards elimination of trachoma as a public health problem, AFE interventions should be implemented for at least three years before re-survey for the approximately 887,000 people in the five EUs in which TF prevalence was ≥ 10%, and for at least one year before re-survey for the nearly 2.8 million people in the 11 EUs in which TF prevalence was 5–9.9%. Although we are unable to report our own data on access to water and sanitation, 2017 data released by the Chad Government and UNICEF suggest that region-level proportions of the population with access to potable water are as low as 12% (Ennedi-Est), and that outside N’Djamena, region-level rates of open defecation range from 61 to 93%. These conditions are associated with high risk of active trachoma,Citation32,Citation33 highlighting the need for the F&E components of the SAFE strategy here.
The TF prevalence estimates from these GTMP-supported surveys are considerably lower than those of previous surveys completed in Chad.Citation13–Citation15 There are a number of possible explanations for this. When surveys were first planned here, it would have been logical to choose to start in districts with higher expected burdens of trachoma – where, in other words, eye health professionals were already aware of cases. There may also, or alternatively, have been a temporal decline in the prevalence of active trachoma in the intervening period,Citation34–Citation37 with older surveys reflecting C. trachomatis transmission intensitiesCitation38 occurring before more recent improvements in access to water, sanitation and health care. The GTMP’s emphasis on standardization of trachoma grading (including grader training and qualification based on examination of real people, rather than projected imagesCitation20) may also have contributed.
Trichiasis is widespread in Chad (), with more than two-thirds of EUs surveyed in 2014–2015 having trichiasis prevalence estimates above the WHO elimination threshold. Establishing a public health-level response to trichiasis throughout the widely dispersed communities in these EUs will require considerable capacity building for delivery of high-quality trichiasis surgery and programme management, as well as community-based efforts to generate awareness and encourage uptake of services.Citation39,Citation40 The excess burden of trichiasis among females (), also noted elsewhere,Citation25,Citation41,Citation42 compels us to ensure that such efforts particularly serve women. Experience in other countries can inform strategies to improve use of eye care services by women.Citation43,Citation44 The association noted here between higher prevalence of trichiasis and greater ratio of trichiasis prevalence in women to trichiasis prevalence in men cannot be explained from our data alone. We note that this was not a pre-specified hypothesis of the current work, and suggest only that further investigation is indicated.
Our work has a number of limitations. First, in five EUs, we did not quite reach the estimated sample size requirement. We report confidence intervals here, however, which facilitates objective assessment of the likely repeatability of our estimates. In future surveys in Chad, the sampling approach will be revised slightly to reflect the smaller-than-expected mean number of children encountered per household. Second, we recruited these marginally low numbers of examinees despite what was apparently an extraordinarily high enrolment rate: 99% of enumerated residents. We wonder whether field teams, fearing criticism for incomplete enrolment, may have failed to register absentees: anecdotally, this occurred in other constituent projects of the GTMP, but obtaining definitive proof was difficult.Citation20 Third, this survey work was commenced prior to the inclusion of examination for trachomatous conjunctival scarring (TSCitation4) in standard GTMP protocols,Citation45 as later recommended by a global scientific meeting.Citation29 It is therefore likely that some of the trichiasis cases included in our prevalence estimates were due to conditions other than trachomaCitation28,Citation29; this may explain part of the association between the overall prevalence of trichiasis and the ratio of gender-specific prevalence estimates. We also did not ask about previous management of trichiasis, so the trichiasis prevalence estimates reported here include both cases known and unknown to the health system.Citation19 These refinements can be helpful in influencing whether or not public-health-level interventions are needed against trichiasis.Citation46 Fourth, as noted in the results section, three EU pairs were combined at the data cleaning stage; the main implication of this is that the resulting EU populations (like that for N’Djamena suburbs) are larger than the recommended 100,000–250,000 people.Citation19 Fifth, as also already noted, data from two EUs in Moundou, Logone Occidental Region, could not be approved due to missing GPS data; as a consequence, results from this EU are not included in the current report. Sixth, because household-level questions were not used as set out in the survey protocol, we are unable to report data on access to water and sanitation. Though unfortunate, as much as this situation reveals a weakness in one part of fieldwork execution, it also demonstrates strength in fieldwork supervision.
Subsequent to completing these surveys, in addition to expanding SAFE interventions, the Ministry of Health commenced planning to re-map Moundou as well as to undertake mapping in some of, but not all, the EUs in which surveys were not attempted in 2014–2015. Undertaking those surveys will contribute to the completion of nationwide mapping of suspected-trachoma-endemic areas of Chad, and help chart a course towards national elimination of trachoma as a public health problem.Citation47
Disclosure Statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017. doi:10.1016/S2214-109X(17)30393-5.
- Mabey DC, Solomon AW, Foster A. Trachoma. Lancet. 2003;362(9379):223–229. doi:10.1016/S0140-6736(03)13914-1.
- Solomon AW, Holland MJ, Burton MJ, et al. Strategies for control of trachoma: observational study with quantitative PCR. Lancet. 2003;362(9379):198–204. doi:10.1016/S0140-6736(03)13909-8.
- Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bull World Health Organ. 1987;65:477–483.
- Grayston JT, Wang SP, Yeh LJ, Kuo CC. Importance of reinfection in the pathogenesis of trachoma. Rev. Infect. Dis. 1985;7:717–725.
- Gambhir M, Basanez MG, Burton MJ, et al. The development of an age-structured model for trachoma transmission dynamics, pathogenesis and control. PLoS Negl Trop Dis. 2009;3(6):e462. doi:10.1371/journal.pntd.0000462.
- West SK, Munoz B, Mkocha H, Hsieh YH, Lynch MC. Progression of active trachoma to scarring in a cohort of Tanzanian children. Ophthalmic Epidemiol. 2001;8:137–144.
- Francis V, Turner V. Achieving Community Support for Trachoma Control (WHO/PBL/93.36). Geneva: World Health Organization; 1993.
- Kuper H, Solomon AW, Buchan J, Zondervan M, Foster A, Mabey D. A critical review of the SAFE strategy for the prevention of blinding trachoma. Lancet Infect Dis. 2003;3:372–381.
- Solomon AW, Zondervan M, Kuper H, Buchan JC, Mabey DCW, Foster A. Trachoma Control: A Guide for Programme Managers. Geneva: World Health Organization; 2006.
- Solomon AW, Peeling RW, Foster A, Mabey DC. Diagnosis and assessment of trachoma. Clin Microbiol Rev. 2004;17(4):982–1011. doi:10.1128/CMR.17.4.982-1011.2004.
- Resnikoff S, Lamarque D. Compte rendu d’enquete: sondage sur la trachome et les cecites a Mao et Bol (Tchad). Bull OCEAC. 1985;71:47–56.
- Resnikoff S. Compte rendu d’enquête sur la situation ophtalmologique dans les préfectures du Biltine et du Ouaddaï. Bull OCEAC. 1987;79:109–115.
- Madani MO, Huguet P, Mariotti SP, et al. [Trachoma in Chad: results of an epidemiological survey]. Sante. 2003;13(1):9–15.
- Dézoumbé D, Djada DA, Négrel AD. Resultats preliminaires de quatre enquetes epidemiologiques de prevalence du trachome (2001-2005): Note de synthèse. N’Djamena: Programme National de Lutte Contre la Cécité, Ministère de la Santé; 2005.
- Dawson CR, Jones BR, Tarizzo ML. Guide to Trachoma Control in Programmes for the Prevention of Blindness. Geneva: World Health Organization; 1981.
- Solomon AW, Emerson PM, Resnikoff S. Trachoma then and now: update on mapping and control. Community Eye Health. 2017;30:90–91.
- Smith JL, Haddad D, Polack S, et al. Mapping the global distribution of trachoma: why an updated atlas is needed. PLoS Negl Trop Dis. 2011;5(6):e973. doi:10.1371/journal.pntd.0001370.
- World Health Organization. Report of the 3rd global scientific meeting on trachoma, Johns Hopkins University, Baltimore, MA, 19-20 July 2010 (WHO/PBD/2.10). Geneva: World Health Organization; 2010.
- Solomon AW, Willis R, Pavluck AL, et al. Quality assurance and quality control in the global trachoma mapping project. Am J Trop Med Hyg. 2018;99(4):858–863. doi:10.4269/ajtmh.18-0082.
- Solomon AW, Kurylo E. The global trachoma mapping project. Community Eye Health. 2014;27:18.
- Solomon AW, Pavluck A, Courtright P, et al. The global trachoma mapping project: methodology of a 34-country population-based study. Ophthalmic Epidemiol. 2015;22(3):214–225. doi:10.3109/09286586.2015.1037401.
- Mpyet C, Kello AB, Solomon AW. Global elimination of trachoma by 2020: A work in progress. Ophthalmic Epidemiol. 2015;22(3):148–150. doi:10.3109/09286586.2015.1045987.
- République du Tchad. Recensement général de la population. N’Djamena: Institut national de la statistique, des études économiques et démographiques; 2009.
- Bero B, Macleod C, Alemayehu W, et al. Prevalence of and risk factors for trachoma in Oromia regional state of Ethiopia: results of 79 population-based prevalence surveys conducted with the global trachoma mapping project. Ophthalmic Epidemiol. 2016;23(6):392–405. doi:10.1080/09286586.2016.1243717.
- Phiri I, Manangazira P, Macleod CK, et al. The burden of and risk factors for trachoma in selected districts of Zimbabwe: results of 16 population-based prevalence surveys. Ophthalmic Epidemiol. 2018;25(Sup 1):181–191. doi:10.1080/09286586.2017.1298823.
- Courtright P, Gass K, Lewallen S, et al. Global Trachoma Mapping Project: Training for Mapping of Trachoma (Version 2). London: International Coalition for Trachoma Control; 2013. http://www.trachomacoalition.org/node/357].
- World Health Organization Strategic and Technical Advisory Group on Neglected Tropical Diseases. Technical Consultation on Trachoma Surveillance. September 11−12, 2014, Task Force for Global Health, Decatur, USA (WHO/HTM/NTD/2015.02). Geneva: World Health Organization; 2015.
- World Health Organization Alliance for the Global Elimination of Trachoma by 2020. Second Global Scientific Meeting on Trachomatous Trichiasis. Cape Town, 4-6 November 2015 (WHO/HTM/NTD/2016.5). Geneva: World Health Organization; 2016.
- Polack SR, Solomon AW, Alexander ND, et al. The household distribution of trachoma in a Tanzanian village: an application of GIS to the study of trachoma. Trans R Soc Trop Med Hyg. 2005;99(3):218–225. doi:10.1016/j.trstmh.2004.06.010.
- World Health Organization. Validation of Elimination of Trachoma as a Public Health Problem (WHO/HTM/NTD/2016.8). Geneva: World Health Organization; 2016.
- Garn JV, Boisson S, Willis R, et al. Sanitation and water supply coverage thresholds associated with active trachoma: modeling cross-sectional data from 13 countries. PLoS Negl Trop Dis. 2018;12(1):e0006110. doi:10.1371/journal.pntd.0006110.
- Oswald WE, Stewart AE, Kramer MR, et al. Active trachoma and community use of sanitation, Ethiopia. Bull World Health Organ. 2017;95(4):250–260. doi:10.2471/BLT.16.177758.
- Jha H, Chaudary JS, Bhatta R, et al. Disappearance of trachoma from Western Nepal. Clin Infect Dis. 2002;35(6):765–768. doi:10.1086/342298.
- Chidambaram JD, Alemayehu W, Melese M, et al. Effect of a single mass antibiotic distribution on the prevalence of infectious trachoma. Jama. 2006;295(10):1142–1146. doi:10.1001/jama.295.10.1142.
- Dolin PJ, Faal H, Johnson GJ, et al. Reduction of trachoma in a sub-Saharan village in absence of a disease control programme. Lancet. 1997;349(9064):1511–1512.
- Dolin PJ, Faal H, Johnson GJ, Ajewole J, Mohamed AA, Lee PS. Trachoma in The Gambia. Br J Ophthalmol. 1998;82:930–933.
- Migchelsen SJ, Sepulveda N, Martin DL, et al. Serology reflects a decline in the prevalence of trachoma in two regions of The Gambia. Sci Rep. 2017;7(1):15040. doi:10.1038/s41598-017-15056-7.
- Burton M, Solomon A. What’s new in trichiasis surgery? Community Eye Health. 2004;17:52–53.
- Gupta KM, Harding JC, Othman MS, Merbs SL, Gower EW. Why do patients refuse trichiasis surgery? Lessons and an education initiative from Mtwara Region, Tanzania. PLoS Negl Trop Dis. 2018;12(6):e0006464. doi:10.1371/journal.pntd.0006464.
- Elshafie BE, Osman KH, Macleod C, et al. The epidemiology of trachoma in Darfur states and Khartoum state, sudan: results of 32 population-based prevalence surveys. Ophthalmic Epidemiol. 2016;23(6):381–391. doi:10.1080/09286586.2016.1243718.
- Cromwell EA, Courtright P, King JD, Rotondo LA, Ngondi J, Emerson PM. The excess burden of trachomatous trichiasis in women: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2009;103(10):985–992. doi:10.1016/j.trstmh.2009.03.012.
- Mousa A, Courtright P, Kazanjian A, Bassett K. A community-based eye care intervention in Southern Egypt: impact on trachomatous trichiasis surgical coverage. Middle East Afr J Ophthalmol. 2015;22(4):478–483. doi:10.4103/0974-9233.167808.
- West S, Nguyen MP, Mkocha H, et al. Gender equity and trichiasis surgery in the Vietnam and Tanzania national trachoma control programmes. Br J Ophthalmol. 2004;88(11):1368–1371. doi:10.1136/bjo.2004.041657.
- Courtright P, Gass K, Lewallen S, et al. Global Trachoma Mapping Project: Training for Mapping of Trachoma (Version 3). London: International Coalition for Trachoma Control; 2014. http://www.trachomacoalition.org/node/122].
- Khan AA, Florea VV, Hussain A, et al. Prevalence of Trachoma in Pakistan: results of 42 population-based prevalence surveys from the global trachoma mapping project. Ophthalmic Epidemiol. 2018. In preparation.
- Courtright P, Rotondo LA, MacArthur C, et al. Strengthening the links between mapping, planning and global engagement for disease elimination: lessons learnt from trachoma. Br J Ophthalmol. 2018;102(10):1324–1327. doi:10.1136/bjophthalmol-2018-312476.
Appendix
The Global Trachoma Mapping Project Investigators are: Agatha Aboe (1,11), Liknaw Adamu (4), Wondu Alemayehu (4,5), Menbere Alemu (4), Neal D. E. Alexander (9), Berhanu Bero (4), Simon J. Brooker (1,6), Simon Bush (7,8), Brian K. Chu (2,9), Paul Courtright (1,3,4,7,11), Michael Dejene (3), Paul M. Emerson (1,6,7), Rebecca M. Flueckiger (2), Allen Foster (1,7), Solomon Gadisa (4), Katherine Gass (6,9), Teshome Gebre (4), Zelalem Habtamu (4), Danny Haddad (1,6,7,8), Erik Harvey (1,6,10), Dominic Haslam (8), Khumbo Kalua (5), Amir B. Kello (4,5), Jonathan D. King (6,10,11), Richard Le Mesurier (4,7), Susan Lewallen (4,11), Thomas M. Lietman (10), Chad MacArthur (6,11), Colin Macleod (3,9), Silvio P. Mariotti (7,11), Anna Massey (8), Els Mathieu (6,11), Siobhain McCullagh (8), Addis Mekasha (4), Tom Millar (4,8), Caleb Mpyet (3,5), Beatriz Muñoz (6,9), Jeremiah Ngondi (1,3,6,11), Stephanie Ogden (6), Alex Pavluck (2,4,10), Joseph Pearce (10), Serge Resnikoff (1), Virginia Sarah (4), Boubacar Sarr (5), Alemayehu Sisay (4), Jennifer L. Smith (11), Anthony W. Solomon (1,2,3,4,5,6,7,8,9,10,11), Jo Thomson (4), Sheila K. West (1,10,11), Rebecca Willis (2,9).
Key: (1) Advisory Committee, (2) Information Technology, Geographical Information Systems, and Data Processing, (3) Epidemiological Support, (4) Ethiopia Pilot Team, (5) Master Grader Trainers, (6) Methodologies Working Group, (7) Prioritisation Working Group, (8) Proposal Development, Finances and Logistics, (9) Statistics and Data Analysis, (10) Tools Working Group, (11) Training Working Group.
