ABSTRACT
Purpose: A number of previous administrative-district-level baseline trachoma prevalence estimates in Zambia required verification. We used methodologies and systems for trachoma surveys considered to represent international best practice in order to generate reliable estimates of the prevalence of trachoma.
Methods: Between March 2016 and July 2017, we undertook 32 population-based prevalence surveys covering 47 administrative districts. In each of the 32 evaluation units (EUs), we selected 31 households in each of 24 clusters. In selected households, trained, certified graders examined all residents aged 1 year and above for evidence of trachomatous inflammation—follicular (TF) and trichiasis. In eyes that had trichiasis, the presence or absence of trachomatous scarring (TS) was recorded, and the subject was asked about previous trichiasis management recommendations from health workers.
Results: Five EUs (encompassing seven administrative districts) had prevalence estimates of trichiasis+TS unknown to the health system in ≥15-year-olds of ≥0.2%, and require public-health-level implementation of trichiasis surgery services. Eleven EUs (encompassing 16 administrative districts) had TF prevalence estimates in 1–9-year-olds of ≥5%. Intervention with the A, F and E components of the SAFE strategy for trachoma elimination is required for nearly 1.5 million people.
Conclusion: Trachoma is a public health problem in some parts of Zambia. The Ministry of Health will continue to partner with other stakeholders to implement the multi-sectoral SAFE strategy. Consideration should be given to re-surveying other suspected-endemic administrative districts in which surveys using older methodologies returned TF prevalence estimates ≥5%.
Introduction
Zambia was an enthusiastic supporter of the 1998 adoption of World Health Assembly Resolution 51.11, which called for the global elimination of blinding trachoma.Citation1 The country continues to support that goal, through active participation in the World Health Organization (WHO) Alliance for Global Elimination of Trachoma by 2020,Citation2,Citation3 and development of strategic plans to eliminate trachoma domestically. This article describes data generation exercises carried out in order to facilitate that planning process.
Trachoma is a neglected tropical diseaseCitation4 caused by the ocular biovarCitation5,Citation6 of the intracellular bacterium Chlamydia trachomatis. It is common in populations that have inadequate access to water and sanitation.Citation7–Citation10 In Zambia, as elsewhere, such people tend to live in remote and rural areas, and to be very poor.Citation11–Citation15
Ocular C. trachomatis is transmitted in eye and nose secretions via fingers, fomites (such as face towels and clothing) and eye-seeking flies,Citation7 particularly between members of the same household.Citation16,Citation17 Infection may be associated with active (inflammatory) trachoma, which often meets the criteria for trachomatous inflammation—follicular (TF) and/or trachomatous inflammation—intense (TI), signs defined within the WHO simplified trachoma grading scheme.Citation18 Both ocular C. trachomatis infection and active trachoma are more common and more intense in pre-school-age children,Citation19,Citation20 with immunological factorsCitation21 and reduced exposure patterns possibly responsible for their lower prevalence in older individuals. Repeated episodes of infection and associated inflammation are needed for the development of significant conjunctival scarring (TS) and for the trachomatous trichiasis (TT) that, in some individuals, supervenes.Citation22 Mathematical modelling suggests that development of these two signs may require more than 100 and 150 C. trachomatis infections, respectively.Citation23
Blindness from trachoma is prevented using the SAFE strategy,Citation24–Citation27 which includes surgery for TT, antibiotics to clear infection, and facial cleanliness and environmental improvement to reduce transmission.Citation28 The S component of SAFE should be offered to anyone with TT. The A, F and E components of SAFE are administered to whole populations in which the TF prevalence in 1–9-year-olds is ≥5%. Programmatic planning for public-health-level approaches for reducing both the prevalence of TT and the prevalence of TF relies on prevalence estimates of these signs, which should be generated through population-based surveys.Citation29
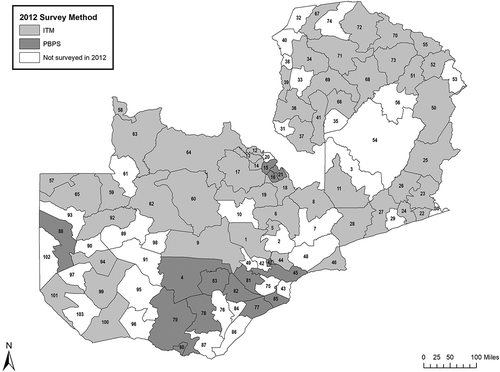
In 2012, just prior to the launch of the Global Trachoma Mapping Project (GTMP),Citation30 prevalence surveys were undertaken in each of 65 administrative districts across all 10 provinces of Zambia (). These surveys used a variety of approaches,Citation31 specifically cluster sampling to generate population-based prevalence estimates according to WHO guidelines,Citation32 and the then-newly-proposed integrated threshold mapping (ITM) methodology.Citation33 Some of the prevalence estimates that these surveys produced differed markedly from pre-survey expectations. In particular, all nine surveyed administrative districts of Copperbelt Province, which was not historically understood to be trachoma-endemic, had estimates of TF prevalence in 1–9-year-olds that exceeded 10% [unpublished Ministry of Health data]; the near-absence of trichiasis in adults examined in these districts as part of the same surveys could be interpreted as a pointer to recent introduction of trachoma to this population, or a need to reconfirm the TF prevalence estimates. The National Blindness Prevention Committee therefore recommended implementation of a further tranche of surveys in selected districts of Zambia.
Figure 1. Trachoma prevalence surveys undertaken in Zambia, 2012, using either the integrated threshold mapping (ITM) methodology, or a population-based prevalence survey (PBPS) approach. Key to districts: 1. Chibombo; 2. Chisamba; 3. Chitambo; 4. Itezhi tezhi; 5. Kabwe Rural; 6. Kapiri Mposhi; 7. Luano; 8. Mkushi; 9. Mumbwa; 10. Ngabwe; 11. Serenje; 12. Chililbombwe; 13. Chingola; 14. Kalulushi; 15. Kitwe; 16. Luanshya; 17. Lufwanyama; 18. Masaiti; 19. Mpongwe; 20. Mufulira; 21. Ndola; 22. Chadiza; 23. Chipata; 24. Katete; 25. Lundazi; 26. Mambwe; 27. Nyimba; 28. Petauke; 29. Sinda; 30. Vubwi; 31. Chembe; 32. Chienge; 33. Chipili; 34. Kawambwa; 35. Lunga; 36. Mansa; 37. Milenge; 38. Mwansabombwe; 39. Mwense; 40. Nchelenge; 41. Samfya; 42. Chilanga; 43. Chirundu; 44. Chongwe; 45. Kafue; 46. Luangwa; 47. Lusaka; 48. Rufunsa; 49. Shibuyunji; 50. Chama; 51. Chinsali; 52. Isoka; 53. Mafinga; 54. Mpika; 55. Nakonde; 56. Shiwangandu; 57. Chavuma; 58. Ikelenge; 59. Kabompo; 60. Kasempa; 61. Manyinga; 62. Mufumbwe; 63. Mwinilunga; 64. Solwezi; 65. Zambezi; 66. Chilubi; 67. Kaputa; 68. Kasama; 69. Luwingu; 70. Mbala; 71. Mporokoso; 72. Mpulungu; 73. Mungwi; 74. Nsama; 75. Chikankata; 76. Choma; 77. Gwembe; 78. Kalomo; 79. Kazungula; 80. Livingstone; 81. Mazabuka; 82. Monze; 83. Namwala; 84. Pemba; 85. Siavonga; 86. Sinazongwe; 87. Zimba; 88. Kalabo; 89. Kaoma; 90. Limulunga; 91. Luampa; 92. Lukulu; 93. Mitete; 94. Mongu 95. Mulobezi; 96. Mwandi; 97. Nalolo; 98. Nkeyema; 99. Senanga; 100. Sesheke; 101. Shang’ombo; 102. Sikongo; 103. Sioma.
Materials and methods
The survey methodology used was based on that of the GTMP,Citation34 as modified and refined by Tropical Data (www.tropicaldata.org).Citation35,Citation36 Our approaches were consistent with WHO recommendations for trachoma prevalence surveys.Citation32,Citation37
Survey teams
Each team was composed of a grader (Ophthalmic Clinical Officer or Ophthalmic Nurse), a recorder (Grade 12 school-leaver), a village guide and a driver. Graders, recorders and team supervisors (ophthalmologists) were trained using the standardized five-day training system detailed in the Tropical Data training manual.Citation38 Only participants who passed stringent tests of competency proceeded to take part in the surveys.Citation34
Sample size
Surveys were powered primarily based on considerations relevant to TF prevalence, with trichiasis prevalence a secondary outcome. Planned sample sizes for each evaluation unit (EU) were consistent with guidance recently published by WHO.Citation39 We sought to have 95% confidence to estimate an expected TF prevalence of 4% with absolute precision of 2%, using a design effect of 2.63, and inflating the result by 20% to account for non-response. This meant that in each EU we needed to include at least the number of households in which 1164 1–9-year-olds would be resident, expecting to examine 970 of them.
Delineation of evaluation units and selection of clusters, households, and individuals
EUs, which were each composed of one or more administrative districts, were framed to encompass populations of roughly 100,000–250,000 people by either taking one administrative district per EU or combining two or more adjacent similar administrative districts. In each EU, 24 clusters (wards) were systematically selected using a probability-proportional-to-ward-size methodology.Citation32 In each selected cluster, 31 households were randomly selected, using compact segment sampling via random draw. In selected households, all residents aged 1 year or above were eligible to participate.
Fieldwork
Fieldwork was completed between March 2016 and July 2017. Provincial and district health offices facilitated community awareness and sensitisation exercises prior to planned survey team visits, using radio messages and community health workers (CHWs). CHWs then served as survey guides. Graders used 2.5× magnifying loupes and sunlight to examine all consenting household residents aged 1 year or above for signs of trachoma. Return visits were arranged to examine residents who were absent at the time of the primary visit.
Data management
Data were entered directly into Android smartphones running the Tropical Data app, a custom-built evolution of the LINKS Android smartphone data collection tool (Task Force for Global Health, Atlanta, GA, USA; https://linkssystem.org).Citation34,Citation40 At the end of each field day, the recorder uploaded data to the Tropical Data server using a secure, encrypted connection. The Data & Analytics Team checked and cleaned the data while field teams were still in the field; designated health ministry officials reviewed the cleaned data and approved analyses.Citation34 As previously described, these analyses included age standardization of TF prevalence estimates, age- and gender-standardization of trichiasis prevalence estimates, and generation of 95% confidence intervals for each prevalence estimate by bootstrapping, with replacement, the adjusted cluster-level proportions of each sign, over 10,000 replications.Citation34 Owing to current uncertainty over whether it is appropriate to define the trichiasis elimination prevalence threshold counting only those individuals in which TS is found in the same eye as the trichiasis—advanced by some authorities as a possible way to distinguish trachomatous from non-trachomatous diseaseCitation41—we present here prevalence estimates for all trichiasis, all trichiasis unknown to the health system, and trichiasis+TS unknown to the health system, each in ≥15-year-olds.
Prevalence categories for TF and trichiasis were provided to the Global Atlas of Trachoma to facilitate planning and global surveillance.Citation42,Citation43
Ethical considerations
The University of Zambia Biomedical Ethics and Research Committee (reference number 009-03-16) and the London School of Hygiene & Tropical Medicine Research Ethics Committee (6319, 8355) approved the surveys. Provincial and district health offices and local leaders, such as ward councillors and village headmen, were informed and engaged. Survey teams obtained informed verbal consent to proceed from the head of each selected household and informed verbal consent for examination from adults. For examination of minors, the head of the household gave informed verbal consent. Consent was documented in the data collection tool. Examinees with active trachoma were given 1% tetracycline eye ointment to apply to both eyes twice daily for 6 weeks. Examinees with trichiasis were referred to the nearest appropriate health facility for management.
Results
The 32 surveyed EUs had an estimated total population of 5,025,494, of which 98,454 residents (43,987 males, 54,467 females) were enumerated and 91,788 (93%) consenting individuals (39,719 males, 52,069 females) were examined ( 1) in 23,491 households of 757 clusters. A total of 320 individuals refused to participate, 6334 were absent on the day that a field team visited their household and 12 were not examined for other reasons. Resulting trachoma prevalence estimates are shown in 1, alongside comparisons, where available, of previous trachoma prevalence estimates. and display the EU-level prevalence estimates generated by this tranche of mapping.
Table 1. Number of ≥15-year-olds and number of 1–9-year-olds resident, examined, absent and refused in selected households; prevalence of all trichiasis; prevalence of all trichiasis unknown to the health system; prevalence of trichiasis+TS unknown to the health system; and prevalence of trachomatous inflammation—follicular (TF); by evaluation unit, trachoma prevalence surveys, Zambia, 2016–2017, with comparison to prevalence estimates from previous surveys, where available.
Figure 2. Prevalence of trichiasis + trachomatous scarring (TS) unknown to the health system (UTTHS), in ≥15-year-olds, by evaluation unit, trachoma prevalence surveys, Zambia, 2016–2017.
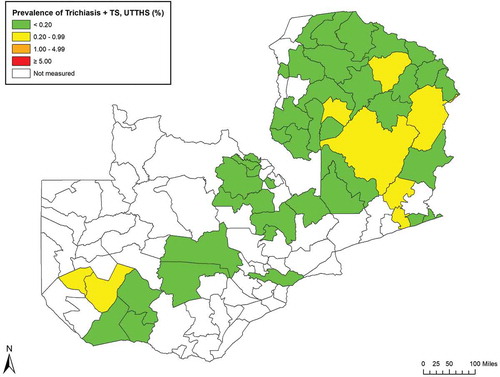
Figure 3. Prevalence of trachomatous inflammation—follicular (TF) in 1–9-year-olds, by evaluation unit, trachoma prevalence surveys, Zambia, 2016–2017.
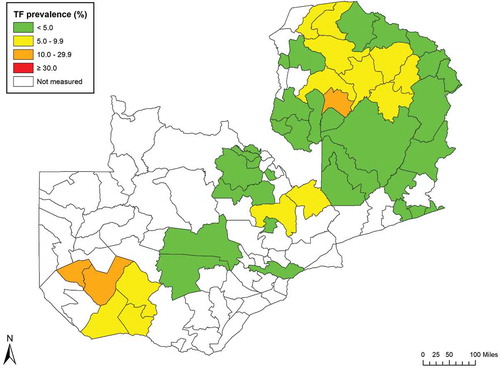
Eleven EUs (34% of EUs mapped, 16 districts, total population 1,473,707) had TF prevalence estimates in children of ≥5%. Eight EUs (12 districts, total population 986,620) had prevalence estimates of trichiasis unknown to the health system in adults of ≥0.2%, of which five EUs (seven districts, total population 618,204) had prevalence estimates of trichiasis+TS unknown to the health system of ≥0.2%. In these populations, trachoma is a public health problem, and interventions are needed.
Discussion
Control, elimination or eradication of a neglected tropical disease like trachoma are thought to deliver multiple benefits to endemic populations. Besides decreasing suffering from the targeted disease, the reduced morbidity that ensues plus the action of implementing control interventions each improves our collective likelihood of delivering results on a range of sustainable development goals.Citation44–Citation46 In particular, accessing the remote communities in which trachoma is found establishes a beachhead for universal health coverage. Even without considering these knock-on effects, trachoma elimination is objectively cheap,Citation47 cost-effective,Citation48,Citation49 and likely to result in economic gains that significantly exceed the cost of programme implementation.Citation50,Citation51
In Zambia, after early pilot work,Citation52 trachoma elimination has been underway in earnest since 2007, when the first comprehensive tranche of baseline surveys was initiated. From 2007 to 2012, surveys were conducted using several qualitatively and quantitatively different approaches, including ITM. (Relevant data from 2007 to 2012 are included, as comparators for the results of the 2016–2017 surveys, in 1.) Later analyses suggested that the use of ITM carried some risk of district misclassification,Citation29 and in the present work, we reverted to the use of cluster-sampled, population-based surveys. Our highly standardized, quality-controlled and quality-assuredCitation36 methodologies are considered to provide highly reliable dataCitation53; we believe, on this basis, that the prevalence estimates generated here supersede those produced in 2012.
Our teams mapped a total of 32 EUs covering 47 administrative districts ( 1). In many of these districts, trachoma is still a disease of public health significance. Particular note is made of the two EUs containing Chilubi (Northern Province) and Nalolo and Senanga (Western Province), which had TF prevalence estimates of >10%. Chilubi is mainly an island administrative district and has different demographic characteristics to other administrative districts of the Northern Region. Nalolo and Senanga are adjacent to other administrative districts with known high burdens of disease [unpublished Ministry of Health data]. For both of these EUs, implementation of the full SAFE strategy is now underway.
In the EUs surveyed here, the prevalence of trichiasis+TS unknown to the health system was generally not markedly lower than the prevalence of trichiasis unknown to the health system. There were only two EUs (Mulobezi, Mwandi and Sesheke of Western Province; and Nakonde of Muchinga Province) in which decisions on whether or not to initiate public-health-level trichiasis surgery interventions would differ using these two metrics. Further data and global policy decisions are awaited.
Zambia is now better placed than ever before to eliminate trachoma. The government is strongly focused on health investment, which is seen to target socioeconomic development by stimulating individual, grass-roots productivity.Citation54 Implementation of SAFE, a comprehensive, multi-sectoral strategy, can catalyze development partnerships whilst offering primary, secondary and tertiary prevention against trachoma blindness. For many participating communities, previous opportunities to access quality-assured antibiotics and/or modern surgery will have been limited prior to trachoma programme entry. Bilateral agencies and non-governmental organizations fund interventions against NTDs in ZambiaCitation55; both political will and partner support are therefore in place. Parallel initiatives that may alleviate poverty and thereby reduce trachoma risk, including foreign direct investment (which seems to have greatest impact in poorer environmentsCitation56), social cash transferCitation57 and rural electrification,Citation58 are also being pursued.
In health promotion and disease prevention programmes, community participation and empowermentCitation59 and implementation of a range of behaviour change techniquesCitation60 are key. Zambia’s recent re-commissioning of public health nurse and community health assistant training courses are likely to contribute to future trachoma elimination efforts.
Tremendous progress has been made against trachoma globally in the last few decades.Citation61 Zambia’s Ministry of Health is encouraged to complete mapping for trachoma in the remaining districts of northwestern province, mobilize resources to implement the SAFE strategy where needed, and continue to lead and coordinate stakeholders keen to assist the country to eliminate trachoma as a public health problem nationwide.
Acknowledgments
The authors are grateful to Girija Sankar and Paul M. Emerson of the International Trachoma Initiative for their support to the Zambia trachoma elimination programme.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- World Health Assembly. Global elimination of blinding trachoma. 51st World Health Assembly, Geneva; May 16, 1998; Resolution WHA51.11; Geneva, World Health Organization.
- World Health Organization. WHO alliance for the global elimination of trachoma by 2020: progress report on elimination of trachoma, 2014-2016. Wkly Epidemiol Rec. 2017;92(26): 359–368.
- World Health Organization. Report of the twentieth meeting of the WHO alliance for the global elimination of trachoma by 2020. Geneva: World Health Organization; 2018.
- Feasey N, Wansbrough-Jones M, Mabey DC, Solomon AW. Neglected tropical diseases. Br Med Bull. 2010;93:179–200. doi:10.1093/bmb/ldp046.
- Caldwell HD, Wood H, Crane D, et al. Polymorphisms in Chlamydia trachomatis tryptophan synthase genes differentiate between genital and ocular isolates. J Clin Invest. 2003;111(11):1757–1769. doi:10.1172/JCI17993.
- Hadfield J, Harris SR, Seth-Smith HMB, et al. Comprehensive global genome dynamics of Chlamydia trachomatis show ancient diversification followed by contemporary mixing and recent lineage expansion. Genome Res. 2017;27(7):1220–1229. doi:10.1101/gr.212647.116.
- Mabey DC, Solomon AW, Foster A. Trachoma. Lancet. 2003;362(9379):223–229. doi:10.1016/S0140-6736(03)13914-1.
- Garn JV, Boisson S, Willis R, et al. Sanitation and water supply coverage thresholds associated with active trachoma: modeling cross-sectional data from 13 countries. PLoS Negl Trop Dis. 2018;12(1):e0006110. doi:10.1371/journal.pntd.0006110.
- Oswald WE, Stewart AE, Kramer MR, et al. Active trachoma and community use of sanitation, Ethiopia. Bull World Health Organ. 2017;95(4):250–260. doi:10.2471/BLT.16.177758.
- Polack S, Kuper H, Solomon AW, et al. The relationship between prevalence of active trachoma, water availability and its use in a Tanzanian village. Trans R Soc Trop Med Hyg. 2006;100(11):1075–1083. doi:10.1016/j.trstmh.2005.12.002.
- Habtamu E, Wondie T, Aweke S, et al. Trachoma and relative poverty: a case-control study. PLoS Negl Trop Dis. 2015;9(11):e0004228. doi:10.1371/journal.pntd.0004228.
- Berhane Y, Worku A, Bejiga A, et al. Prevalence of trachoma in Ethiopia. Ethiop J Health Dev. 2007;21(3):211–215.
- Sallam TA, Raja’a YA, Al-Zubiery TK, et al. Chlamydia trachomatis infections among Yemeni school pupils in relation to environmental conditions. Saudi Med J. 2003;24(1):84–87.
- Smith JL, Sivasubramaniam S, Rabiu MM, Kyari F, Solomon AW, Gilbert C. Multilevel analysis of trachomatous trichiasis and corneal opacity in Nigeria: the role of environmental and climatic risk factors on the distribution of disease. PLoS Negl Trop Dis. 2015;9(7):e0003826. doi:10.1371/journal.pntd.0003826.
- Sukwa TY, Ngalande TC, Mwandu DH, Siziya S, Mukunyandela M. Prevalence and distribution of trachoma in the Luapula Valley, Zambia. East Afr Med J. 1992;69:34–36.
- Blake IM, Burton MJ, Bailey RL, et al. Estimating household and community transmission of ocular Chlamydia trachomatis. PLoS Negl Trop Dis. 2009;3(3):e401. doi:10.1371/journal.pntd.0000401.
- Polack SR, Solomon AW, Alexander ND, et al. The household distribution of trachoma in a Tanzanian village: an application of GIS to the study of trachoma. Trans R Soc Trop Med Hyg. 2005;99(3):218–225. doi:10.1016/j.trstmh.2004.06.010.
- Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bull World Health Organ. 1987;65:477–483.
- Solomon AW, Holland MJ, Burton MJ, et al. Strategies for control of trachoma: observational study with quantitative PCR. Lancet. 2003;362(9379):198–204. doi:10.1016/S0140-6736(03)13909-8.
- Solomon AW, Peeling RW, Foster A, Mabey DC. Diagnosis and assessment of trachoma. Clin Microbiol Rev. 2004;17(4):982–1011. doi:10.1128/CMR.17.4.982-1011.2004.
- Hu VH, Holland MJ, Burton MJ. Trachoma: protective and pathogenic ocular immune responses to Chlamydia trachomatis. PLoS Negl Trop Dis. 2013;7(2):e2020. doi:10.1371/journal.pntd.0002020.
- Grayston JT, Wang SP, Yeh LJ, Kuo CC. Importance of reinfection in the pathogenesis of trachoma. Rev Infect Dis. 1985;7:717–725.
- Gambhir M, Basanez MG, Burton MJ, et al. The development of an age-structured model for trachoma transmission dynamics, pathogenesis and control. PLoS Negl Trop Dis. 2009;3(6):e462. doi:10.1371/journal.pntd.0000462.
- Kuper H, Solomon AW, Buchan J, Zondervan M, Foster A, Mabey D. A critical review of the SAFE strategy for the prevention of blinding trachoma. Lancet Infect Dis. 2003;3:372–381.
- Ngondi J, Onsarigo A, Matthews F, et al. Effect of 3 years of SAFE (surgery, antibiotics, facial cleanliness, and environmental change) strategy for trachoma control in southern Sudan: a cross-sectional study. Lancet. 2006;368(9535):589–595. doi:10.1016/S0140-6736(06)69202-7.
- Ngondi J, Gebre T, Shargie EB, et al. Evaluation of three years of the SAFE strategy (surgery, antibiotics, facial cleanliness and environmental improvement) for trachoma control in five districts of Ethiopia hyperendemic for trachoma. Trans R Soc Trop Med Hyg. 2009;103(10):1001–1010. doi:10.1016/j.trstmh.2008.11.023.
- Hammou J, El Ajaroumi H, Hasbi H, Nakhlaoui A, Hmadna A, El Maaroufi A. In Morocco, the elimination of trachoma as a public health problem becomes a reality. Lancet Glob Health. 2017;5(3):e250–e251. doi:10.1016/S2214-109X(17)30023-2.
- Francis V, Turner V. Achieving Community Support for Trachoma Control. Geneva: World Health Organization; 1993. (WHO/PBL/93.36).
- Smith JL, Sturrock HJ, Olives C, Solomon AW, Brooker SJ. Comparing the performance of cluster random sampling and integrated threshold mapping for targeting trachoma control, using computer simulation. PLoS Negl Trop Dis. 2013;7(8):e2389. doi:10.1371/journal.pntd.0002389.
- Solomon AW, Kurylo E. The global trachoma mapping project. Community Eye Health. 2014;27:18.
- Ngondi J, Reacher M, Matthews F, Brayne C, Emerson P. Trachoma survey methods: a literature review. Bull World Health Organ. 2009;87(2):143–151.
- Solomon AW, Zondervan M, Kuper H, Buchan JC, Mabey DCW, Foster A. Trachoma Control: A Guide for Programme Managers. Geneva: World Health Organization; 2006.
- Pelletreau S, Nyaku M, Dembele M, et al. The field-testing of a novel integrated mapping protocol for neglected tropical diseases. PLoS Negl Trop Dis. 2011;5(11):e1380. doi:10.1371/journal.pntd.0001370.
- Solomon AW, Pavluck A, Courtright P, et al. The global trachoma mapping project: methodology of a 34-country population-based study. Ophthalmic Epidemiol. 2015;22(3):214–225. doi:10.3109/09286586.2015.1037401.
- Hooper PJ, Millar T, Rotondo LA, Solomon AW. Tropical data: a new service for generating high quality epidemiological data. Community Eye Health. 2016;29:38.
- Solomon AW, Willis R, Pavluck AL, et al. Quality assurance and quality control in the global trachoma mapping project. Am J Trop Med Hyg. 2018;99(4):858–863. doi:10.4269/ajtmh.18-0082.
- World Health Organization Strategic and Technical Advisory Group on Neglected Tropical Diseases. Technical consultation on trachoma surveillance. Task Force for Global Health, Decatur, USA; September 11−12, 2014; Geneva: World Health Organization; 2015. (WHO/HTM/NTD/2015.02).
- Courtright P, MacArthur C, Macleod C, et al. Tropical Data: Training System for Trachoma Prevalence Surveys (Version 1). London: International Coalition for Trachoma Control; 2016. http://tropicaldata.knowledgeowl.com/help/training-system-for-trachoma-prevalence-surveys.
- World Health Organization. Design Parameters for Population-Based Trachoma Prevalence Surveys. Geneva: World Health Organization; 2018. (WHO/HTM/NTD/PCT/2018.07)
- Pavluck A, Chu B, Mann Flueckiger R, Ottesen E. Electronic data capture tools for global health programs: evolution of LINKS, an Android-, web-based system. PLoS Negl Trop Dis. 2014;8(4):e2654. doi:10.1371/journal.pntd.0002654.
- World Health Organization Alliance for the Global Elimination of Trachoma by 2020. Second Global Scientific Meeting on Trachomatous Trichiasis, Cape Town; November 4–6, 2015. Geneva: World Health Organization; 2016. (WHO/HTM/NTD/2016.5).
- Smith JL, Haddad D, Polack S, et al. Mapping the global distribution of trachoma: why an updated atlas is needed. PLoS Negl Trop Dis. 2011;5(6):e973. doi:10.1371/journal.pntd.0001370.
- Smith JL, Flueckiger RM, Hooper PJ, et al. The geographical distribution and burden of trachoma in Africa. PLoS Negl Trop Dis. 2013;7(8):e2359. doi:10.1371/journal.pntd.0002359.
- Fitzpatrick C, Bangert M, Engels D. Sustainable development goals: diseases that neglect no goals. Nature. 2016;535(7613):493. doi:10.1038/535493c.
- Bangert M, Molyneux DH, Lindsay SW, Fitzpatrick C, Engels D. The cross-cutting contribution of the end of neglected tropical diseases to the sustainable development goals. Infect Dis Poverty. 2017;6(1):73. doi:10.1186/s40249-017-0288-0.
- Boisson S, Engels D, Gordon BA, et al. Water, sanitation and hygiene for accelerating and sustaining progress on neglected tropical diseases: a new Global Strategy 2015–20. Int Health. 2016;8(Suppl 1):i19–i21. doi:10.1093/inthealth/ihv073.
- Fitzpatrick C, Fleming FM, Madin-Warburton M, et al. Benchmarking the cost per person of mass treatment for selected neglected tropical diseases: an approach based on literature review and meta-regression with web-based software application. PLoS Negl Trop Dis. 2016;10(12):e0005037. doi:10.1371/journal.pntd.0005037.
- Baltussen R, Smith A. Cost effectiveness of strategies to combat vision and hearing loss in sub-Saharan Africa and South East Asia: mathematical modelling study. Bmj. 2012;344:e615. doi:10.1136/bmj.e615.
- Solomon AW. Don’t let misinformation derail the trachoma elimination programme. Bmj. 2012;344:e2579. doi:10.1136/bmj.e2579.
- International Coalition for Trachoma Control. The End in Sight: 2020 INSight. Atlanta: International Coalition for Trachoma Control; 2011.
- Redekop WK, Lenk EJ, Luyendijk M, et al. The socioeconomic benefit to individuals of achieving the 2020 targets for five preventive chemotherapy neglected tropical diseases. PLoS Negl Trop Dis. 2017;11(1):e0005289. doi:10.1371/journal.pntd.0005289.
- Astle WF, Wiafe B, Ingram AD, Mwanga M, Glassco CB. Trachoma control in Southern Zambia–an international team project employing the SAFE strategy. Ophthalmic Epidemiol. 2006;13(4):227–236. doi:10.1080/09286580600718974.
- Engels D. The global trachoma mapping project: a catalyst for progress against neglected tropical diseases. Ophthalmic Epidemiol. 2016;23(sup1):1–2. doi:10.1080/09286586.2016.1257139.
- de la Fuente A, Murr A, Rascón E. Mapping Subnational Poverty in Zambia. Washington, DC: World Bank; 2015. https://openknowledge.worldbank.org/handle/10986/21783.
- United States Agency for International Development. Neglected Tropical Diseases Donor Landscape: Preventive Chemotherapy Diseases. Washington, DC: United States Agency for International Development; 2015.
- Gohou G, Soumaré I. Does foreign direct investment reduce poverty in Africa and are there regional differences? World Dev. 2012;40(1):75–95. doi:10.1016/j.worlddev.2011.05.014.
- Tembo G, Freeland N, Chimai B, Schüring E. Social cash transfers and household welfare: evidence from Zambia’s oldest scheme. Appl Econ Finance. 2014;1(1):13–26. doi:10.11114/aef.v1i1.354.
- Haanyika CM. Rural electrification in Zambia: a policy and institutional analysis. Energy Policy. 2008;36(3):1044–1058. doi:10.1016/j.enpol.2007.10.031.
- Sutter EE, Ballard RC. Community participation in the control of trachoma in Gazankulu. Soc Sci Med. 1983;17:1813–1817.
- Delea MG, Solomon H, Solomon AW, Freeman MC. Interventions to maximize facial cleanliness and achieve environmental improvement for trachoma elimination: a review of the grey literature. PLoS Negl Trop Dis. 2018;12(1):e0006178. doi:10.1371/journal.pntd.0006178.
- Solomon AW, Emerson PM, Resnikoff S. Trachoma then and now: update on mapping and control. Community Eye Health. 2017;30:90–91.
