ABSTRACT
Purpose
To systematically review and critically appraise clinical practice guidelines (CPGs) and summarise the recommendations for non-infectious and infectious conjunctivitis
Methods
CPGs published on non-infectious and infectious conjunctivitis between 2010 and March 2020 were reviewed, evaluated, and selected using nine items from the Appraisal of Guidelines for Research and Evaluation II tool (4, 7, 8, 10, 12, 13, 15, 22 and 23). CPGs with an average score for items 4, 7, 8, 12, or 22 below 3 and/or a sum of the two researchers’ average score for all nine items less than 45 were excluded. Two authors independently extracted and validated the data using standardised forms.
Results
Fifteen CPGs from five sources remained for data extraction. CPGs consistently recommended non-pharmacological interventions (artificial tears, cold compress, avoidance or removal of allergens) for non-infectious conjunctivitis and pharmacological interventions (topical anti-histamine, mast-cell stabiliser and dual-acting agent) for allergy types. Observation without treatment was strongly recommended for non-herpetic viral and bacterial infections. Systemic and topical anti-viral was consistently recommended for herpetic viral conjunctivitis, while systemic and topical antibiotics were recommended for chlamydial and gonorrhoeal conjunctivitis. The methods used to assess the level of evidence and the strength of recommendation varied among CPGs.
Conclusions
There are a number of high-quality CPGs for non-infectious and infectious conjunctivitis. While there were a number of consistencies in the recommendations provided within these CPGs, several inconsistencies were also identified. Many of which related to the scope of practise of the targeted end-user of the particular guideline.
Introduction
The World Health Organization (WHO) launched the first World Report on VisionCitation1 in 2019. It highlighted the increasing need for, and the role of, eye care in attaining the Sustainable Development Goals. The Report recommended coordinated and concerted global action towards strengthening the integration of eye care in health systemsCitation2 to address inequities in access to, and provision of, eye care services across the population.Citation2
To facilitate the integration of eye care into health systems, the WHO is developing a priority, evidence-based, Package of Eye Care Interventions (PECI) to be used by countries to plan, budget, and integrate eye care interventions into national health insurance schemes and policies. The methodology for developing the PECI was designed and published by the WHO in collaboration with Cochrane Eyes and Vision (CEV).Citation3
Conjunctivitis is inflammation of the conjunctiva due to allergic or immunological reactions, infection (viral, bacterial or parasitic), mechanical irritation, neoplasia, or contact with toxic substances. Ocular allergy is the commonest form of non-infectious conjunctivitis and can significantly impact productivity and quality of life.Citation4,Citation5 Some less common severe forms of ocular allergy can also be sight-threatening.Citation6,Citation7 The prevalence of ocular allergy (seasonal and perennial allergic conjunctivitis) has been increasing worldwide.Citation4
Viral and bacterial conjunctivitis are the two commonest forms of infectious conjunctivitis, with viral infection responsible for up to 80% of all acute conjunctivitis casesCitation8–13 and bacterial infection account for between 50–75% of cases of infectious conjunctivitis in children.Citation14 While most cases are self-limiting, conjunctivitis is one of the leading reasons people seek eye care due to the associated symptoms.Citation15 Hence, effective interventions may significantly reduce direct and opportunity costs associated with seeking care. This study aims to conduct a systematic review and critical appraisal of CPGs and summarise the recommendations for non-infectious and infectious conjunctivitis.
Methods and analysis
Eligibility criteria
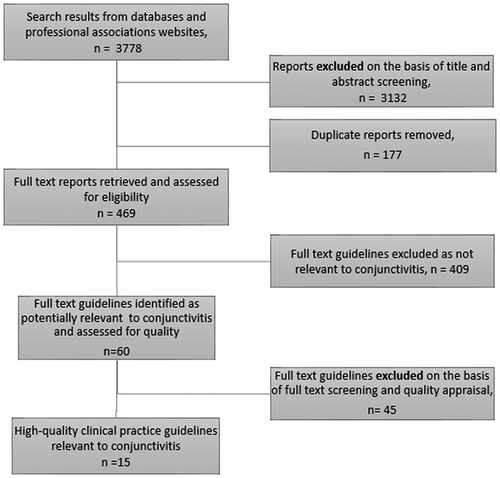
We conducted this systematic review of CPGs using the methodology presented by Keel et al.Citation3 Exclusion criteria for each stage of screening are provided in .
Table 1. Exclusion criteria for screening of clinical practice guidelines.
Search methods and screening
An information specialist from CEV (IG) designed and conducted a single, systematic literature search on professional ophthalmology and optometry associations’ websites for relevant guidelines (Supplementary material 1). MEDLINE, Embase, CINAHL, Global Health, Global Index Medicus and guideline databases on 9 Mar 2020 were also searched (Supplementary material 2). All the searches were limited to the last ten years and English language. Two authors (GL and SS) independently screened the titles and abstracts of articles identified from the systematic literature searches. We used Abstrackr to track the inclusion/exclusion decisions and highlight disagreements between the authors. All disagreements were resolved by discussion between the two authors, a methodologist from CEV (JE) and a representative from the WHO (SK). CPGs that were identified as potentially relevant to conjunctivitis were included in the full-text screening.
Two authors (VFC/ACY) independently screened the full-texts of CPGs potentially relevant to non-infectious and infectious conjunctivitis. CPGs were excluded if there were no listing author affiliations or significant or absent declarations of conflicts of interest and not deemed relevant to infectious or non-infectious conjunctivitis on full-text screening. Discrepancies were resolved through discussion between the two authors or, in the event a consensus could not be reached, by a discussion with a third author (AAB).
Quality assessment
The same two authors who conducted the full-text screening then independently evaluated the quality of the CPGs using the “Appraisal of Guidelines for Research and Evaluation” (AGREE II) tool.Citation16 We used Items 4, 7, 8, 10, 12, 13, 15, 22 and 23 to select the guidelines ().
Table 2. Description of Items 4, 7, 8, 10, 12, 13, 15, 22, and 23 of Appraisal of Guidelines for Research and Evaluation II (AGREE II) tool.
These items were selected based on a consensus finding process among three researchers and address the domains of stakeholder involvement, the rigour of development, clarity of presentation, applicability and editorial independence. Each item was rated on a 7-point scale of importance (1–strongly disagree to 7–strongly agree). If an item’s rating differed by more than 2 points between the two researchers, the two researchers discussed the results to reach a consensus. Following evaluation with the AGREE II tool, we excluded the guidelines if (i) the average score of the two researchers for items 4, 7, 8, 12, or 22 was below 3; (ii) the sum of the average score of the two researchers for all nine items (4, 7, 8, 10, 12, 13, 15, 22 and 23) was less than 45.Citation17
The protocol specified including a maximum of 5 CPGs for each eye condition, prioritising the final selection according to quality, publication year and comprehensiveness. However, many guidelines addressed specific forms of conjunctivitis. Hence, we included more than 5 CPGs per condition in data extraction after considering relevant information. This decision has been reached by agreement of the whole group.
Data collection
One author (ACY) extracted data and validated it by a second senior author (VFC). We used a standardised form which comprised information on the recommendation (type of recommendation, dosage, target group, and others), the strength of recommendation and the quality of the evidence used to inform the recommendation.Citation18 In the event of disagreement, a third author (AAB) was involved, and an agreement reached by discussion. The process was repeated for all the CPGs until agreement on the recommended eye care interventions was reached. Interventions extracted from CPGs were broadly grouped into pharmacological and non-pharmacological measures and then categorised into types of intervention. Within each category, interventions were further organised into specific medication groups where appropriate.
Data synthesis and analysis
We used textual descriptive synthesis to identify the scope and consistency (congruence in content) of the CPGs recommendations. The recommended interventions were synthesised to provide an overview of the specific eye conditions, and the consistency of recommendations and the level of evidence were assessed. The reporting of this systematic review was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Results
The data base search yielded 3778 titles and abstracts, for screening and 60 (1.59%) eligible CPGs were selected for full paper review, among which 45 were excluded because they were not relevant to the topic of interest,Citation19–54 possible conflicts of interest were not clearly stated and/or the author affiliations were not listed,Citation55–59 the average score of the two researchers for AGREE II items 4, 7, 8, 12, or 22 was less than three and/or the sum of the average score of the two researchers for all nine items was less than 45.Citation60–63 () We finally included the following 15 CPGs:
Atopic Keratoconjunctivitis (AKC). The College of Optometrists. 2019.Citation64
CL-associated Papillary Conjunctivitis (CLAPC), Giant Papillary Conjunctivitis (GPC). The College of Optometrists. 2017.Citation65
Conjunctivitis (Acute Allergic). The College of Optometrists. 2019.Citation66
Seasonal Allergic Conjunctivitis (Hay Fever Conjunctivitis); Perennial Allergic Conjunctivitis. The College of Optometrists. 2019.Citation67
Conjunctivitis Medicamentosa (also Dermatoconjunctivitis medicamentosa). The College of Optometrists. 2019.Citation68
Consensus Document on Allergic Conjunctivitis (DECA). Sánchez-Hernández et al. 2015.Citation69
Conjunctivitis – Allergic. The National Institute for Health and Care Excellence. 2017.Citation70
Conjunctivitis (Bacterial). The College of Optometrists. 2018.Citation71
Conjunctivitis (Viral, non-herpetic). The College of Optometrists. 2019.Citation72
Conjunctivitis, Chlamydial (Adult inclusion conjunctivitis). The College of Optometrists. 2019.Citation73
Ophthalmia Neonatorum. The College of Optometrists. 2018.Citation74
Vernal Keratoconjunctivitis (Spring catarrh). The College of Optometrists. 2019.Citation75
Conjunctivitis Preferred Practice Pattern. American Academy of Ophthalmology. 2018.Citation76
Ocular Prophylaxis for Gonococcal Ophthalmia Neonatorum. US Preventive Services Task Force. 2019.Citation77
Conjunctivitis – Infective. The National Institute for Health and Care Excellence. 2018.Citation78
The AGREE II ratings for the selected CPGs was 49.5 (IQR 0) ().
Table 3. Selected guidelines and their respect of the criteria used to reach the final choice.
Guideline development process
All of the included CPGs were developed based on a systematic literature search of relevant evidence. However, the methods used to assess the level of evidence and the strength of recommendation varied. The National Institute for Health and Care Excellence (NICE)Citation70,Citation78 and the College of Optometrists (COO)Citation64–67,Citation71,Citation72,Citation74,Citation75 assessed the level of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE); the Consensus Document for Allergic Conjunctivitis (DECA)Citation69 used Scottish Intercollegiate Guidelines Network (SIGN); the American Academy of Ophthalmology (AAO)Citation76 used both GRADE and SIGN. In contrast, the US Preventive Services Task Force (USPSTF)Citation77 used none of these.
Summary of recommended interventions for conjunctivitis
Comparing recommendations for a specific eye condition across CPGs was not possible due to the differences of guideline intended users. The end-users for NICE, COO and USPSTF guidelines were mainly primary care personnel; AAO was developed for ophthalmologists, while DECA for primary and secondary care practitioners. Interventions beyond primary care were not comprehensively included in NICE and COO guidelines.
Supplementary and Supplementary present the interventions recommended for non-infectious conjunctivitis and infectious conjunctivitis. Nine CPGs for non-infectious conjunctivitis (Supplementary) and seven CPGs for infectious conjunctivitis (Supplementary ) were identified.
Non-infectious conjunctivitis
Non-pharmacological interventions
All the CPGs included non-pharmacological interventions for non-infectious conjunctivitis. Avoidance or removal of allergens, cold compress and artificial tears were the most common strong recommendations across the CPGs from the COO (Supplementary ). Avoidance of eye rubbing was recommended for acute presentations, seasonal allergic conjunctivitis (SAC) and perennial allergic conjunctivitis (PAC). Lid hygiene was recommended for rosacea conjunctivitis, atopic keratoconjunctivitis (AKC), SAC and PAC. Discontinuing contact lens wear and re-examine contact lens care regimens were recommended for contact lens-induced conjunctivitis. Warm compression was recommended explicitly for rosacea conjunctivitis (Supplementary ).
Pharmacological interventions
Systemic anti-histamine was inconsistently recommended across CPGs for vernal kerato-conjunctivitis (VKC), AKC, SAC and PAC. For topical anti-histamine, it was recommended by all CPGs for mild and acute allergic conjunctivitis, AKC, VKC, SAC and PAC (Supplementary ) (strongly recommended in AAO and COO guidelines). A combination of topical anti-histamine and vaso-constrictor was recommended for allergic conjunctivitis that was not manageable by non-pharmacological interventions (NICE guideline) and strongly recommended for mild SAC and PAC (AAO guideline) (Supplementary ). All CPGs recommended topical mast-cell stabiliser for intermittent mild allergic conjunctivitis, AKC, VKC, giant papillary conjunctivitis (GPC), superior limbic keratoconjunctivitis (SLK), and persistent or recurrent SAC and PAC (Supplementary ). For conditions that were acute, persistent, recurrent, or not resolved by non-pharmacological interventions, a topical dual-acting agent was recommended. Besides topical corticosteroid, immunosuppression was strongly recommended for severe non-infectious conjunctivitis (Supplementary ). NSAIDs were also recommended for SAC and PAC (Supplementary ). Allergen-specific immunotherapy was consistently recommended across DECA, NICE, and AAO guidelines for SAC and PAC if the symptoms were persistent, recurrent or severe. For AKC and VKC, in which topical interventions were ineffective, supratarsal injection of corticosteroid was recommended (Supplementary ). For SLK caused by laxity of the superior bulbar conjunctiva, topical mast-cell stabiliser and immunosuppressor were recommended (Supplementary ). Besides, topical hypertonic 5% saline and mucolytic were suggested if the condition was associated with filamentary keratitis. Rosacea conjunctivitis was recommended to be managed by systemic and topical antibiotic besides topical corticosteroid and topical immunosuppression (Supplementary ).
Infectious conjunctivitis
Non-pharmacological interventions
Five types of non-pharmacological interventions were recommended for conjunctivitis caused by infection. Observation without treatment was strongly recommended in non-herpetic viral conjunctivitis and bacterial conjunctivitis (except chlamydial and gonorrhoeal conjunctivitis) due to the usually self-limiting nature of the conditions. Cold compress, artificial tears and lubricating ointments were suggested for symptomatic relief of infectious conjunctivitis. Lid hygiene was strongly recommended where mucopurulent discharge with crusting on the lids is present (Supplementary ).
Pharmacological interventions
Antibacterial
Ocular antibiotic prophylaxis was recommended for neonates in CPGs related to ophthalmia neonatorum (ON) (recommended with high certainty in USPSTF guideline) (Supplementary ). For neonates infected by chlamydia, systemic erythromycin or topical azithromycin 1.5% was recommended; those infected by gonorrhoea were recommended systemic Penicillin G or cephalosporin; and neonates with other types of bacterial infection were recommended topical erythromycin, azithromycin 1.5% or chloramphenicol 0.5%. For chlamydial conjunctivitis in adults, systemic azithromycin and doxycycline were recommended, while in gonorrhoeal conjunctivitis, systemic ceftriaxone or spectinomycin was recommended in addition to the interventions used to manage chlamydial infection. In bacterial conjunctivitis, which did not resolve within three days, topical chloramphenicol 0.5% drops or 1% ointment, fusidic acid 1% drops or azithromycin 1.5% were strongly recommended. Topical gentamycin or moxifloxacin was included as interventions for infection related to contact lens wear (strongly recommended by COO guideline). In settings where medical supply is limited, povidone-iodine 1.25% may be used in mild bacterial or chlamydia infection (Supplementary ).
Anti-viral
For non-herpetic viral conjunctivitis, anti-viral medications were considered to be ineffective. However, symptomatic relief interventions such as topical anti-histamine (strongly recommended by COO guideline) and corticosteroid can be adopted, particularly if there was pseudomembrane formation. In herpetic viral infection among neonates, systemic and topical Acyclovir were recommended. For adults with herpes simplex or varicella zoster infection, systemic Acyclovir, Valacyclovir or Famciclovir were recommended, and additional topical Gancyclovir 0.15% gel or Trifluridine 1% were recommended for varicella zoster conjunctivitis (Supplementary ).
Other interventions
In a rare condition, specific interventions such as surgical intervention and the use of proteolytic enzyme were recommended.
Discussion
This study has systematically identified, appraised and summarised current CPGs to manage infectious and non-infectious conjunctivitis. While there were robustly designed CPGs, we have excluded some of them because they did not meet our inclusion criteria. Low scores were given to CPGs that did not use systematic methods to search for evidence or did not report the criteria used to select the evidence, even though the recommendations were well presented to inform clinicians.Citation60–63 Among the high-quality CPGs, NICECitation70,Citation78 scored the highest (average score: 61/63) attributed by the comprehensive description of the guideline development process: the composition of the research team and their expertise, the systematic and rigorous strategies used in searching for evidence, the declaration of funding, and the handling of potential conflict of interests of the development group members.
All the included high-quality CPGs were up to date, and some were published in 2020.Citation67,Citation74,Citation75 DECACitation69 included literature spanning the last ten years, and AAO reviewed the guideline every 5 years to update new evidence. The identified CPGs also covered a range of population groups (newborns, children and adults) and conjunctivitis sub-types (infectious and non-infectious conjunctivitis including various types of infections and allergies).
We faced challenges when extracting information about the recommended interventions. We observed that different CPGs categorised the various types of conjunctivitis differently. For instance, in CPGs related to allergic conjunctivitis, DECACitation69 grouped the conditions based on severity and frequency, while COO,Citation64,Citation65,Citation67,Citation68,Citation75 NICE,Citation70 and AAOCitation76 categorised conditions according to the allergy subtypes. This made it challenging to compare recommendations across the CPGs, and thus uncertainties for practitioners may remain.
We observed that recommendations varied according to the CPG’s end-users (primary care, optometrists, and ophthalmologists). For instance, antibacterial medications were recommended for chlamydial conjunctivitis in AAOCitation76 but not in COOCitation73 guidelines. We found that these interventions were instead categorised as possible management by ophthalmologists with no level of evidence and strength of recommendation reported. This categorisation was possibly due to the end-users for COO were mainly practising at the primary level, and conditions such as chlamydial infection will typically be referred to ophthalmological care for further investigation and management. This was similar to non-infectious conjunctivitis, in which immunotherapy was not included as management in NICECitation70 and COOCitation64,Citation65,Citation67,Citation68,Citation75 guidelines but appeared in AAOCitation76 and DECA.Citation69
There were further inconsistencies between CPGs concerning the rating of the level of evidence and strength of recommendation. For example, the AAO guidelineCitation76 rated the level of evidence as good and strongly recommended topical anti-histamine (second generation) or a combination of anti-histamine with vasoconstrictor for management of mild allergic conjunctivitis. However, COOCitation66 rated the evidence for topical anti-histamine to manage mild allergic conjunctivitis as low, although strong recommendation was reported. The COOCitation67 graded evidence as high and strongly recommended prescribing systemic anti-histamine in managing SAC and PAC. In contrast, AAOCitation76 reported that the adverse effect from systemic anti-histamine (drying of the ocular surface) may worsen the condition of allergic conjunctivitis and did not include it as a recommendation. In addition, due to the undesirable effect, this intervention was not recommended by NICE.Citation70 The inconsistencies in the recommendations in three different CPGs further emphasises the need for standardisation in reviewing and rating the available evidence.
In AAO,Citation76 the use of a combination of anti-histamine and vasoconstrictor was strongly recommended as an initial approach for mild SAC and PAC, while in NICE,Citation70 this intervention was recommended only for allergies that non-pharmacological interventions cannot resolve. Topical anti-histamine was recommended for mild allergic conjunctivitis in DECACitation69 and mild SAC and PAC in AAO.Citation76 However, NICECitation70 recommended topical anti-histamine only in patients who were not responding to non-pharmacological interventions.
Despite being easily implemented at the primary care level, interventions to relieve symptoms of conjunctivitis (artificial tears and cold compression) were inconsistently addressed across the CPGs. One possible reason could be that there was a lack of high-quality evidence to support non-pharmacological interventions. Therefore, CPG development groups tend to omit these interventions. We suggest that a more robust research methodology could be employed in testing these interventions in future research. Secondly, only the ten COO guidelines have reported the level of evidence and the strength of recommendation for each of the specified interventions. We were uncertain of the quality of evidence and the strength of recommendation for some of the other guidelines. We highly recommend development groups adhere to a standard protocol or guide to ensure high-quality guidelines.
A number of high-quality CPGs for both non-infectious and infectious conjunctivitis were identified. While there were a number of consistencies in the recommendations provided within these CPGs, a number of inconsistencies were also identified. The inconsistencies for some types of conjunctivitis related to the scope of practice of the targeted end-user of the particular guideline. The standardised rating of the level of evidence and strength of recommendation among guideline bodies would aid in further analysis of CPGs for conjunctivitis.
Financial support
None.
Acknowledgments
None.
Disclosure Statement
No conflicting relationship exists for any author.
References
- World Health Organization. World report on vision. 2019.https://www.who.int/publications/i/item/world-report-on-vision
- World Health Organization. Universal health coverage (UHC). https://www.who.int/news-room/fact-sheets/detail/universal-health-coverage-(uhc) ( accessed 15 Nov 2020).
- Keel S, Evans JR, Block S, et al. Strengthening the integration of eye care into the health system: Methodology for the development of the WHO package of eye care interventions. BMJ Open Ophthalmol. 2020;5(1):533. doi:10.1136/bmjophth-2020-000533.
- Rosario N, Bielory L. Epidemiology of allergic conjunctivitis. Curr Opin Allergy Clin Immunol. 2011;11(5):471–476. doi:10.1097/ACI.0b013e32834a9676.
- Virchow JC, Kay S, Demoly P, et al. Impact of ocular symptoms on quality of life (QoL), work productivity and resource utilisation in allergic rhinitis patients - An observational, cross sectional study in four countries in Europe. J Med Econ. 2011;14(3):305–314. doi:10.3111/13696998.2011.576039.
- De Smedt S, Wildner G, Kestelyn P. Vernal keratoconjunctivitis: an update. Br J Ophthalmol. 2013;97(1):9–14. doi:10.1136/bjophthalmol-2011-301376.
- Chen JJ, Applebaum DS, Sun GS, et al. Atopic keratoconjunctivitis: a review. J Am Acad Dermatol. 2014;70(3):569–575. doi:10.1016/j.jaad.2013.10.036.
- Stenson S, Newman R, Fedukowicz H. Laboratory studies in acute conjunctivitis. Arch Ophthalmol. 1982;100(8):1275–1277. doi:10.1001/archopht.1982.01030040253009.
- Harding SP, Mallinson H, Smith JLS, et al. Adult follicular conjunctivitis and neonatal ophthalmia in a liverpool eye hospital, 1980-1984. Eye. 1987;1(4):512–521. doi:10.1038/eye.1987.77.
- Harding SP, Mallinson H, Smith JLS, et al. Adult follicular conjunctivitis and neonatal ophthalmia in a liverpool eye hospital, 1980-1984. Eye. 1987;1(4):512–521. doi:10.1038/eye.1987.77.
- Woodland RM, Darougar S, Thaker U, et al. Causes of conjunctivitis and keratoconjunctivitis in Karachi, Pakistan. Trans R Soc Trop Med Hyg. 1992;86(3):317–320. doi:10.1016/0035-9203(92)90328-A.
- Fitch CP, Rapoza PA, Owens S, et al. Epidemiology and diagnosis of acute conjunctivitis at an inner-city hospital. Ophthalmology. 1989;96(8):1215–1220. doi:10.1016/S0161-6420(89)32749-7.
- Bielory BP, O’Brien TP, Bielory L. Management of seasonal allergic conjunctivitis: guide to therapy. Acta Ophthalmol. 2012;90(5):399–407. doi:10.1111/j.1755-3768.2011.02272.x.
- Høvding G. Acute bacterial conjunctivitis. Acta Ophthalmol. 2008;86(1):5–17. doi:10.1111/j.1600-0420.2007.01006.x.
- Shields T, Sloane PD. A comparison of eye problems in primary care and ophthalmology practices. Fam Med. 1991;23:544–546.
- Brouwers MC, Hanna S, University M et al. The AGREE II instrument [electronic version]. 2017. www.agreetrust.org (accessed 15 Nov 2020).
- Rauch A, Negrini S, Cieza A. Toward strengthening rehabilitation in health systems: Methods used to develop a WHO package of rehabilitation interventions. Arch Phys Med Rehabil. 2019;100(11):2205–2211. doi:10.1016/j.apmr.2019.06.002.
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi:10.1136/bmj.39489.470347.ad.
- The Royal College of Ophthalmologists. Ophthalmic services guidance ophthalmic imaging. 2016. https://www.rcophth.ac.uk/wp-content/uploads/2014/12/Ophthalmic-Imaging-November-2016.pdf (accessed 13 Nov 2020).
- The Royal College of Ophthalmologists. Primary eye care, community ophthalmology and general ophthalmology. 2019.https://www.rcophth.ac.uk/wp-content/uploads/2019/02/Primary-Eye-Care-Community-Ophthalmology-and-General-Ophthalmology-2019.pdf+&cd=1&hl=en&ct=clnk&gl=uk (accessed 13 Nov 2020).
- Welsh Government. Children’s Vision Wales Pathway (4-5 year olds).
- Ruben JB, Granet DB, Blocker RJ, et al. Instrument-based pediatric vision screening policy statement. Pediatrics. 2012;130:983–986. doi:10.1542/peds.2012-2548.
- Drake B, Paterson R, Tabin G, et al. Wilderness medical society practice guidelines for treatment of eye injuries and illnesses in the wilderness. Wilderness Environ Med. 2012;23(4):325–336. doi:10.1016/j.wem.2012.08.015.
- The Royal College of Ophthalmologists. Ophthalmology Service Guidance Theatre Procedures. 2018.
- Donahue SP, Arthur B, Neely DE, et al. Guidelines for automated preschool vision screening: a 10-year, evidence-based update. J AAPOS. 2013;17(1):4–8. doi:10.1016/j.jaapos.2012.09.012.
- Swaminathan M, Jayaraman D, Jacob N. Visual function assessment, ocular examination, and intervention in children with developmental delay: a systematic approach. Part 1 Indian J Ophthalmol. 2019;67(2):196–203. doi:10.4103/ijo.IJO_524_18.
- Welsh Government. Wales eye healthcare service-eye health examinations wales (EHEW): a clinical manual with protocols. For optometrists and ophthalmic medical practitioners (OMPs). 2014. www.eyecare.wales.nhs.uk. (accessed 13 Nov 2020).
- National Institute for Health and Care Excellence. Red eye. 2016.https://cks.nice.org.uk/topics/red-eye/ (accessed 13 Nov 2020).
- Donahue SP, Ruben JB. US Preventive Services. Task force vision screening recommendations. Pediatrics. 2011;127:(3). 569–570. doi:10.1542/peds.2011-0020.
- Donahue SP, Baker CN. Procedures for the evaluation of the visual system by pediatricians. Pediatrics. 2016;137(1):e20153597. doi:10.1542/peds.2015-3597.
- Delpero WT, Robinson BE, Gardiner JA, et al. Evidence-based clinical practice guidelines for the periodic eye examination in children aged 0–5 years in Canada. Can J Ophthalmol. 2019;54(6):751–759. doi:10.1016/j.jcjo.2019.09.003.
- The Royal College of Ophthalmologists. Ophthalmic Services Guidance - Prevention of transmission of blood-borne viruses in ophthalmic surgery.
- Fauquert J-L, Jedrzejczak-Czechowicz M, Rondon C, et al. Conjunctival allergen provocation test : guidelines for daily practice. Allergy. 2017;72(1):43–54. doi:10.1111/all.12986.
- Jin J. Vision screening in children. JAMA - J Am Med Assoc. 2017;318(9):878. doi:10.1001/jama.2017.11386.
- NICE Pathways. Eye conditions overview. 2020.https://pathways.nice.org.uk/pathways/eye-conditions/eye-conditions-overview.pdf+&cd=3&hl=en&ct=clnk&gl=uk (accessed 13 Nov 2020).
- Cotter SA, Cyert LA, Miller JM, et al. Vision Screening for Children 36 to . Optom Vis Sci. 2015;92(1):6–16. doi:10.1097/OPX.0000000000000429.
- Ophthalmic Services Guidance Managing an Outbreak of Postoperative Endophthalmitis. London. 2016.
- Clinical Council for Eye Health Commissioning. Strategy 2016-2018 commissioning for the needs of the patients. Enhancing eye health services. https://www.college-optometrists.org/asset/879614C6-5711-486D-91C8EB54ADDCA6F5/+&cd=1&hl=en&ct=clnk&gl=uk ( accessed 13 Nov 2020).
- Feder RS, Olsen TW, Prum JBE, et al. Comprehensive adult medical eye evaluation preferred practice pattern® guidelines. Ophthalmology. 2016;123(1):209–236. doi:10.1016/j.ophtha.2015.10.047.
- Wallace DK, Morse CL, Melia M, et al. Pediatric eye evaluations preferred practice pattern®: i. vision screening in the primary care and community setting; ii. comprehensive ophthalmic examination. Ophthalmology. 2018;125(1):184–227. doi:10.1016/j.ophtha.2017.09.032.
- Scott CA, Catania LJ, Larkin KM, et al. Care of the patient with ocular surface disorders. St. Louis:: American Optometric Association 2010. https://www.sdeyes.org/docs/CPG-10.pdf (accessed 12 Nov 2020).
- Wilson BJ, Courage S, Bacchus M, et al. Screening for impaired vision in communitydwelling adults aged 65 years and older in primary care settings. CMAJ. 2018;190(19):E588–94. doi:10.1503/cmaj.171430.
- AOA Evidence-Based Optometry Guideline Development Group. Comprehensive adult eye and vision examination. St. Louis:: American Optometric Association 2015. https://webcache.googleusercontent.com/search?q=cache:8HjSwYDG4FwJ:https://www.aoa.org/AOA/Documents/Practice%2520Management/Clinical%2520Guidelines/EBO%2520Guidelines/Comprehensive%2520Adult%2520Eye%2520and%2520Vision%2520Exam.pdf+&cd=2&hl=en&ct=clnk&gl=uk (accessed 12 Nov 2020).
- AOA Evidence-Based Optometry Guideline Development Group. Comprehensive pediatric eye and vision examination. St. Louis: 2015. https://www.aoa.org/AOA/Documents/Practice%2520Management/Clinical%2520Guidelines/EBO%2520Guidelines/Comprehensive%2520Pediatric%2520Eye%2520and%2520Vision%2520Exam.pdf+&cd=1&hl=en&ct=clnk&gl=uk (accessed 12 Nov 2020).
- The Royal Australian and New Zealand College of Ophthalmologists. Infection control guidelines to prevent nosocomial epidemic keratoconjunctivitis (EKC). 2010. www.ranzco.edu (accessed 12 Nov 2020).
- The Royal Australian and New Zealand College of Ophthalmologists. Referral guidelines for eye and vision problems in infants and children. NSW: 2015. www.ranzco.edu (accessed 12 Nov 2020).
- Kumar CM, Eke T, Dodds C, et al. Local anaesthesia for ophthalmic surgery - New guidelines from the royal college of anaesthetists and the royal college of ophthalmologists. Eye. 2012;26(6):897–898. doi:10.1038/eye.2012.82.
- The Royal College of Ophthalmologists. Ophthalmic services for Children. 2012. http://www.rcophth.ac.uk/page.asp?section=444§ionTitle=Quality+Standards (accessed 13 Nov 2020).
- M A, Canadian Paediatric Society, Community Paediatrics Committee. Vision screening in infants, children and youth. Can. Paediatr. Soc. https://www.cps.ca/en/documents/position/children-vision-screening ( accessed 13 Nov 2020).
- Grossman DC, Curry SJ, Owens DK, et al. Vision screening in children aged 6 months to 5 years: US preventive services task force recommendation statement. JAMA - J Am Med Assoc. 2017;318(9):836–844. doi:10.1001/jama.2017.11260.
- Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for impaired visual acuity in older adults: US preventive services task force recommendation statement. J Am Med Assoc. 2016;315(9):908–914. doi:10.1001/jama.2016.0763.
- Calonge N, Petitti DB, Curry S, et al. Vision screening for children 1 to 5 years of age: US preventive services task force recommendation statement. Pediatrics. 2011;127:340–346. doi:10.1542/peds.2010-3177.
- Anwar Z, Wellik SR, Galora A. Glaucoma therapy and ocular surface disease: current literature and recommendations. Curr Opin Ophthalmol. 2013;24(2):136–143. doi:10.1097/ICU.0b013e32835c8aba.
- Jin J. Prevention of gonococcal eye infection in newborns. JAMA - J Am Med Assoc. 2019;321(4):414. doi:10.1001/jama.2018.21434.
- Lian K-Y, Napper G, Stapleton FJ, et al. Infection control guidelines for optometrists 2016. Clin Exp Optom. 2017;100(4):341–356. doi:10.1111/cxo.12544.
- Gindala W, Lewis K. Management of common eye conditions in a primary health care setting: a guide for South Sudan health workers. South Sudan Med J. 2017;10:17–22.
- US Preventive Services Task Force. Vision screening for children 1 to 5 years of age: US Preventive Services Task Force Recommendation statement. Pediatrics. 2011;127(2):340-6. .
- British and Irish Orthoptic Society. Vision screening. https://www.orthoptics.org.uk/policy-and-campaigns/vision-screening/ ( accessed 13 Nov 2020).
- Child vision screening. https://www.gov.uk/government/publications/child-vision-screening ( accessed 13 Nov 2020).
- Moore DL, MacDonald NE; Canadian Paediatric Society, Infectious Diseases and Immunization Committee. Preventing ophthalmia neonatorum. Paediatr Child Health. 2015;20(2):93-6.
- Takamura E, Uchio E, Ebihara N, et al. Japanese guidelines for allergic conjunctival diseases 2017. Allergol Int. 2017;66(2):220–229. doi:10.1016/j.alit.2016.12.004.
- Agarwal P, Gupta S, Sharma A, et al. Preferred practice patterns for presumed viral kerato-conjunctivitis in Central India. Natl J Commun Med. 2014;5:54–56.
- Hammerschlag MR. Chlamydial and gonococcal infections in infants and children. Clin Infect Dis. 2011;53(suppl_3):S99–102. doi:10.1093/cid/cir699.
- The College of Optometrists. Atopic Keratoconjunctivitis (AKC). https://www.college-optometrists.org/guidance/clinical-management-guidelines/atopic-keratoconjunctivitis-akc-.html ( accessed 12 Nov 2020).
- The College of Optometrists. CL-associated Papillary Conjunctivitis (CLAPC), Giant Papillary Conjunctivitis (GPC). https://www.college-optometrists.org/guidance/clinical-management-guidelines/cl-associated-papillary-conjunctivitis-.html ( accessed 12 Nov 2020).
- The College of Optometrists. Conjunctivitis (Acute Allergic). https://www.college-optometrists.org/guidance/clinical-management-guidelines/conjunctivitis-acute-allergic-.html ( accessed 12 Nov 2020).
- The College of Optometrists. Seasonal allergic conjunctivitis (hay fever conjunctivitis); perennial allergic conjunctivitis. https://www.college-optometrists.org/guidance/clinical-management-guidelines/seasonal-allergic-conjunctivitis.html ( accessed 12 Nov 2020).
- The College of Optometrists. Conjunctivitis medicamentosa (also dermatoconjunctivitis medicamentosa). https://www.college-optometrists.org/guidance/clinical-management-guidelines/conjunctivitis-medicamentosa.html ( accessed 12 Nov 2020).
- Sánchez-Hernández MC, Montero J, Rondon C, et al. Consensus document on allergic conjunctivitis (DECA). J Investig Allergol Clin Immunol. 2015;25:94–106. https://europepmc.org/article/med/25997302. accessed 13 Nov 2020).
- National Institute for Health and Care Excellence. Conjunctivitis - allergic. 2020.https://cks.nice.org.uk/topics/conjunctivitis-allergic/ (accessed 13 Nov 2020).
- The College of Optometrists. Conjunctivitis (bacterial). https://www.college-optometrists.org/guidance/clinical-management-guidelines/conjunctivitis-bacterial-.html ( accessed 12 Nov 2020).
- The College of Optometrists. Conjunctivitis (viral, non-herpetic). https://www.college-optometrists.org/guidance/clinical-management-guidelines/conjunctivitis-viral-non-herpetic-.html ( accessed 12 Nov 2020).
- The College of Optometrists. Conjunctivitis, chlamydial (adult inclusion conjunctivitis). https://www.college-optometrists.org/guidance/clinical-management-guidelines/conjunctivitis-chlamydial.html ( accessed 12 Nov 2020).
- The College of Optometrists. Ophthalmia neonatorum. https://www.college-optometrists.org/guidance/clinical-management-guidelines/ophthalmia-neonatorum.html ( accessed 12 Nov 2020).
- The College of Optometrists. Vernal keratoconjunctivitis (spring catarrh). https://www.college-optometrists.org/guidance/clinical-management-guidelines/vernal-keratoconjunctivitis-spring-catarrh-.html ( accessed 12 Nov 2020).
- Varu DM, Rhee MK, Akpek EK, et al. Conjunctivitis preferred practice pattern®. Ophthalmology. 2019;126:94–169. doi:10.1016/j.ophtha.2018.10.020.
- Guirguis-Blake JM, Evans CV, Rushkin M Ocular prophylaxis for gonococcal ophthalmia neonatorum: updated evidence report and systematic review for the US preventive services task force. JAMA - J. Am. Med. Assoc. 2019;321: 404–406. doi:10.1001/jama.2018.17847
- National Institute for Health and Care Excellence. Conjunctivitis - infective. 2018.https://cks.nice.org.uk/topics/conjunctivitis-infective/ (accessed 13 Nov 2020).