ABSTRACT
Purpose
To determine the prevalence of trachoma in each of the 21 local government areas (LGAs) of Adamawa State, Nigeria.
Methods
A population-based cross-sectional survey was conducted in each of the 21 LGAs of Adamawa State between 2017 and 2019. With the support of Tropical Data (TD), surveys were planned and implemented in accordance with World Health Organization (WHO) recommendations. A two-stage cluster sampling technique was used in each LGA, 25 or 30 clusters were selected with a probability of selection proportionate to cluster size, and in each of these clusters, 25 or 30 households were enrolled for the survey. All residents aged 1 year and older within selected households were examined by TD-certified graders for trachomatous inflammation – follicular (TF) and trachomatous trichiasis (TT) using the WHO simplified grading scheme. Additionally, data were collected on household water and sanitation access.
Results
All 21 LGAs had TF prevalence in 1–9-year-olds below 5%. The prevalence of TT unknown to the health system in people aged ≥15 years was ≥0.2% in three of the 21 LGAs. Access to improved water and sanitation facilities was <80% in the majority of the surveyed LGAs. Only 12 of the 21 LGAs had ≥50% household-level improved latrine access, and only Yola North had ≥80% household-level improved latrine access.
Conclusion
There is no need for mass treatment with antibiotics for trachoma elimination purposes in any of these LGAs. There is a need for active TT case finding and provision of community-based TT surgical services in three LGAs. Furthermore, engagement with water and sanitation agencies is needed to augment access to improved water and sanitation facilities across the State; this will help to avoid the recrudescence of active trachoma in the State.
Introduction
Trachoma is the leading infectious cause of blindness worldwide.Citation1 In 1996, the World Health Organization (WHO) Alliance for the Global Elimination of Trachoma by 2020 (GET2020) set the year 2020 as the target date for the global elimination of trachoma as a public health problemCitation2; this has recently been updated to 2030.Citation3 The recommended intervention package to eliminate trachoma is the SAFE strategy (Surgery for trachomatous trichiasis (TT), antibiotics to clear infection, and promotion of Facial cleanliness and Environmental improvement to reduce transmission).Citation4,Citation5 The WHO recommends mapping of trachoma and implementation of elimination activities at the district level; for trachoma elimination purposes, this is defined as the normal unit of health administration with a population of 100,000–250,000 people.Citation6 In Nigeria, this equates to a Local Government Area (LGA). The decision to implement the SAFE strategy and two of the three criteria to declare elimination of trachoma as a public health problem (prevalence of trachomatous inflammation – follicular (TF) <5% in 1–9-year-olds and prevalence of TT < 0.2% in ≥15-year-olds), are all based on district-level, population-based prevalence data.Citation5,Citation7
Adamawa State is located in the north-eastern zone of Nigeria; it has 21 LGAs and shares boundaries with Gombe, Taraba, and Borno States while on its eastern border lies the Far Northern and Adamaoua Regions of Cameroon. Trachoma is a notifiable disease in Adamawa State, although cases are seldom reported.Citation8 Community-based surveys are needed to accurately estimate prevalence. Prior to this series of surveys, Adamawa State had not been mapped for trachoma, largely due to security challenges, and it did not have an existing trachoma programme. Surrounding administrative areas in both Nigeria and Cameroon had previously been mapped and documented to have trachoma requiring interventions.Citation9–12
This study sought to determine the LGA-level prevalence of TF and TT in Adamawa so that the government and its partners could determine whether a trachoma elimination program would be required. Additionally, given the association between ocular Chlamydia trachomatis transmission intensity and (a) limited access to water for hygiene purposesCitation13 and (b) increased availability of breeding sites for the human-faeces-breeding trachoma vector Musca sorbens,Citation14–16 household-level access to water, sanitation and hygiene (WASH) facilities was also assessed.
Materials and methods
From 2017 to 2019, we conducted LGA-level, baseline, and cross-sectional population-based prevalence surveys for trachoma in Adamawa State. Fourteen LGAs were surveyed in 2017 and seven in 2019. WHO protocol recommendationsCitation17 and standardised implementation guidelines from Tropical Data (TD) were followed, including TD’s standardised trachoma grading certification. Versions 1 (2017) and 2 (2019) of the TD training systemCitation18,Citation19 were used. We implemented standard quality assurance and quality control measures for trachoma surveys that have been described in detail elsewhere.Citation20
Sample size calculation
As is customary for trachoma surveys, the TF prevalence in 1–9-year-olds was considered the primary outcome measure, and the number of households that should be included in each constituent survey was determined on the basis of the number needed to have reasonable confidence in enrolling and examining sufficient children to estimate TF prevalence with acceptable precision. TT prevalence in ≥15-year-olds was estimated amongst ≥15-year-olds examined in the same households.
The sample size of 1–9-year-olds for estimating the TF prevalence in each LGA was determined using the single-population-proportion-for-precision formula,Citation17,Citation21 whereby sample size (n) is equal to Deff × NRIF × Z × (p(1 − p))/(dCitation2). For our calculations, the design effect (Deff) was set as 2.65; the non-response inflation factor (NRIF) was set as 1.2; the z-score for the risk of alpha-error (Z) was 1.96; the expected proportion of children with TF (p) was 10% and the absolute precision required (d) was ±3%.Citation21 The calculated target sample size was therefore 1225 children.
Sampling technique
The evaluation unit in this survey series was the LGA. For each of the 21 LGAs, a two-stage cluster sampling strategy was used to select the study population. Villages were first-stage clusters. A list of the villages in each LGA was used as the sampling frame, from which 25 clusters (in 14 LGAs – for the 2017 surveys), and 30 clusters (in 7 LGAs – for the 2019 surveys) were selected using systematic sampling with a probability-proportional-to-village-size methodology.Citation5 In 2017, the cluster number was determined based on each survey team being able to reliably survey 25 households in 1 day, and there being about 2 children aged 1–9 years in each rural household in northern Nigeria (1225/(25 × 2) = 24.5).Citation22 In 2019, the number of households per day was expanded to 30, and the cluster number was increased to 30 to be more certain of meeting the sample size requirement for estimating both TF and TT prevalence.Citation17 The second-stage clusters were households. These were selected using a compact segment sampling method. Briefly, villages were divided into segments that contained approximately 25 or 30 households (in 2017 and 2019, respectively) and one segment was selected by drawing lots. All the households in the selected segment were invited to take part in the survey.
The WHO simplified grading scheme, as set out in 1987,Citation23 was used to grade trachoma. All graders were ophthalmic nurses who had participated in a TD grader qualifying workshop and passed both the slide-based and live patient inter-grader agreement tests with kappa statistics ≥0.70 for the diagnosis of TF.Citation18,Citation19 Data recorders also completed analogous TD processes of standardised training, testing, and certification.
Within each household, all the residents aged ≥1 year who had lived in the household for at least 6 months at the time of survey, including those absent at the time of the survey team’s visit, were enumerated and invited to be examined. Household members present at the time of the survey and willing to participate were examined for TT and TF. The teams returned at the end of the day to examine consenting persons who had been absent earlier.
Each grader used a 2.5 × magnifying loupe to examine participants under daylight illumination. In any eye graded as having TT, the grader also assessed whether trachomatous scarring (TS) was present.Citation24 For the purposes of this paper, we defined TT as the presence of trichiasis in either the upper or lower eyelid, with or without TS. Those with TT were asked whether they had been offered and/or received management for their TT. We defined a case of TT unknown to the health system as someone with TT who had not yet been offered management or who could not remember being offered management in at least one eye. In 2019 surveys, follicle size guidesCitation25 were used to help graders consistently make a distinction between follicular inflammation that met or did not meet the definition of TF.Citation23
Data collection and data management
Data were collected, stored, and managed using the TD Android phone-based data collection app, and were encrypted and transferred in near real-time to TD servers for cleaning, analysis, and storage.
Household-level data
GPS coordinates for each selected household were recorded. Additional data on water and sanitation facilities were collected by administering a questionnaire – adapted from the WHO/United Nations Children’s Fund (UNICEF) Joint Monitoring Program (JMP) for Water Supply and Sanitation household questionnaireCitation18,Citation19,Citation21 – to the household head or their proxy. Household latrine and handwashing facilities (if present) were inspected to capture details of their type (latrines) or presence/absence of water (hand washing facility). Water sources and sanitation facilities were categorised as improved or unimproved, as per the WHO/UNICEF JMP definitions used for monitoring progress towards the Sustainable Development Goals.Citation26 A household was defined as a compound head together with all individuals normally resident in the compound and eating from the same pot.
Ethics
Survey protocols were approved in advance by the Ethics Committee of the Ministry of Health of Adamawa State, the National Health Research Ethics Committee of Nigeria (NHREC/01/01/2007) and the Observational/Interventions Research Ethics Committee of the London School of Hygiene & Tropical Medicine (16105). After field teams explained the examination protocol to each adult in an appropriate language, informed verbal consent for enrolment and examination was obtained. Verbal consent was agreed by the responsible ethics committees to be culturally appropriate in Adamawa, where adult literacy rates are low. Individuals aged ≥18 years consented for themselves; heads of households gave consent for the participation of minors (<18 years). Consent (or its refusal) was formally documented in the TD data collection app by the data recorder, who had a unique identification number. Individuals with active (inflammatory) trachoma were given two tubes of 1% tetracycline eye ointment, and they or their carer instructed to apply it to the lower conjunctival fornix of both eyes twice daily for 6 weeks; persons with TT were referred for eyelid surgery at the nearest facility to the village. Examiners cleaned their hands with an alcohol-based skin cleaning agent after the examination of each participant.
Data analysis
Data were analysed in R (R Foundation for Statistical Computing, Vienna, Austria).Citation27 Cluster-level TF prevalence was adjusted for age in one-year bands using the most recent census data.Citation28 LGA-level TF prevalence estimates were generated as the mean of the adjusted cluster-level TF prevalence estimates. Similarly, cluster-level TT prevalence was adjusted for age and gender in five-year age bands using the most recent census data, and LGA-level TT prevalence generated as the mean of adjusted cluster-level prevalence estimates. Confidence intervals (CIs) were calculated by taking the 2.5th and 97.5th centiles of the distribution of prevalence estimates generated by repeatedly resampling cluster-level estimates over 10,000 iterations.
Results
Surveys were conducted in 21 LGAs; in February 2017 (14 LGAs) and March 2019 (7 LGAs). A total of 76,096 people were examined; 54% were female. The ages of persons examined ranged from 1 to 100 years. A total of 35,731 children aged 1–9 years were enumerated, of whom 35,338 (99%) were examined, and 36,517 adults aged ≥15 years were enumerated, of whom 33,040 (90%) were examined. The age and gender distribution of the study population for all LGAs combined is shown in .
Table 1. Age and gender distribution of study participants surveyed for trachoma in 21 LGAs of Adamawa State, Nigeria, February 2017–March 2019.
Prevalence of trachoma
The prevalence of TF in children aged 1–9 years ranged from 0.4% in Maiha LGA to 3.6% in Yola North LGA. There was no LGA with a TF prevalence of ≥5% (, ). The prevalence of TT unknown to the health system in persons aged ≥15 years was <0.2% in 18 LGAs. In Fufore, Numan, and Toungo LGAs, the prevalence of TT unknown to the health system was ≥0.2% ( and ). The backlog of TT in Adamawa State, which has an estimated total population of 3,168,10128, was calculatedCitation29 to be 2,008 ().
Figure 1. Prevalence of trachomatous inflammation-follicular (TF) in 1–9-year-olds, by Local Government Area, Adamawa State, Nigeria, February 2017–March 2019. The boundaries and names shown and the designations used on this map do not imply the expression of any opinion whatsoever on the part of the authors, or the institutions with which they are affiliated, concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.
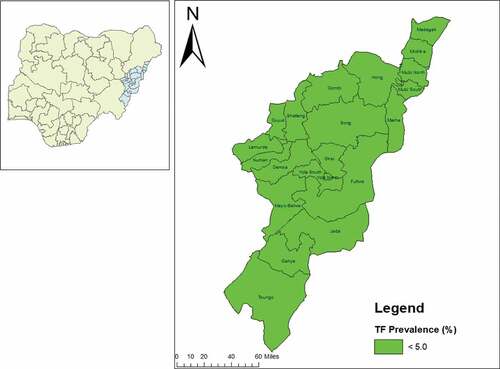
Figure 2. Prevalence of trachomatous trichiasis (TT) unknown to the health system in persons aged ≥15 years, by Local Government Area, Adamawa State, Nigeria, February 2017–March 2019. The boundaries and names shown and the designations used on this map do not imply the expression of any opinion whatsoever on the part of the authors, or the institutions with which they are affiliated, concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.
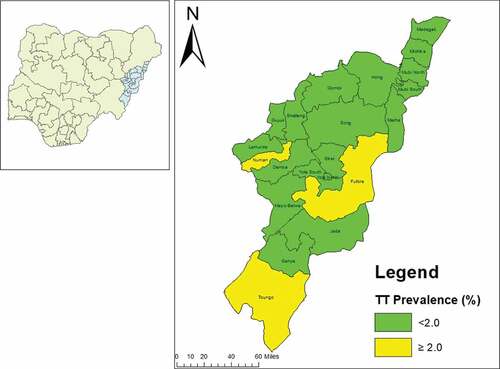
Table 2. Local government area-level prevalence of trachomatous inflammation-follicular (TF) in 1–9-year-olds, Adamawa State, Nigeria, February 2017–March 2019.
Table 3. Prevalence of trachomatous trichiasis (TT) unknown to the health system and TT backlog in 21 LGAs of Adamawa State, Nigeria, February 2017–March 2019.
Water and sanitation coverage
The proportion of households with access to improved drinking water sources was the lowest in Guyuk LGA (35%) and highest in Hong LGA (87%) (). Only two of the 21 LGAs had ≥80% of the households with access to an improved drinking water source. Drinking water access in the household or within 30 minutes of the household (regardless of status of the source as improved or unimproved) ranged from 59% in Michika to 89% in Madagali. Similarly, there was poor access to improved latrines across the State, with only 12 of 21 LGAs having ≥50% household-level improved latrine access and only 1 LGA having ≥80% household-level improved latrine access (). Household–level access to improved sanitation was lowest in Song (29%) and highest in Yola North (95%).
Table 4. Household access to improved drinking water source and improved latrines, by local government area (LGA), Adamawa State, Nigeria, February 2017–March 2019.
Discussion
This study suggests that TF is below the target for elimination as a public health problem in all LGAs in Adamawa State. According to current guidelines, no LGA in the State requires mass drug administration (MDA) of antibiotics for trachoma elimination.Citation5 This is comparable to findings reported in neighbouring Gombe State,Citation10 where none of the 11 LGAs surveyed required antibiotic MDA; Taraba State,Citation9 where only one out of 13 surveyed LGAs required antibiotic MDA; and Northern Cameroon,Citation11 where only 3 of 15 surveyed health districts required antibiotic MDA for trachoma elimination purposes. However, Madagali LGA does border some suspected endemic LGAs in Borno State, in addition to some previously trachoma-endemic districts in Cameroon, and therefore cross-border partnership is important to maintain these LGAs below the elimination prevalence threshold.Citation30
To reduce the possibility of active trachoma recrudescence and maintain the low TF prevalence seen in this survey series, access to water and sanitation should be sustained and improved upon in all of Adamawa’s LGAs. In these surveys, inadequate access to improved water and sanitation facilities was recorded. The exception to this was Yola North, where 95% of households had access to improved sanitation. Community awareness should be raised on the health benefits of using improved sanitation; higher levels of access are associated with lower risk of trachoma.Citation15,Citation31–33 Water and sanitation agencies and other partners should engage with community leaders on this issue and determine ways to sustainably improve access.Citation34 Such improvements would be likely to have substantial health benefits beyond trachomaCitation35 and contribute towards the attainment of Sustainable Development Goals three (to improve global health and well-being) and six (to provide universal clean water and improved sanitation facilities).Citation36,Citation37
The prevalence of TT unknown to the health system was above the target for elimination of trachoma as a public health problem in Fufore, Numan, and Toungo LGAs. This finding is intriguing, given the low TF prevalence. Possible explanations could be due to the inclusion of lower eyelid trichiasis in the TT definition or that these present-day TT prevalences reflect past high TF prevalences, which have since been reduced to below elimination thresholds as a result of MDA. Even though only a few hundred people are in need of TT surgery in each one, the prevalence of TT meets the definition of a public health problem in these LGAs. It is therefore important for Adamawa State to undertake active TT case finding, train, and equip TT surgeons and deploy them to provide community-based services for these LGAs. These services should be durable: assuming that the trichiasis is due to trachoma, it is likely that people will continue to develop incident TT for some years.
This study was undertaken in an area that, like neighbouring Borno State,Citation38 has faced security challenges for over a decade. During the period of the survey, several modifications and safety measures were undertaken; (i) In the second phase of the survey (2019) involving seven LGAs, the number of selected clusters was increased from 25 to 30 as the number of children encountered in the initial LGAs was too low. (ii) In some instances, no more than five villages had to be changed (another random selection of villages from a predefined list) as preselected villages came under attack by insurgents just as the survey team was due to deploy. To circumvent these security challenges and complete the surveys, the survey coordinator had to adopt a fluid itinerary that changed according to the security situation; survey teams were trained on local security conditions; satellite phones were carried; and a dedicated Security and Safety Officer was placed in charge of gathering and disseminating security-related information. We also worked with residents who advised field teams on the safest routes to take to selected villages and used local vehicles to avoid being marked out as visitors.
There were limitations to this study. Reliance on self-reporting for determining access to water could have led to inaccuracies, although direct observation was employed where possible to minimise this. Further, estimates of TT surgical backlog from survey data like these are acknowledged to be inaccurate because of the relatively small number of people examined in the age groups most at risk of TT. It is also worth noting that upper and lower eyelid trichiasis were not recorded separately during these surveys, precluding an analysis of the results in line with the new definition of TT agreed at the 4th Global Scientific Meeting on Trachoma, November 2018.Citation39,Citation40
Conclusion
These surveys show that in Adamawa State, prevalence of trachoma is generally low. No MDA intervention is required, and active TT case finding and other targeted surgical interventions are warranted in only three of the 21 surveyed LGAs. Behavioural practices that encourage facial cleanliness and infrastructure improvements in water access and human waste management should continue to guard against an increase in active trachoma prevalence in the future.
Disclaimer
The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated, USAID or the United States Government.
Acknowledgments
We thank the leadership and staff of the Adamawa State Ministry of Health for their generous and unstinting collaboration; in-country TD trainers; our hard-working field teams; supporting NGDOs; the TD team and the residents of participating communities. We are grateful to Robert Butcher for reviewing the first draft of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Global Health. 2017;5:e1221–e1234. doi:10.1016/S2214-109X(17)30393-5.
- Future approaches to trachoma control: report of a global scientific meeting, Geneva, 17-20 June 1996. (WHO/PBL/96.56) Geneva: World Health Organization. 1997.
- Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021–2030 ( available at: https://apps.who.int/iris/handle/10665/338565, accessed 28 January 2021). Geneva: World Health Organization; 2020
- Francis V, Turner V Achieving community support for trachoma control (WHO/PBL/93.36). Geneva: World Health Organization; 1993.
- Solomon AW, Zondervan M, Kuper H, et al. Trachoma Control: A Guide for Programme Managers. Geneva: World Health Organization; 2006.
- Report of the 3rd Global Scientific Meeting on Trachoma, Johns Hopkins University, Baltimore, MA, 19-20 July 2010 (WHO/PBD/2.10). Geneva: World Health Organization. 2010.
- Validation of Elimination of Trachoma as a Public Health Problem. Geneva: World Health Organization; 2016.
- Kumur J, Siya T, Adamu B, et al. Analysis of notifiable disease reported in Adamawa State, Nigeria. Int J Sci Technol Res. 2017;6(7):219–220.
- Umar MM, Mpyet C, Muhammad N, et al. Prevalence of trachoma in 13 local government areas of Taraba State, Nigeria. Ophthalmic Epidemiol. 2018;25(sup1):18–24. doi:10.1080/09286586.2017.1368670.
- Mpyet C, Muhammad N, Adamu MD, et al. Trachoma mapping in Gombe State, Nigeria: results of 11 local government area surveys. Ophthalmic Epidemiol. 2016;23(6):406–411. doi:10.1080/09286586.2016.1230633.
- Noatina BN, Kagmeni G, Souleymanou Y, et al. Prevalence of trachoma in the North Region of Cameroon: results of a survey in 15 health districts. PLoS Negl Trop Dis. 2014;8:6. doi:10.1371/journal.pntd.0002932.
- Noatina BN, Kagmeni G, Mengouo MN, et al. Prevalence of trachoma in the Far North region of Cameroon: results of a survey in 27 health districts. PLoS Negl Trop Dis. 2013;7:5. doi:10.1371/journal.pntd.0002240.
- Cairncross S. Trachoma and water. Community Eye Health. 1999;12:58.
- Miller K, Pakpour N, Yi E, et al. Pesky trachoma suspect finally caught. Br J Ophthalmol. 2004;88(6):750–751. doi:10.1136/bjo.2003.038661.
- Garn JV, Boisson S, Willis R, et al. Sanitation and water supply coverage thresholds associated with active trachoma: modeling cross-sectional data from 13 countries. PLoS Negl Trop Dis. 2018;12(1):e0006110. doi:10.1371/journal.pntd.0006110.
- Last A, Versteeg B, Shafi Abdurahman O, et al. Detecting extra-ocular Chlamydia trachomatis in a trachoma-endemic community in Ethiopia: identifying potential routes of transmission. PLoS Negl Trop Dis. 2020;14(3):e0008120.doi:10.1371/journal.
- World Health Organization Strategic and Technical Advisory Group on Neglected Tropical Diseases. Design Parameters for Population-based Trachoma Prevalence Surveys. Geneva: World Health Organization; 2018.
- Courtright P, MacArthur C, Macleod C, et al. Tropical Data: Training System for Trachoma Prevalence Surveys (Version 1). London: International Coalition for Trachoma Control; 2014.
- Courtright P, MacArthur C, Macleod C, et al. Tropical Data: Training System for Trachoma Prevalence Surveys (Version 2). London: International Coalition for Trachoma Control; 2016.
- Solomon AW, Willis R, Pavluck AL, et al. Quality assurance and quality control in the global trachoma mapping project. Am J Trop Med Hyg. 2018;99(4):858–863. doi:10.4269/ajtmh.18-0082.
- Solomon AW, Pavluck A, Courtright P, et al. The global trachoma mapping project: methodology of a 34-country population-based study. Ophthalmic Epidemiol. 2015;22(3):214–225.doi:10.3109/09286586.2015.
- National Population Commission. 2006 Housing Characteristics and Amenities Tables – Priority Tables (LGA) Volume II. Abuja, Nigeria: Federal Government of Nigeria.
- Thylefors B, Dawson CR, Jones BR, et al. A simple system for the assessment of trachoma and its complications. Bull World Health Organ. 1987;65(4):477.
- World Health Organization. Second global scientific meeting on trachomatous trichiasis. Cape Town, 4-6 November 2015 (WHO/HTM/NTD/2016.5). 2016, Geneva: World Health Organization
- Solomon AW, Le Mesurier RT, Williams WJ. A diagnostic instrument to help field graders evaluate active trachoma. Ophthalmic Epidemiol. 2018;25(5–6):399–402. doi:10.1080/09286586.2018.1500616.
- World Health Organization. Core Questions on Drinking Water and Sanitation for Household Surveys. Geneva: World Health Organization; 2006.
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. https://www.R–project.org/.
- National Population Commision. National and State Population and Housing Tables: Priority Table 1&43. Abuja: NPC; 2006.
- Solomon AW, Bella ALF, Negussu N, et al. How much trachomatous trichiasis is there? A guide to calculating district–level estimates. Community Eye Health. 2019;31(104):S5.
- International Trachoma Initiative. Trachoma Atlas (Accessed at https://www.trachomaatlas.org). Task Force for Global Health: Decatur, 2021.
- Oswald WE, Stewart AE, Kramer MR, et al. Active trachoma and community use of sanitation, Ethiopia. Bull World Health Organ. 2017;95(4):250–260. doi:10.2471/BLT.16.177758.
- Courtright P, Sheppard J, Lane S, et al. Latrine ownership as a protective factor in inflammatory trachoma in Egypt. Br J Ophthalmol. 1991;75(6):322–325. doi:10.1136/bjo.75.6.322.
- Haile M, Tadesse Z, Gebreselassie S, et al. The association between latrine use and trachoma: a secondary cohort analysis from a randomized clinical trial. Am J Trop Med Hyg. 2013;89(4):717–720. doi:10.4269/ajtmh.13-0299.
- World Health Organization. WASH and Health Working Together: A ‘How-to’ Guide for Neglected Tropical Disease Programmes. Geneva:WorldHealth Organization; 2018. accessed at https://apps.who.int/wash-health-toolkit/.
- Mills JE, Cumming O. The Impact of Water, Sanitation and Hygiene on Key Health and Social Outcomes. New York, NY,USA: Sanitation and Hygiene Applied Research for Equity (SHARE) and UNICEF. UNICEF; 2016:1–110.
- Gupta J, Vegelin C. Sustainable development goals and inclusive development. International environmental agreements: Politics, law and economics. 2016;16(3):433–448. doi:10.1007/s10784-016-9323-z.
- Boisson S, Engels D, Gordon BA, et al. Water, sanitation and hygiene for accelerating and sustaining progress on neglected tropical diseases: a new Global Strategy 2015–20. Int Health. 2016;8(suppl_1):i19–i21. doi:10.1093/inthealth/ihv073.
- Adamu MD, Am J, Orji P, et al. Baseline prevalence of trachoma in 13 local government areas of Borno State, Nigeria. (in preparation).
- Solomon AW, Kello AB, Bangert M, et al. The simplified trachoma grading system, amended. Bull World Health Organ. Oct 1 2020;98(10):698–705. doi:10.2471/BLT.19.248708.
- World Health Organization. Report of the 4th global scientific meeting on Trachoma, Geneva, 27–29 November 2018. Geneva: World Health Organization. accessed at https://apps.who.int/iris/bitstream/handle/10665/325121/WHO-CDS-NTD-PCT-2019.03-eng.pdf?ua=1
