ABSTRACT
Cancer patients show the signs and symptoms of a failing circadian organization. Melatonin, secreted only during nightly darkness, promotes nightly sleep and synchronizes daily molecular and physiologic clocks. Using a placebo-controlled randomized double-blind clinical trial, we determined whether the hypothesized proper physiologic daily timing, the evening, is essential for cancer patients to benefit from melatonin. Eighty-four advanced non-small cell lung cancer (NSCLC) patients from two centers (42 each) receiving standard etoposide/cisplatin therapy, were randomly assigned to one of three arms (I, 8AM & 8PM placebo; II, 8AM 20 mg melatonin and 8PM placebo; III, 8AM placebo and 8PM 20 mg melatonin. Each patient was followed until death. After adjusting for covariates, the Cox proportional hazards regression analysis found that the overall survival was enhanced only among those patients randomized to receive evening melatonin (p = 0.031 HR = 0.39). Survival benefit in the PM melatonin arm was optimized in patients who reported normal sleep quality. After adjusting for significant covariates at baseline, we found an overall survival advantage for PM melatonin when compared against placebo. Moreover, PM melatonin’s therapeutic effect was optimal in patients who self-reported normal sleep quality.
Introduction
There is accumulating evidence that a dysfunctional daily time keeping system contributes to the pathogenesis of many diseases (Marcheva et al. Citation2010; Sulli et al. Citation2019). Melatonin is a daily time-keeping hormone found in organisms that range from algae to mammals (Zhao et al. Citation2019). Its fundamental function is to help coordinate an organism’s physiological, cytokinetic and metabolic activities, so that the right things are done at the right times of day. In both diurnal and nocturnal mammals, melatonin is mainly produced in the pineal gland and secreted into the systemic blood only during darkness at night.
Nighttime pineal melatonin production is driven by the central circadian pacemaker located in the paired suprachiasmatic nuclei (SCN) of the anterior hypothalamus. The endogenous oscillatory activity of the SCN is synchronized by light/dark cycle information transmitted via non-visual neural pathways from specialized blue light (dusk and dawn sensitive) retinal ganglion photoreceptors in the retinae of the eyes. As the chemical expression of darkness, melatonin is the most reliable output signal reflecting the circadian activity of the SCN (Zhao et al. Citation2019). Daytime light exposure serves to reset the central circadian clock each day while light exposure during the night not only disrupts circadian activity of the SCN but also specifically suppresses the nocturnal production of melatonin by the pineal gland as a function of light intensity, wavelength and duration. Daily melatonin dynamics affect many host biologic processes, including the daily sleep activity pattern, mood, sexual maturation and reproduction, metabolism and immune function (Brzezinski Citation1997). Further, circadian disruption by light at night that occurs in rotating shift work, increases the risk of breast, colorectal and prostate cancer by more than 50% and accelerates cancer growth progression and spread (Levin et al. Citation2005; Talib Citation2018). Conversely, the presence of cancer itself is commonly associated with symptomatic daily time-keeping and sleep disruption as well as daytime fatigue (Mehnert Citation2018).
Melatonin administration has been shown to produce a variety of benefits among cancer patients, including a reduction in the frequency or severity of treatment-related adverse events (Del et al. Citation2013; Lissoni et al. Citation1999; Chen et al. Citation2014; Hansen et al. Citation2014; Rasmussen et al. Citation2015; Sookprasert et al. Citation2014; Ben-David et al. Citation2016; Kurdi & Muthukalai Citation2016). Melatonin-treated cancer patients also experienced relief of anxiety, improvement in sleep quality and diminished cachexia with progressing cancer (Lissoni et al. Citation1996). Virtually, no melatonin-related toxicities have been reported (Lissoni et al. Citation1996, Citation1999). Lissoni et al. has reported among patients with metastatic non-small cell lung cancer (NSCLC) receiving cisplatin and etoposide that evening melatonin was associated with greater tumor regression, longer survival, and better tolerance (Lissoni et al. Citation1992). We have previously reported that the extent of cancer-associated circadian disruption at baseline among advanced NSCLC patients correlates with the rate of cancer progression, diminishment of quality of life (QoL), depression, anxiety, and poor sleep quality (Du-Quiton et al. Citation2010; Grutsch et al Citation2011a; Grutsch et al. Citation2011b). Two meta-analyses of clinical trials using various doses of melatonin and unstipulated treatment timing reported that melatonin reduces the relative risk of death at 1 year by an average of 37%, doubles the frequency of complete response, and reduces the prevalence of chemotherapy-induced leukopenia, nausea/vomiting, hypotension, and thrombocytopenia (Seely et al. Citation2012; Wang et al. Citation2012).
Experimentally, the time of day of melatonin administration is critical. Melatonin administered during the mid-dark phase upregulates tumor melatonin receptors, suppresses tumor growth, and minimizes circadian rhythm disturbances much more than when it is given during the mid-light phase (Grutsch et al. Citation2011b). Herein, for the first time, we performed a prospective, double-blind, randomized, controlled trial to evaluate whether the time of day of melatonin administration would impact overall survival in patients with advanced NSCLC concurrently receiving standard chemotherapy.
Materials and methods
Study site
The trial was conducted concurrently at Cancer Treatment Centers of America® (CTCA) at Midwestern Regional Medical Center (MRMC) in Zion, Illinois and the WJB Dorn Veterans Medical Center (VAMC) in Columbia, South Carolina from June 2002 to April 2006. The study was performed after the approval of MRMC IRB in August 2001 and VAMC IRB in June 2001 and in accord with an assurance approved by the Department of Health and Human Services.
Patients
Patients, between 18 and 80 years, with a pathologically confirmed diagnosis of Stage IIIB, or IV NSCLC, bi-dimensionally measurable disease and an Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1, or 2, were included. Informed consent was obtained from each participant. Untreated patients and patients who had failed to benefit from one prior chemotherapy treatment regimen were eligible. Ineligible patients included those with medical conditions that precluded administration of chemotherapy, such as inadequate renal function with serum creatinine >221 mmol × 10−1, inadequate hepatic function with bilirubin >34.2 mmnol × 10−1, uncontrolled congestive heart failure, uncontrolled hypertension, arrhythmia or angina, carcinomatous meningitis, or uncontrolled infection. Patients with a history of uncontrolled brain metastases or other primary cancer were also ineligible. If patients were on melatonin prior to study, a 2-week washout period was implemented.
Study design and intervention
The study was a randomized, double-blind, three-arm study in patients with advanced NSCLC. Arm 1 was placebo at 8AM and placebo at 8PM; Arm 2 was melatonin 20 mg at 8AM and placebo at 8PM; and arm 3 was placebo at 8AM and melatonin 20 mg at 8PM. All patients were treated with an identical dose and schedule of cytotoxic chemotherapy: cisplatin 25 mg/m2/day followed by etoposide 100 mg/m2/day (intravenous infusion over three consecutive days). Therapy was repeated every 28 days. Response to therapy was evaluated clinically and radiologically every third cycle. Treatment was continued until progressive disease (PD), development of unacceptable toxicity, patient withdrawal, or completion of six cycles of treatment. Further therapy after PD was permitted, when indicated in the judgment of the attending oncologist. Dose of chemotherapy was unaltered if patient’s recovery white blood cell (WBC) counts were >4,000 per microliter and platelet levels >100,000 per microliter. If levels failed to meet these cut-offs, chemotherapy was either delayed for up to 2 weeks or the dose reduced by 25%. Patients who stopped chemotherapy remained on melatonin/placebo therapy and continued to be evaluated every 3 months until death. An adequate nutrition program was provided to each patient. Symptomatic and other palliative therapies were encouraged.
Extensive data published in the literature indicates that 20 mg doses of melatonin are safely administered to patients with advanced cancer while providing significant benefit as seen in Lissoni’s studies (Lissoni et al. Citation1992; Lissoni et al. Citation1999; Lissoni Citation1999). As a result, a dose of 20 mg was utilized in this study.
Outcomes
The main outcome measures for this analysis was overall survival from first treatment and sleep quality. The secondary outcome measures were objective tumor response and QoL. Tumor response was evaluated using the response evaluation criteria in solid tumors (RECIST) 1.0. QoL was evaluated using European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (QLQ-C30) and Ferrans and Powers Quality of Life Index (QLI). Sleep quality was measured using Pittsburg Sleep Quality Index (PSQI). These questionnaires have been described in detail elsewhere (Buysse et al.Citation1989; Carpenter at al. Citation1998; Osoba et al. Citation1998; Ferrans Citation2007; Gupta et al. Citation2008; Cocks et al. Citation2011).
Statistical analysis
Blinding and randomization
A random number generator produced eight blocks of nine at each study site that was balanced for three patients per treatment arm. Each participating site received its own randomization table from the study statistician (DP) sent only to the study pharmacist. The melatonin and placebo pills were identical in appearance to maintain the strict double blind. The study pharmacists allocated patients to interventions after patient signed the consent form. Consequently, the treatment team, patients and their caregivers were blinded to the treatment. The blind was broken to the data analysts only after the last patient had died.
Sample size estimation
Multiple randomized clinical trials investigating melatonin’s survival benefits in patients with advanced metastatic disease consistently reported that melatonin induced a statistically significant result at p < 0.05 level with 15 patients per arm and at p < 0.001 level with 30–40 patients per arm (21, 22). The sample size estimation was therefore based on the following assumptions: 80% power, alpha 0.05, and a median progression-free survival of 4 months in the control arm and 9 months in one of the experimental arms. This yielded an overall sample size 97 patients for a three-arm study.
Statistical model building
Issues of slow patient accrual plus the impossibility of determining time to tumor progression in the radiological images of 23 patients with pleural effusions eliminated time to tumor progression as an outcome. Consequently, the primary endpoint was switched to overall survival because we could ascertain the date of death for all patients. This decision occurred after patient accrual had ceased, consequently, no new power calculation was done. Survival curves stratified by melatonin treatment were evaluated using the Kaplan–Meier method and log rank test. Cox multivariate regression was performed to evaluate the effect of adjuvant melatonin vs placebo after adjusting for the following covariates: site, age, gender, tumor stage, PSQI components sleep efficiency and sleep quality, QLI and QLQ-C30. The likelihood ratio score and Wald tests were used to evaluate a model against the null model.
There were missing data. Sixteen patients failed to respond to most/all baseline surveys, in addition to one patient who did not respond due to early death. These 17 patients were removed from the analysis; therefore, the full data for studying multivariate survival models were available for 67 patients. All statistical tests were two-sided. Data were analyzed using SAS version 9.1 (Cary, NC, USA).
Results
Patient characteristics
A total of 84 advanced NSCLC patients were randomly assigned to receive placebo at 8AM and at 8PM (n = 29); melatonin 20 mg at 8AM and placebo at 8PM (n = 27); and placebo at 8AM and melatonin 20 mg at 8PM (n = 28). All patients had expired at the time of the analysis (January 2013). There were no statistically significant differences between treatment arms by age, gender, tumor stage, and performance status (). In addition, the treatment arms were statistically indistinguishable for all baseline QLQ-C30 and QLI scores, except for baseline PSQI global sleep score which was superior in the placebo group (). As reported previously, there were systematic differences in the demographic and clinical status of participants between the two sites (19, 20).
Table 1. Patient characteristics by three treatment arms
Table 2. Baseline mean QoL and sleep quality scores by three treatment arms
Sleep quality and quality of life
The number of patients providing baseline data was 74, 75 and 71 for QLQ-C30, QLI and PSQI respectively. At 3-month follow-up, the corresponding numbers were 56, 48 and 50, respectively. The loss to QoL follow-up was independent of the treatment arm. shows the absolute change in these patients’ self-reported Qol and PSQI global sleep scores from baseline to 3-month follow-up. This allowed us to measure the change in various instruments related to trial arm.
Table 3. Mean change (± s.e.m.) in QoL and sleep quality scores and tumor response by three treatment arms
Most of the 13 scales of QLQ-C30 showed rather small changes in scores between baseline and follow-up. Melatonin had no apparent effect. Only one scale, dyspnea, was related to melatonin. The frequency and severity of lung cancer-associated dyspnea showed significant differences in the mean change from baseline to 3 months (; p = 0.037). The melatonin treated patients’ dyspnea symptoms improved; while placebo treated patients reported a clinically large deterioration in dyspnea.
There was no change in scores from baseline to 3 months in the four domains that compose the QLI. Clinically significant change is defined by a 4-point difference in the QLI scores between baseline and follow-up (31). Compared to the general population, however, these cancer patients’ baseline mean score in QLI health and physical functioning domain was eight points or nearly two standard deviations lower (worse) at 16.2. There was no difference in the other three QLI domain scores (social and economic, psychological and spiritual, and family) and the corresponding population-based scores.
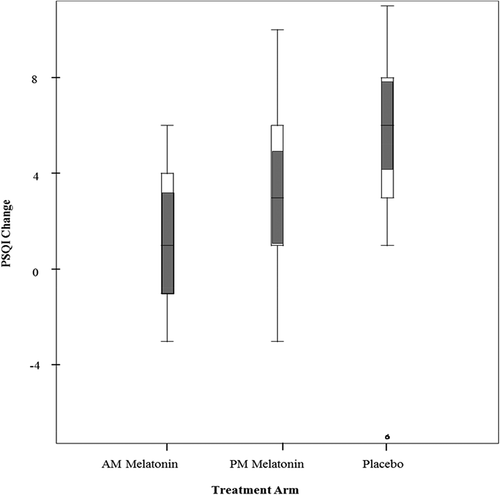
There are 50 subjects for whom we had both baseline and follow-up PSQI data to allow an evaluation of how the change in sleep quality was related to treatment arm. The change in the global PSQI score (mean ± s.e.m.) in the placebo group was 5.0 ± 1.2; in the PM-melatonin group it was 3.1 ± 0.8; and in the AM-Melatonin group it was 1.1 ± 0.8. As expected in lung cancer patients, whose condition deteriorates during disease progression, there was a deterioration of sleep quality in all three arms (higher scores equate to worse sleep quality). However, the deterioration in the placebo group was significantly more pronounced (p = 0.027). The differences between the baseline and 3-month follow-up global sleep score across the three treatment arms are illustrated in the comparative boxplots in .
Tumor response
Tumor response data could be evaluated for only 49 patients. RECIST data could not be collected for 12 patients who died within 3 months of randomization (before first scheduled tumor re-evaluation) and 23 patients who had obscuring pleural effusions which precluded accurate measurements of tumor response. The distribution of tumor responses differed four-fold across treatment groups but not statistically significantly (). The most common response was stable disease. No patient underwent a complete response. The highest prevalence rate for partial response was in the group given PM melatonin.
Overall survival
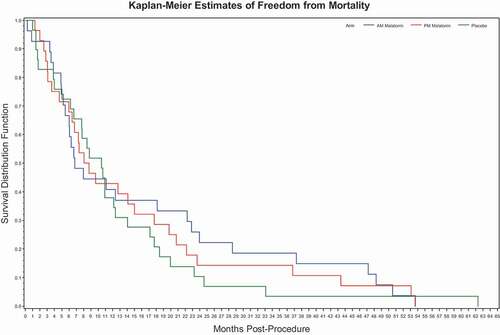
Effect of melatonin: Univariate analysis did not show an effect of melatonin on survival. Rather, and show the overlapping nature of the Kaplan–Meier survival plot where the placebo arm had a slightly longer median survival but a shorter mean survival. In the multivariate model, PM melatonin, when compared against placebo, was statistically significant with respect to survival, with a p-value of 0.031, as shown in . Adjusting for the other variables in this multivariate model, PM melatonin had a large estimated reduction in hazard ratio against placebo of 1–0.61 = 0.39 or 39%. This was in contrast to AM melatonin that was highly insignificant. One subpopulation that appeared to show a beneficial effect of PM melatonin was the patients starting with a global PSQI score less than 7 (i.e., suggesting normal quality sleep coming into the trial). For these subjects, the placebo group had a median survival of 10.4 months (mean = 11.0) compared with the PM Melatonin group’s median survival of 17.6 months (mean = 20.4). As it turned out, none of the 20 patients with a total baseline PSQI of less than 7 were randomized to the AM Melatonin group, which may provide a partial explanation for why PM but not AM administration was seen as significant in the multivariate models.
Table 4. Mortality through days post treatment
Table 5. Multivariate Cox regression analysis for overall survival
Figure 2. Overall survival stratified by treatment arm. Univariate Survival analysis derived from N = 84.
Sleep quality
Two of the seven components of the PSQI, sleep quality and sleep efficiency, had a statistically significant effect on survival. Sleep quality had a significantly positive impact on survival time while baseline sleep efficiency had a negative effect. An increase (improvement) of one unit in sleep quality yielded a large decrease of the hazard rate by 1–0.536 = 0.464 or 46.4%, but sleep efficiency elevated the hazard rate of death by 40% (). The sleep efficiency outcome would be expected due to the percentage of non-sleep time in bed that a progressively sicker patient would experience.
Ferrans and powers QLI
Among the four QLI domains, only the family domain had a significantly positive impact on survival (). The amount of decreased hazard rate per unit increase (improvement) of this index appears small – though a 10-unit difference (on a 30-unit scale) is estimated to reduce the hazard rate by 32%.
QLQ-C30
Of the 13 scales of baseline QLQ-C30, three were found to be significantly positively associated with survival: role and cognitive functional scales and the insomnia symptom item (). No scales or symptom items were associated with shortening survival. Approximately a 30-point swing (improvement) in these indices would be required to halve the hazard rate.
Biological and site effects
Increasing age was found to have a significantly negative impact on survival. An age increase of one decade hiked the estimated hazard by 105% (). No gender differences were found. No survival differences were found between stages 3 and 4 cancer patients. The hazard rate of MRMC was 2.5 times that of the VA, but this difference was accounted for by the higher proportion of MRMC patients undergoing second-line therapy, which was double that of the VAMC patient population.
Discussion
The rationale of this clinical trial was based on the following observations 1) sleep quality is related to survival of cancer patients, 2) cancer patients with a disrupted circadian organization have a poorer than expected lifespan, 3) melatonin is safe and commonly used to reset the circadian organization in subjects with circadian disruption (Innominato et al. Citation2009; Cohen et al. Citation2012, 2016; Innominato et al Citation2014; Levi et al Citation2014; Palesh et al Citation2014; Collins et al. Citation2017; Mehnert et al Citation2018).
Non-small cell lung cancer participants of this trial experienced circadian disruption as the repeated interruptions of their night-time sleep span by high levels of activity and during scheduled periods of daily wakefulness with repeated episodes of low activity (Levin et al. Citation2005; Grutsch Citation2011a). These patients’ baseline actigraphy data revealed extensive circadian disruption in the robustness (amplitude) and the day-to-day phase stability of their rest/activity rhythms. Moreover, actigraphy data showed the magnitude of circadian disruption quantitatively reflected cancer stage, patient performance status, and prior treatment status (Levin et al. Citation2005). The level of circadian disruption increases as the patient’s disease progresses. As a result, these patients are ideal subjects to test melatonin’s therapeutic benefits.
This clinical trial design focused on whether melatonin’s clinical benefits were contingent on the circadian timing of its administration; that is, melatonin administration in the evening versus morning, versus placebo. A second strength of this trial design was its multivariate survival analysis which controlled for known clinical, demographic, and QoL prognostic covariates. The multivariate analysis found no survival benefit among AM melatonin patients, but PM melatonin patients experienced a reduced risk of death of 39%, which is clinically significant. The greatest survival benefit occurred among the PM melatonin arm patients who reported normal quality sleep at baseline. Two PSQI components, sleep quality and sleep efficiency, had large effects on patient survival, wherein sleep quality decreased the hazard rate of death by 46%, while sleep efficiency elevated the hazard rate of death by 40% (Buysse et al. Citation1989). Univariate survival analysis in this and another trial did not find a melatonin survival benefit, which emphasizes that cofactors can mask melatonin’s survival benefit among these patients (Sookprasert et al. Citation2014).
Between the baseline and follow-up measurements, both AM and PM melatonin significantly slowed the progressive deterioration in sleep quality that is routinely experienced by these patients (Buysse et al. Citation1989). Melatonin affected only 1 of the 18 scales that composes the EORTC QLQ c30 and Ferrans/Powers Quality of Life Index questionnaires. Both AM and PM melatonin diminished the intensity of shortness of breath, one of the most common and severe lung cancer symptoms, while the placebo group reported a clinically significant intensification of this symptom (p = 0.037).
There was no evidence that the melatonin’s positive clinical benefits for shortness of breath and sleep quality were mediated by a circadian mechanism. Survival analysis showed that administering melatonin at the appropriate circadian time produced a clinically significant survival benefit; however, no patients with normal sleep scores were randomized to the AM arm. Unfortunately, we do not have the data to show whether AM or PM administered melatonin improved the stability of the participants’ circadian sleep/activity rhythm. A significant limitation of this analysis is that there was no biomarker for the sleep/wake circadian rhythm, such as actigraphy (Innominato et al. Citation2018). Consequently, we cannot link changes in the patients’ sleep/wake rhythms to improved patient outcomes.
There are further limitations of this study: (1) it was originally powered to detect whether melatonin prolonged progression-free survival but it was not originally powered to definitively measure melatonin’s therapeutic benefits on overall survival, (2) incomplete baseline and follow-up patient self-reported data (an unescapable problem due the aggressive and debilitating nature of this disease), (3) two centers had patient populations with different clinical attributes, and (4) the chemotherapy used in the protocol was standard of care when this trial was conducted but advances in the treatment in lung cancer has made this treatment protocol obsolete.
Nonetheless, a daily oral dosage of 20 mg of evening melatonin but not morning melatonin, nor twice daily placebo, correlated with improved survival of advanced NSCLC patients with no perceptible side effects. Evening and morning melatonin produced salutary effects upon nighttime sleep quality and diminished intensity of shortness of breath, which are also important patient benefits. These results suggest that oncologists should investigate the clinical benefits of interventions that restore circadian organization of patients with advanced cancer
Acknowledgments
We would like to thank William Faloon of Life Extension Foundation for providing melatonin and placebo for this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ben-David MA, Elkayam R, Gelernter I, Pfeffer RM. 2016. Melatonin for prevention of breast radiation dermatitis: a phase II, prospective, double-blind randomized trial. Isr Med Assoc J. 18(3–4):188–192.
- Brzezinski A. 1997. Melatonin in humans. N Engl J Med. 336(3):186–195. https://doi.org/10.1056/NEJM199701163360306.
- Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. 1989. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 28(2):193–213. https://doi.org/10.1016/0165-1781(89)90047-4.
- Carpenter JS, Andrykowski MA. 1998. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 45(1):5–13. https://doi.org/10.1016/S0022-3999(97)00298-5.
- Chen WY, Giobbie-Hurder A, Gantman K, Savoie J, Scheib R, Parker LM, Schernhammer ES. 2014. A randomized, placebo-controlled trial of melatonin on breast cancer survivors: impact on sleep, mood, and hot flashes. Breast Cancer Res Treat. 145(2):381–388. https://doi.org/10.1007/s10549-014-2944-4.
- Cocks K, King MT, Velikova G, Martyn St-James M, Fayers PM, Brown JM. 2011. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the research and treatment of cancer quality of life questionnaire core 30. J Clin Oncol. 29(1):89–96. https://doi.org/10.1200/JCO.2010.28.0107.
- Cohen L, Cole SW, Sood AK, Prinsloo S, Kirschbaum C, Arevalo JM, Jennings NB, Scott S, Vence L, Wei Q. 2012. Depressive symptoms and cortisol rhythmicity predict survival in patients with renal cell carcinoma: role of inflammatory signaling. PLoS One. 7(8):e42324. https://doi.org/10.1371/journal.pone.0042324. Epub 2012/08/08.
- Collins KP, Geller DA, Antoni M, Donnell DM, Tsung A, Marsh JW, Krane A, Antoni M, Marsh JW, Burke LE. 2017. Sleep duration is associated with survival in advanced cancer patients. Sleep Med. 32:208–212. https://doi.org/10.1016/j.sleep.2016.06.041.
- De Leon CF, Grady KL, Eaton C, Rucker-Whitaker C, Janssen I, Calvin J, Powell LH. 2009. Quality of life in a diverse population of patients with heart failure: baseline findings from the heart failure adherence and retention trial (HART). J Cardiopulm Rehabil Prev. 29(3):171–178. https://doi.org/10.1097/HCR.0b013e31819a0266.
- Del FE, Dev R, Hui D, Palmer L, Bruera E. 2013. Effects of melatonin on appetite and other symptoms in patients with advanced cancer and cachexia: a double-blind placebo-controlled trial. J Clin Oncol. 31(10):1271–1276. https://doi.org/10.1200/JCO.2012.43.6766.
- Du-Quiton J, Wood PA, Burch JB, Grutsch JF, Gupta D, Tyer K, Lis CG, Levin RD, Quiton DF, Reynolds JL, et al. 2010. Actigraphic assessment of daily sleep-activity pattern abnormalities reflects self-assessed depression and anxiety in outpatients with advanced non-small cell lung cancer. Psychooncology. 19(2):180–189. https://doi.org/10.1002/pon.1539.
- Ferrans CE. 2007. Differences in what quality-of-life instruments measure. J Natl Cancer Inst Monogr. (37):22–26. https://doi.org/10.1093/jncimonographs/lgm008.
- Grutsch JF, Ferrans C, Wood PA, Du-Quiton J, Quiton DF, Reynolds JL, Ansell CM, Oh EY, Daehler MA, Levin RD. 2011a. The association of quality of life with potentially remediable disruptions of circadian sleep/activity rhythms in patients with advanced lung cancer. BMC Cancer. 11(1):11–193. https://doi.org/10.1186/1471-2407-11-193.
- Grutsch JF, Wood PA, Du-Quiton J, Reynolds JL, Lis CG, Levin RD, Ann Daehler M, Gupta D, Quiton DF, Hrushesky WJ. 2011b. Validation of actigraphy to assess circadian organization and sleep quality in patients with advanced lung cancer. J Circadian Rhythms. 9(0):4–9. https://doi.org/10.1186/1740-3391-9-4.
- Gupta D, Grutsch JF, Lis CG. 2008. Comparison of two quality of life instruments for cancer patients: the ferrans and powers quality of life index and the European Organisation for the research and treatment of cancer quality of life questionnaire C30. J Soc Integr Oncol. 6(1):13–18.
- Hansen MV, Andersen LT, Madsen MT, Hageman I, Rasmussen LS, Bokmand S, Rosenberg J, Gögenur I. 2014. Effect of melatonin on depressive symptoms and anxiety in patients undergoing breast cancer surgery: a randomized, double-blind, placebo-controlled trial. Breast Cancer Res Treat. 145(3):683–695. https://doi.org/10.1007/s10549-014-2962-2.
- Innominato PF, Focan C, Gorlia T, Moreau T, Garufi C, Waterhouse J, Giacchetti S, Coudert B, Iacobelli S, Genet D, et al. 2009. Circadian rhythm in rest and activity: a biological correlate of quality of life and a predictor of survival in patients with metastatic colorectal cancer. Cancer Res. 69(11):4700–4707. https://doi.org/10.1158/0008-5472.CAN-08-4747.
- Innominato PF, Komarzynski S, Palesh OG, Dallmann R, Bjarnason GA, Giacchetti S, Ulusakarya A, Bouchahda M, Haydar M, Ballesta A, et al. 2018. Circadian rest-activity rhythm as an objective biomarker of patient-reported outcomes in patients with advanced cancer. Cancer Med. 7(9):4396–4405. https://doi.org/10.1002/cam4.1711.
- Innominato PF, Roche VP, Palesh OG, Ulusakarya A, Spiegel D, Lévi FA. 2014. The circadian timing system in clinical oncology. Ann Med. 46(4):191–207. https://doi.org/10.3109/07853890.2014.916990.
- Kurdi MS, Muthukalai SP. 2016. The efficacy of oral melatonin in improving sleep in cancer patients with insomnia: a randomized double-blind placebo-controlled study. Indian J Palliat Care. 22(3):295–300. https://doi.org/10.4103/0973-1075.185039.
- Lévi F, Dugué PA, Innominato P, Karaboué A, Dispersyn G, Parganiha A, Giacchetti S, Moreau T, Focan C, Waterhouse J. 2014. Wrist actimetry circadian rhythm as a robust predictor of colorectal cancer patients survival. Chronobiol Int. 31(8):891–900. https://doi.org/10.3109/07420528.2014.924523.
- Levin RD, Daehler MA, Grutsch JF, Quiton J, Lis CG, Peterson C, Gupta D, Watson K, Layer D, Huff-Adams S. 2005. Circadian function in patients with advanced non-small-cell lung cancer. Br J Cancer. 93(11):1202–1208. https://doi.org/10.1038/sj.bjc.6602859.
- Lissoni P, Barni S, Ardizzoia A, Paolorossi F, Crispino S, Tancini G, Tisi E, Archili C, De Toma D, Pipino G. 1992. Randomized study with the pineal hormone melatonin versus supportive care alone in advanced nonsmall cell lung cancer resistant to a first-line chemotherapy containing cisplatin. Oncology. 49(5):336–339. https://doi.org/10.1159/000227068.
- Lissoni P, Barni S, MandalA M, Ardizzoia A, Paolorossi F, Vaghi M, Longarini R, Malugani F, Tancini G. 1999. Decreased toxicity and increased efficacy of cancer chemotherapy using the pineal hormone melatonin in metastatic solid tumour patients with poor clinical status. Eur J Cancer. 35(12):1688–1692. https://doi.org/10.1016/S0959-8049(99)00159-8.
- Lissoni P, Paolorossi F, Tancini G, Barni S, Ardizzoia A, Brivio F, Zubelewicz B, Chatikhine V. 1996. Is there a role for melatonin in the treatment of neoplastic cachexia?. Eur J Cancer. 32A(8):1340–1343. https://doi.org/10.1016/0959-8049(96)00136-0.
- Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Martha H, et al. 2010. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 466(7306):627–631. https://doi.org/10.1038/nature09253.
- Mehnert A, Hartung TJ, Friedrich M, Vehling S, Brähler E, Härter M, Keller M, Schulz H, Wegscheider K, Weis J. 2018. One in two cancer patients is significantly distressed: prevalence and indicators of distress. Psychooncology. 27(1):75–82. https://doi.org/10.1002/pon.4464.
- Osoba D, Rodrigues G, Myles J, Zee B, Pater J. 1998. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 16(1):139–144. https://doi.org/10.1200/JCO.1998.16.1.139.
- Palesh O, Aldridge-Gerry A, Zeitzer JM, Koopman C, Neri E, Giese-Davis J, Jo B, Kraemer H, Nouriani B, Spiegel D. 2014. Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep. 37(5):837–842. https://doi.org/10.5665/sleep.3642.
- Rasmussen CR, Olsen MK, Johnsen AT, Petersen MA, Lindholm H, Andersen L, Villadsen B, Groenvold M, Pedersen L. 2015. Effects of melatonin on physical fatigue and other symptoms in patients with advanced cancer receiving palliative care: a double-blind placebo-controlled crossover trial. Cancer. 121(20):3727–3736. https://doi.org/10.1002/cncr.29563.
- Seely D, Wu P, Fritz H, Kennedy DA, Tsui T, Seely AJ, Mills E. 2012. Melatonin as adjuvant cancer care with and without chemotherapy: a systematic review and meta-analysis of randomized trials. Integr Cancer Ther. 11(4):293–303. https://doi.org/10.1177/1534735411425484.
- Sloan JA, Frost MH, Berzon R, Dueck A, Guyatt G, Moinpour C, Sprangers M, Ferrans C, Cella D, Clinical Significance Consensus Meeting Group. 2006. The clinical significance of quality of life assessments in oncology: a summary for clinicians. Support Care Cancer. 14(10):988–998. https://doi.org/10.1007/s00520-006-0085-y.
- Sookprasert A, Johns NP, Phunmanee A, Pongthai P, Cheawchanwattana A, Johns J, Konsil J, Plaimee P, Porasuphatana S, Jitpimolmard S. 2014. Melatonin in patients with cancer receiving chemotherapy: a randomized, double-blind, placebo-controlled trial. Anticancer Res. 34(12):7327–7337.
- Sulli G, Lam MTY, Panda S. 2019. Interplay between circadian clock and cancer: new frontiers for cancer treatment. Trends Cancer. 5(8):475–494. https://doi.org/10.1016/j.trecan.2019.07.002.
- Talib WH. 2018. Melatonin and cancer hallmarks. Molecules. 23(3):518. https://doi.org/10.3390/molecules23030518.
- Wang YM, Jin BZ, Ai F, Duan CH, Lu YZ, Dong TF, Qing-lin Fu QL. 2012. The efficacy and safety of melatonin in concurrent chemotherapy or radiotherapy for solid tumors: a meta-analysis of randomized controlled trials. Cancer Chemother Pharmacol. 9(5):1213–1220. https://doi.org/10.1007/s00280-012-1828-8.
- Zhao D, Yu Y, Shen Y, Liu Q, Zhao Z, Sharma R, Reiter RJ. 2019. Melatonin synthesis and function: evolutionary history in animals and plants. Front Endocrinol (Lausanne). 10:249. https://doi.org/10.3389/fendo.2019.00249.