ABSTRACT
Diurnal preference for evening time has been associated with poorer physical and mental health outcomes. In the current report, perceived loneliness and brain structure (hippocampal and amygdala volumes) were compared in a large (N = 4684) sample of morning- and evening-type individuals. Definite eveningness was associated with increased odds for reporting self-perceived loneliness and lonely evening-types had significantly smaller right hippocampal volume as compared to morning and more socially connected evening types. These data add to the mounting body of evidence linking an evening profile with increased risk for psychiatric disorder.
Introduction
Eveningness, a preference to rise late, retire late and plan daily activities for later in the day, has been associated with physical and mental health. Compared to morning types, individuals with a preference for evening time (late chronotype) consistently display poorer health outcomes. For example, eveningness has been associated with type-2 diabetes, cardiovascular disease and increased mortality (Knutson and Von Schantz Citation2018; Almoosawi et al. Citation2019). Consistent evidence also suggests that eveningness is linked to a number of psychiatric disorders including depression (Kivelä et al. Citation2018; Norbury and Evans Citation2019). Data from large population-based studies and meta-analyses suggest that evening types are more likely to report modest to severe depressive symptoms, a diagnosis of depression and use of prescribed antidepressant medication (Norbury et al. Citation2004; Merikanto et al. Citation2013, Citation2015; Antypa et al. Citation2016; Au and Reece Citation2017; Norbury Citation2019, Citation2021b).
In the healthy population, eveningness is associated with personality traits such as neuroticism (Duggan et al. Citation2014), Machiavellianism (Jonason et al. Citation2013) and openness to experience (Randler et al. Citation2017). By contrast, morningness is associated with increased conscientiousness, openness and agreeability (Duggan et al. Citation2014). Previous work has also reported negative biases in emotional processing (Berdynaj et al. Citation2016; Horne et al. Citation2017) and impaired emotion regulation in evening-types (Antypa et al. Citation2017; Watts and Norbury Citation2017; Berg et al. Citation2018) although (Taylor et al. Citation2020) reported no difference between evening-types and non-evening types (operationalised as a Composite Scale of Morningness score > 26) using a standardised laboratory-based emotion regulation task. Taken together, the bulk of current evidence suggests that eveningness is associated with poorer physical and mental health outcomes, reduced well-being and emotional processing styles that may confer risk for psychiatric disorder.
A number of theories, spanning a broad range of scientific fields, have been posited to account for the relationship between diurnal preference and mental-health and well-being (McClung Citation2013; Logan and McClung Citation2019). One, potentially important mediator, less frequently reported in the extant literature is loneliness. Loneliness, or social isolation, reflects the disparity between an individual’s actual and preferred social relations (Peplau and Perlman Citation1982) and is different to solitude which is an individual choice to be without company either for personal growth or to step back from societal demands (Cacioppo et al. Citation2015). Social connectedness is strongly related to health and well-being and a lack of social connectedness has been observed to contribute to a number of physical and psychiatric disorders including depression (Cacioppo and Cacioppo Citation2018). To date, however, little research has explored loneliness in the context of diurnal preference. One study, Wills et al., reported higher levels of social isolation (as determined by the Multidimensional Scale of Perceived Social Support) and depression symptomatology in evening-type student athletes (Wills et al. Citation2019). Similarly, Walsh and colleagues (Walsh et al. Citation2021) observed reduced social support in young adult evening-types as compared to morning- and neither-type peers. In a more recent study (Norbury Citation2021a) it was observed that eveningness was associated with increased odds for reporting self-perceived loneliness (as indexed by the Three-Item Loneliness Scale (Hughes et al. Citation2004) after adjusting for age, sex and sleep quality. Interpretation of these data are limited, however, due to the different instruments employed, the samples studied (student athletes; (Wills et al. Citation2019) and, with the exception of Walsh et al., who included 3160 participants, relatively small sample sizes (respectively, N = 184 and N = 151). The first aim of the current work, therefore, is to determine if perceived loneliness differs between a large sample of older adult morning and evening-type individuals.
Evidence from Magnetic Resonance Imaging (MRI) studies are beginning to shed light on the structural substrates of loneliness. Using Voxel-Based Morphometry (VBM) Kanai et al. (Citation2012) observed a negative correlation between grey matter volume and loneliness (as measured by the UCLA Loneliness Scale) in 108 healthy volunteers aged between 18 and 32 (Kanai et al. Citation2012). In another VBM study, Kong and colleagues (Kong et al. Citation2015) reported that loneliness was positively associated with grey matter volume in left dorsolateral prefrontal cortex (DLPFC) in a large sample of young adults (N = 308, age range 18–27) which the authors suggested may reflect impaired emotion regulation. In a prospective study designed to explore the impact of increased social support (delivered by a number of exercise interventions) on regional brain volume in 247 community-dwelling older adults (mean age 65 years) Ehlers et al., reported reductions in perceived loneliness but no changes in regional brain volume. The authors did observe, however, that larger amygdalae volume at baseline was associated with greater reductions in loneliness (Ehlers et al. Citation2017). More recently Düzel et al. (Citation2019) observed in a large sample of older adults (N = 319, age 61–82 years) that loneliness was negatively correlated with regional grey matter volume in amygdala and hippocampus (Düzel et al. Citation2019). The second aim of the current work, therefore, is to determine if the impact of perceived loneliness on subcortical volume (amygdala and hippocampus) differs between definite morning and definite evening-type individuals.
The present study
Clinical depression is more prevalent in evening-types as compared to morning-types. In the healthy population, current evidence suggests that evening-types show negative-biases in emotional processing and impaired emotion regulation – factors that may contribute to increased risk for depression in these individuals. Social factors may also play an important role but there is only very limited evidence to support this (Wills et al. Citation2019; Norbury Citation2021a). The aims of the current study were to examine associations between diurnal preference, loneliness and grey matter volume in structures subserving emotion regulation (hippocampus and amygdala) in a large sample of healthy older age adults (the UK Biobank). Specifically, it is predicted that: 1) eveningness will be associated with higher perceived loneliness, and 2) higher perceived loneliness in evening-types will be linked to smaller brain volume in brain regions implicated in emotional processing (hippocampus and amygdala).
Methods
Participants
Adults aged 40–70 years enrolled in the UK Biobank Resource. Ethical approval to the UK Biobank was granted by the NHS National Research Ethics Service North West (Reference number: 11/NW/0382). The current study was approved by the UK Biobank Access Committee (Project reference number 30,833).
Measures
Diurnal preference was assessed in the Biobank cohort with the single question: “Do you consider yourself to be definitely a morning person/more a morning than an evening person/more an evening than a morning person/definitely an evening person”. The current study included participants who self-reported as “Definitely an ‘evening’ person” or “Definitely a ‘morning’ person”. Participants who answered “Do not know” or “Prefer not to answer” were excluded. Age, sex sleep duration (determined using the question “About how many hours sleep do you get in every 24 hours, please include naps?” with values provided as integers) and a surrogate for socio-economic status (the Townsend Deprivation Inventory) were also recorded. Mental health was assessed with the following question: “Have you been diagnosed with one or more of the following mental health problems by a professional, even if you don’t have it currently? Participants were also provided with following clarification statement: “By professional we mean: any doctor, nurse or person with specialist training (such as a psychologist or therapist). Please include disorders even if you did not need treatment for them or if you did not agree with the diagnosis”. Participants that endorsed current or previous diagnosis of a mental health problem or preferred not to answer were excluded. Perceived loneliness was assessed using the single question “Do you often feel lonely?” and participants could indicate “Yes, No, Do not know, Prefer not to answer”. Participants that selected “Do not know”, or “Prefer not to answer”, were excluded.
Magnetic resonance imaging
T1-weighted anatomical images for each participant were acquired on a single Siemens Skyra 3 T scanner (Siemens, Erlangen, Germany) fitted with a 32-channel head coil according to previously reported procedures (Miller et al. Citation2016; Alfaro-Almagro et al. Citation2018) with online documentation available here: http://biobank.ctsu.ox.ac.uk/crystal/docs/brain_mri.pdf. Further processing included subcortical segmentation to yield volumes for left and right hippocampus and amygdala (see http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FIRST/UserGuide) and estimation of intracranial volume (ICV) which was achieved by segmenting whole brain images into three tissue types (grey and white matter and cerebrospinal fluid) and summing the total volumes.
Statistical treatment
Sample characteristics were analysed using independent samples t-tests and χ2 tests of independence as appropriate. Binary logistic regression was used with diurnal preference as the predictor and loneliness as the outcome variable. The first model contained only the predictor and outcome variables, models 2,3,4 & 5 controlled for, respectively, sex, age, sleep and TDI. Analysis of Covariance with subcortical structure (left/right hippocampus/amygdala) as the dependent variable and diurnal variance and perceived loneliness as the independent variables adjusted for ICV, sex, age, sleep and TDI were conducted to examine the interaction between diurnal preference and loneliness on brain structure. For each ANCOVA α was set at .0125 (Bonferroni correction for four brain structures). Post-hoc t-tests were also Bonferroni corrected for multiple tests.
Results
Demographics
Basic demographics are presented in split by diurnal preference (definite-morning (DM), N = 3465; definite evening (DE), N = 1219). Morning types were significantly older and less deprived than evening types (M diff = 2 years, t(4682) = 7.94, p < .001, M diff = −0.42, t(4682) = −4.67, p < .001) but groups were similar in terms of sleep duration (M diff = 0.06 hours,, t(4682) = −1.67, p < .096). The proportion of females and males in each group was similar (DM 51% and DE 48% female, χ2(1, N = 4684) = 2.7, p = .10)
Table 1. Sample demographics. TDI = Townsend deprivation index.
Lonliness
Across the entire sample 12.7% of participants endorsed often feeling lonely and this was more common in females (χ2(1, N = 4684) = 9.64, p = .002). Diurnal preference significantly predicted loneliness () and this remained significant when adjusted for demographic factors (please see for details).
Table 2. The association between diurnal preference and loneliness.
Brain structure
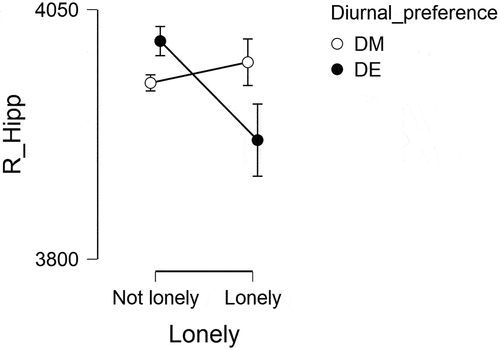
Analysis of covariance demonstrated a significant diurnal preference * loneliness interaction in right hippocampus F(1,4676) = 10.16, p < .001. Post-hoc comparisons showed that DM lonely participants had significantly larger hippocampal volume as compared to DE lonely participants t(1,4676) = 2.77, pBonferroni = 0.034, d = 0.24, 95% CI [0.07, 0.41], DE not lonely participants had significantly greater right hippocampal volume as compared to DE lonely participants t(1,4676) = 3.36, pBonferroni = 0.005 d = 0.25 95% CI [0.11, 0.40] and DM not lonely had significantly greater volume than DE lonely t(1,4676) = 2.73, pBonferroni = 0.038, d = 0.20, 95% CI [0.06, 0.34] (). No significant interactions were observed in left hippocampus or left/right amygdala.
Discussion
The current data were consistent with the first hypothesis that eveningness would be associated with greater perceived loneliness. The data also partially supported the second hypothesis of smaller volume in subcortical structures subserving emotional cognition in lonely evening types – smaller volume was observed in right hippocampus but not left hippocampus or left/right amygdala.
There are several strengths and limitations associated with this work and these should be taken into consideration when interpreting the results. A strength of the work is the relatively large sample size (N = 4684). Limitations include the use of a single question to determine diurnal preference. However, the question presented to participants is similar to the final question of the Reduced Morningness-Eveningness Scale (rMEQ) which alone is a strong predictor of total rMEQ score (Adan and Almirall Citation1991). A further limitation is the use of a single question to determine perceived loneliness (i.e. “Do you often feel lonely?”). Loneliness is not a single entity (Cacioppo et al. Citation2015) but rather comprises dimensions of intimate/emotional (perceived absence of a significant other), relational/social (absence of friendships or family network) and collective (absence of connection to similar others such as teams or group membership). The current work cannot, therefore, speak to which specific components of loneliness may be impacted by diurnal preference. Furthermore, the cross-sectional nature of the current data mean it is not possible to determine if exposure preceded effect.
As noted above, temporal incidence cannot be inferred from the current data set. It is possible, however, that for evening-type individuals social connectedness reduces in late adolescence as young adults tend to transition to a more morning profile. Those individuals that retain a more evening-type may gradually lose network allegiances and group membership as peers take on a schedule more in tune with their changing circadian typology, thereby marooning more evening-prone individuals. This notion is purely speculative and requires further investigation with suitably powered, prospective studies.
In terms of brain structure, it was observed that lonely participants that self-endorsed as definite evening-types had lower right hippocampal volumes as compared to not lonely evening-types and both groups of morning-types (lonely and not lonely). The current findings of reduced right hippocampal volume in lonely evening-types may be consistent with previous work which suggests loneliness is associated with reduced hippocampal/amygdala volume (Düzel et al. Citation2019). The hippocampus is implicated in emotional processing and previous work (D’Agostino et al. Citation2019; Düzel et al. Citation2019) has suggested that differences in processing and regulating emotionally and socially relevant information may reflect differences in brain regions subserving these functions (e.g. hippocampus). More recently, de Lange and colleagues (de Lange et al. Citation2021) observed greater delta brain age (the difference in estimated brain age relative to chronological age) in Biobank participants reporting greater social isolation and loneliness accompanied with a number of unhealthy lifestyle behaviours and socioeconomic deprivation. The authors conclude that both loneliness and social isolation contribute to a risk profile for poor brain health (de Lange et al. Citation2021). The current study adds to this literature and highlights the potential need to consider chronotype when developing interventions to reduce loneliness and support physical and mental health. Of note, eveningness has previously been associated with impaired emotion regulation (Watts and Norbury Citation2017; Berg et al. Citation2018), negative biases in emotional processing (Berdynaj et al. Citation2016; Horne et al. Citation2017) and localised atrophy in the subiculum region of the right hippocampus of younger adults (Horne and Norbury Citation2018). Prolonged loneliness (which may be relevant to the current older adult sample) may lead to higher levels of glucocorticoids and reduced neurotrophic factors and subsequent reductions in hippocampal volume (Sheline Citation2011). In addition or in parallel to this, loneliness would be associated with reduced meaningful social interactions leading to impoverished stimulation of brain regions such as the hippocampus and subsequent atrophy (Mora Citation2013). It should be noted, however, that the observed differences in hippocampal volume were small (Cohen’s d: 0.24–0.25) and the influence of these structural differences on hippocampal function are not known. In addition, the directionality and aetiology of the associations reported here cannot be determined. It is unknown if individuals with a larger hippocampus seek out social connections or if social stimulation and interconnectedness positively impact hippocampal volume.
In conclusion, the current findings suggest that evening-type individuals free from current or previous diagnosis of psychiatric disorder show increased odds for reporting perceived loneliness and smaller right hippocampal volume. Both loneliness and reduced hippocampal volume have been reported in depression and the observation of similar results in never depressed evening types may underpin, in part, the increased vulnerability for depression in these individuals. Future prospective studies are required to test this hypothesis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Adan A, Almirall H. 1991. Horne & Östberg morningness-eveningness questionnaire: a reduced scale. Pers Individ Differ. 12(3):241–253. doi:10.1016/0191-8869(91)90110-W.
- Alfaro-Almagro F, Jenkinson M, Bangerter NK, Andersson JLR, Griffanti L, Douaud G, Sotiropoulos SN, Jbabdi S, Hernandez-Fernandez M, Vallee E, Vidaurre, D, Webster, M, McCarthy, P, Rorden, C, Daducci, A, Alexander, DC, Zhang, H, Dragonu, I, Matthews, PM, Smith, SM, 2018. Image processing and Quality Control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage. 166: 400–424. https://doi.org/10.1016/j.neuroimage.2017.10.034
- Almoosawi S, Vingeliene S, Gachon F, Voortman T, Palla L, Johnston JD, Van Dam RM, Darimont C, Karagounis LG. 2019. Chronotype: implications for epidemiologic studies on chrono-nutrition and cardiometabolic health. Advances in Nutrition. 10(1):30–42. doi:10.1093/advances/nmy070.
- Antypa N, Vogelzangs N, Meesters Y, Schoevers R, Penninx BWJH. 2016. Chronotype associations with depression and anxiety disorders in a large cohort study. Depress Anxiety. 33(1):75–83. doi:10.1002/da.22422.
- Antypa, N., Verkuil, B., Molendijk, M., Schoevers, R., Penninx, B. W. J. H., & Van Der Does, W. (2017). Associations between chronotypes and psychological vulnerability factors of depression. Chronobiology International, 1–11. 10.1080/07420528.2017.1345932
- Au J, Reece J. 2017. The relationship between chronotype and depressive symptoms: a meta-analysis. J Affect Disord. 218:93–104. doi:10.1016/j.jad.2017.04.021.
- Berdynaj D, Boudissa SN, Grieg MS, Hope C, Mahamed SH, Norbury R. 2016. Effect of chronotype on emotional processing and risk taking. Chronobiol Int. 1–13. doi:10.3109/07420528.2016.1146739.
- Berg JFV, Den, Kivelä L, Antypa N. 2018. Chronotype and depressive symptoms in students: an investigation of possible mechanisms. Chronobiol Int. 1–14. doi:10.1080/07420528.2018.1470531.
- Cacioppo JT, Cacioppo S. 2018. The growing problem of loneliness. Lancet (London, England). 391(10119):426. doi:10.1016/S0140-6736(18)30142-9.
- Cacioppo S, Grippo AJ, London S, Goossens L, Cacioppo JT. 2015. Loneliness: clinical import and interventions. Perspect Psychol Sci. 10(2):238–249. doi:10.1177/1745691615570616.
- D’Agostino AE, Abdallah CG, Kattan D, Canli T (2019, April). An fMRI study of loneliness in younger and older adults. Soc Neurosci. 1 0.1 080/17470919.2018.1445027
- de Lange A-MG, Kaufmann T, Quintana DS, Winterton A, Andreassen OA, Westlye LT, Ebmeier KP. 2021. Prominent health problems, socioeconomic deprivation, and higher brain age in lonely and isolated individuals: a population-based study. Behav Brain Res. 414:113510. doi:10.1016/j.bbr.2021.113510.
- Duggan KA, Friedman HS, McDevitt EA, Mednick SC, Arias-Carrion O. 2014. Personality and healthy sleep: the importance of conscientiousness and neuroticism. PLoS ONE. 9(3):e90628. doi:10.1371/journal.pone.0090628.
- Düzel S, Drewelies J, Gerstorf D, Demuth I, Steinhagen-Thiessen E, Lindenberger U, Kühn S. 2019. Structural brain correlates of loneliness among older adults. Sci Rep. 9(1):13569. doi:10.1038/s41598-019-49888-2.
- Ehlers DK, Daugherty AM, Burzynska AZ, Fanning J, Awick EA, Chaddock-Heyman L, Kramer AF, McAuley E. 2017. Regional brain volumes moderate, but do not mediate, the effects of group-based exercise training on reductions in loneliness in older adults. Front Aging Neurosci. 9. doi:10.3389/fnagi.2017.00110.
- Horne C,M, Marr-Phillips SDM, Jawaid R, Gibson EL, Norbury R. 2017. Negative emotional biases in late chronotypes. Biol Rhythm Res. 48(1):151–155. doi:10.1080/09291016.2016.1236461.
- Horne CM, Norbury R. 2018. Exploring the effect of chronotype on hippocampal volume and shape: a combined approach. Chronobiol Int. 1–7. doi:10.1080/07420528.2018.1455056.
- Hughes ME, Waite LJ, Hawkley LC, Cacioppo JT. 2004. A short scale for measuring loneliness in large surveys. Res Aging. 26(6):655–672. doi:10.1177/0164027504268574.
- Jonason PK, Jones A, Lyons M. 2013. Creatures of the night: chronotypes and the Dark Triad traits. Pers Individ Differ. 55(5):538–541. doi:10.1016/j.paid.2013.05.001.
- Kanai R, Bahrami B, Duchaine B, Janik A, Banissy MJ, Rees G. 2012. Brain structure links loneliness to social perception. Curr Biol. 22(20):1975–1979. doi:10.1016/j.cub.2012.08.045.
- Kivelä L, Papadopoulos MR, Antypa N. 2018. Chronotype and psychiatric disorders. Curr Sleep Med Rep. 4(2):94–103. doi:10.1007/s40675-018-0113-8.
- Knutson KL, Von Schantz M. 2018. Associations between chronotype, morbidity and mortality in the UK Biobank cohort. Chronobiol Int. 1–9. doi:10.1080/07420528.2018.1454458.
- Kong X, Wei D, Li W, Cun L, Xue S, Zhang Q, Qiu J. 2015. Neuroticism and extraversion mediate the association between loneliness and the dorsolateral prefrontal cortex. Exp Brain Res. 233(1):157–164. doi:10.1007/s00221-014-4097-4.
- Logan RW, McClung CA. 2019. Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat Rev Neurosci. 20(1):49–65. doi:10.1038/s41583-018-0088-y.
- McClung CA. 2013. How might circadian rhythms control mood? Let me count the ways …. Biol Psychiatry. 74(4):242–249. doi:10.1016/j.biopsych.2013.02.019.
- Merikanto I, Kronholm E, Peltonen M, Laatikainen T, Vartiainen E, Partonen T. 2015. Circadian preference links to depression in general adult population. J Affect Disord. 188:143–148. doi:10.1016/j.jad.2015.08.061.
- Merikanto I, Lahti T, Kronholm E, Peltonen M, Laatikainen T, Vartiainen E, Salomaa V, Partonen T. 2013. Evening types are prone to depression. Chronobiol Int. 30(5):719–725. doi:10.3109/07420528.2013.784770.
- Miller KL, Alfaro-Almagro F, Bangerter NK, Thomas DL, Yacoub E, Xu J, Bartsch AJ, Jbabdi S, Sotiropoulos SN, Andersson JL, et al. 2016. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci. 19(11):1523–1536. doi:10.1038/nn.4393.
- Mora F. 2013. Successful brain aging: plasticity, environmental enrichment, and lifestyle. Dialogues Clin Neurosci. 15(1):45–52.
- Norbury R. 2019. Chronotype, depression and hippocampal volume: cross-sectional associations from the UK Biobank. Chronobiol Int. 36(5):709–716. doi:10.1080/07420528.2019.1578229.
- Norbury R. 2021a. Loneliness in the time of COVID. Chronobiol Int. 1–3. doi:10.1080/07420528.2021.1895201.
- Norbury R. 2021b. Diurnal preference and depressive symptomatology: a meta-analysis. Sci Rep. 11. doi:10.1038/s41598-021-91205-3.
- Norbury R, Evans S. 2019. Time to think: subjective sleep quality, trait anxiety and university start time. Psychiatry Res. 271:214–219. doi:10.1016/j.psychres.2018.11.054.
- Norbury R, Travis MJ, Erlandsson K, Waddington W, Owens J, Ell PJ, Murphy DG. 2004. SPET imaging of central muscarinic receptors with (R,R)[123I]-I-QNB: methodological considerations. Nucl Med Biol. 31(5):583–590. doi:10.1016/j.nucmedbio.2004.01.003.
- Peplau L, Perlman D. 1982. Loneliness: a sourcebook of current theory, research, and therapy. New York: Wiley.
- Randler C, Schredl M, Göritz AS. 2017. Chronotype, sleep behavior, and the big five personality factors. SAGE Open. 7(3):215824401772832. doi:10.1177/2158244017728321.
- Sheline YI. 2011. Depression and the hippocampus: cause or effect? Biol Psychiatry. 70(4):308–309. doi:10.1016/j.biopsych.2011.06.006.
- Taylor BJ, Bowman MA, Brindle A, Hasler BP, Roecklein KA, Krafty RT, Matthews KA, Hall MH. 2020. Evening chronotype, alcohol use disorder severity, and emotion regulation in college students. Chronobiol Int. 1–11. doi:10.1080/07420528.2020.1800028.
- Walsh NA, Repa LM, Garland SN. 2021. Mindful larks and lonely owls: the relationship between chronotype, mental health, sleep quality, and social support in young adults. J Sleep Res. n/a(n/a):e13442. doi:10.1111/jsr.13442.
- Watts AL, Norbury R. 2017. Reduced effective emotion regulation in night owls. J Biol Rhythms. 32(4):369–375. doi:10.1177/0748730417709111.
- Wills C, Athey A, Robbins R, Patterson F, Turner R, Killgore WDS, Tubbs A, Warlick C, Alfonso-Miller P, Grandner MA. 2019. Chronotype and social support among student athletes: impact on depressive symptoms. Sleep. 42(Supplement_1):A361–A362. doi:10.1093/sleep/zsz067.898.