Abstract
Biological motion perception can be assessed using a variety of tasks. In the present study, 8- to 11-year-old children born prematurely at very low birth weight (<1500 g) and matched, full-term controls completed tasks that required the extraction of local motion cues, the ability to perceptually group these cues to extract information about body structure, and the ability to carry out higher order processes required for action recognition and person identification. Preterm children exhibited difficulties in all 4 aspects of biological motion perception. However, intercorrelations between test scores were weak in both full-term and preterm children—a finding that supports the view that these processes are relatively independent. Preterm children also displayed more autistic-like traits than full-term peers. In preterm (but not full-term) children, these traits were negatively correlated with performance in the task requiring structure-from-motion processing, r(30) = −.36, p < .05), but positively correlated with the ability to extract identity, r(30) = .45, p < .05). These findings extend previous reports of vulnerability in systems involved in processing dynamic cues in preterm children and suggest that a core deficit in social perception/cognition may contribute to the development of the social and behavioral difficulties even in members of this population who are functioning within the normal range intellectually. The results could inform the development of screening, diagnostic, and intervention tools.
Children born prematurely at very low birth weight (VLBW; <1500 g) are at increased risk for impairments in cerebral (central) visual function (e.g., Atkinson & Braddick, Citation2007; Atkinson et al., Citation2008; Cooke, Foulder-Hughes, Newsham, & Clarke, Citation2004; Hård, Niklasson, Svensson, & Hellström, Citation2000; Jakobson, Frisk, Knight, Downie, & Whyte, Citation2001; Ramenghi et al., Citation2010). Problems with motion perception have been reported in many studies. These affect relatively “low-level” processes that allow one to detect movement within a small region (i.e., local motion perception; see MacKay et al., Citation2005). But they also affect more complex processes that allow one to group together local motion signals to perceive global movement patterns (“coherent” motion) or motion-defined shapes (e.g., Downie, Jakobson, Frisk, & Ushycky, Citation2003; Jakobson, Frisk, & Downie, Citation2006; MacKay et al., Citation2005; Taylor, Jakobson, Lewis, & Maurer, Citation2009). The difficulties preterm children experience with these tasks frequently co-occur with other problems associated with dysfunction in the dorsal cortical visual stream, including impaired visual search, stereopsis, visuoconstructive, and visuomotor skills (Jakobson et al., Citation2006). Interestingly, despite these problems, children born very prematurely often perform relatively well on tasks requiring static form perception (e.g., Jakobson et al., Citation2001; Taylor et al., Citation2009). These results are consistent with the view that the dorsal stream is more susceptible to developmental perturbations than the ventral stream—a conclusion that gains support from numerous behavioral and neuroimaging studies (e.g., Atkinson & Braddick, Citation2007; Jakobson et al., Citation2006).
Dynamic cues are important for online visuomotor control and other dorsal stream functions, but they also contribute to a range of social perceptual/cognitive functions that allow us to detect the presence of living beings and to understand their actions and intentions. In two investigations carried out in parallel, we used both realistic stimuli and stylized displays to study these important skills in a group of children born preterm at VLBW and in full-term controls. Realistic “life motion” perception was examined using the Child and Adolescent Social Perception measure (CASP; Magill-Evans, Koning, Cameron-Sadava, & Manyk, Citation1995), which requires the visual analysis of videotaped vignettes depicting social scenes involving two or more people. Relative to full-term peers, preterm children showed impaired ability to identify the emotions of characters depicted in these vignettes and to use nonverbal cues from face and body movements to make these determinations (Williamson & Jakobson, Citation2014a). In the second study, we tested the same children on the Happé-Frith animated triangles task (Abell, Happé, & Frith, Citation2000). This task involves stylized “animate motion” displays similar to those first described by Heider and Simmel (Citation1944). Viewers watch animations depicting two triangles that move randomly with respect to one another (random displays), in a seemingly goal-directed way (e.g., one triangle “following” the other; goal-directed displays), or in ways that typical viewers tend to describe using mental state words (e.g., one triangle “coaxing” the other; theory of mind displays). Compared to full-term peers, preterm children overattributed intentionality/mental states to the randomly moving triangles and underattributed intentionality/mental states to triangles in theory of mind displays (Williamson & Jakobson, Citation2014b)—a pattern that suggests impaired ability to use information about how the shapes moved vis-à-vis one another to make social attributions. In both studies, associations between impaired social perceptual/cognitive functioning and poorer social/behavioral outcomes were observed, supporting the view that the ability to process and interpret life motion is an important determinant of social competence (Pavlova, Citation2012).
In the present report, we describe the results of a third experiment that involved the same preterm and full-term children who took part in the work described above (Williamson & Jakobson, Citation2014a, Citation2014b). Our primary goal here was to explore the children’s ability to process the movements of living beings in more detail. In particular, we assessed their ability to carry out four different types of operations that Troje (Citation2008) suggests are involved in this perceptual skill. One involves the analysis of the local motion present in the ballistic movements of the limbs of terrestrial animals. This information provides the visual system with cues that allow us to orient towards and to distinguish a moving animal from its environment quickly, without undertaking a detailed analysis of its shape (Thompson, Hansen, Hess, & Troje, Citation2007; Troje & Chang, Citation2013; Troje & Westhoff, Citation2006). Another operation involves global structure-from-motion (SFM) processing—that is, the ability to perceptually organize the movements of individual dots to reveal an animal’s structure or shape. The third operation involves classification and categorization of actions through the integration of structural and kinematic information. Troje proposes that this mechanism is designed to detect invariants and that, as such, it should operate well regardless of the particular agent, viewing conditions, or style of action. The fourth operation—a style recognition mechanism—is involved in pattern recognition at a subordinate level (cf. Rosch, Citation1988) and is used to retrieve information conveyed by the specific style in which a given agent performs a particular action. Stylistic attributes can carry information about an individual’s identity, gender, age, emotional state, or personality traits (Troje, Citation2002a, Citation2002b; Troje, Sadr, Geyer, & Nakayama, Citation2006; Troje, Westhoff, & Lavrov, Citation2005; Westhoff & Troje, Citation2007). This multicomponent framework for biological motion perception is supported by psychophysical and neuroscience research (Saunders, Citation2011; Troje, Citation2008).
In our investigations into preterm children’s ability to carry out these different operations, we used two types of stimuli—point-light displays and moving stick figures. Point-light displays, first popularized by Gunnar Johansson (Citation1973, Citation1976), depict the movements of markers attached to the head and major joints of an otherwise invisible figure. Humans of all ages are exquisitely sensitive to the motion cues present in these so-called “biological motion” displays (for reviews, see Pavlova, Citation2012; Troje, Citation2008, Citation2013). To study viewers’ ability to extract useful information from the local motion contained in point-light displays, one can spatially scramble the positions of the individual markers. Although it is not possible to reconstruct form cues from these scrambled displays, viewers can reliably extract information about the direction an animal is facing, provided the dot trajectories are presented in their normal (upright) orientation (e.g., Troje & Westhoff, Citation2006). To investigate viewers’ sensitivity to SFM cues, one can measure viewers’ ability to detect a globally coherent figure walking within a mask of moving dots. When the movements of individual dots in the mask mimic the movements of individual dots in the target, the walking figure is camouflaged and viewers are forced to rely on global motion-processing mechanisms to distinguish it from the mask. To study processes required for recognizing specific actions or for learning the idiosyncratic movement styles of specific individuals, one can use moving stick figures in place of point-light displays. That way, performance in the respective tasks reflects only the ability to retrieve semantic information from the way a person moves—independent of the observer’s ability to use SFM for perceptual organization.
Past research examining the effects of prematurity and associated complications on biological motion perception has focused primarily on the question of whether or not preterm children can perceptually organize point-light displays using SFM cues. In a recent study, we showed that 5- to 9-year-old children born at < 32 weeks gestation were impaired, relative to full-term peers, in their sensitivity to global form cues, cues that signal global movement patterns (i.e., global motion coherence), and SFM cues signaling the presence of a globally coherent point-light walker within a mask (Taylor et al., Citation2009). In other work, Pavlova and colleagues (Pavlova, Bidet-Ildei, Sokolov, Braun, & Krägeloh-Mann, Citation2008; Pavlova et al., Citation2005, Pavlova, Marconato, Sokolov Braun, Birbaumer & Krägeloh-Mann, Citation2006; Pavlova, Sokolov, Birbaumer, & Krägeloh-Mann, Citation2006; Pavlova, Staudt, Sokolov, Birbaumer, & Krägeloh-Mann, Citation2003) showed that difficulties preterm children experience with SFM processing are linked to the presence of periventricular leukomalacia (PVL)—the most common ischemic brain injury affecting premature infants (Volpe, Citation1995). Indeed, in their work, sensitivity to the presence of a point-light walker presented within a field of masking dots was specifically correlated with the extent of damage to the parieto-occipital white matter (Pavlova et al., Citation2003). These authors suggested that PVL could interfere with performance on this task (a) by disrupting a cortical-subcortical network involved in visual binding and spatial attention, (b) by disrupting the development of the posterior cerebral cortex, or (c) by disrupting white matter pathways connecting regions involved in biological motion perception, specifically. Pavlova (Citation2012) has gone on to suggest that PVL-induced disruption of subcortico-cortical and cortico-cortical connections within the right temporal cortex may underlie deficits in social perceptual and cognitive skills in preterm children (see Pavlova & Krageloh-Mann, Citation2013, for a recent review of physiological and brain-imaging studies exploring the visual perception of body motion in children born preterm).
By extending our investigations to include not only SFM processing but also the ability to extract useful information from local motion cues and the skills underlying action recognition and person identification, we hoped to gain further insights into the nature of the biological motion perception problems affecting preterm children. We anticipated that these children would experience problems in multiple areas of biological motion perception and that these problems might be most evident in children with more complex medical histories. Given Pavlova’s earlier findings (see Pavlova, Citation2012), we anticipated that problems with SFM processing, in particular, would be linked to the presence of white matter damage.
Links between Biological Motion Perception and “Autistic-Like” Traits
In typical development, biological motion perception is tightly linked with other social perceptual and cognitive skills and with social competence (see Pavlova, Citation2012). Given this, the second major objective of the present research was to explore the relationship between deficits in biological motion perception and social/behavioral outcomes in preterm children. We expected to find that problems in one or more areas of biological motion perception might be related to the presence of “autistic-like” symptoms in this population. If confirmed, this result would be of interest, given that recent reports suggest that children born prematurely are at heightened risk for screening positive for (Kuban et al., Citation2009; Limperopoulos et al., Citation2008; Moore, Johnson, Hennessy, & Marlow, Citation2012) or being diagnosed with (Johnson et al., Citation2010) an autism spectrum disorder. Indeed, among children born weighing < 2000 g, the prevalence of autism spectrum disorder is 5%—a value approximately five times higher than that seen in the general population (Pinto-Martin et al., Citation2011). It has been argued, however, that the “autistic phenotype” of preterm children represents a milder form of the disorder seen in full-term children (Indredavik, Vik, Skranes, & Brubakk, Citation2008) and reflects the effects of brain injuries and altered neurodevelopment associated with very premature birth (Johnson & Marlow, Citation2011; Limperopoulos, Citation2009). In particular, Movsas et al. (Citation2013) have shown that in low-birth-weight children the risk of screening positive for, or being diagnosed with, an autism spectrum disorder is related to white matter injury but not to isolated germinal matrix/intraventricular hemorrhage (another common type of brain injury affecting premature infants; Volpe, Citation1995). Given that reports have independently linked both impairments in biological motion perception (Pavlova, Citation2012) and “autistic-like” symptoms (Movsas et al., Citation2013) to white matter pathology in preterm children, we expected to find an association between these problems in this high-risk population.
We were also curious to know whether links between deficits in biological motion perception and “autistic-like” symptoms in preterm children would be stronger for some operations than for others. In recent work, Parron et al. (Citation2008) reported that the ability to extract emotion from whole-body point-light displays is impaired in children with autism, even though their ability to recognize specific actions and subjective states (such as feeling itchy or cold) from these displays appears to be intact. Learning more about which aspect(s) of biological motion perception are impaired in preterm children will not only provide important insights into the typical and atypical development of the social brain but may also provide useful information for those involved in designing diagnostic tests and intervention strategies for use with this specific population.
METHODS
Participants
Children aged 8–11 years were recruited for this study. In total, data were collected from 33 children born at VLBW and 35 age-matched controls born at term. For demographic information regarding participating children, please see .
Table 1 Demographic and Screening Measures for the Full-Term and Preterm Samples.
VLBW children were recruited through the High-Risk Newborn Follow-Up Programs at Children’s Hospital and at St. Boniface Hospital (both in Winnipeg, Manitoba). With parental consent, the following medical variables were extracted for each child from neonatal medical records: birth weight, gestational age, Apgar score (Apgar, Citation1953) at 5 minutes, duration of mechanical ventilation (days), days on supplemental oxygen, length of hospital stay (days), information regarding the presence/severity of bronchopulmonary dysplasia and retinopathy of prematurity, and results of neonatal cranial ultrasound and any other available brain imaging. Preterm children were excluded if they (a) suffered from major sensory impairment (e.g., blindness or deafness) and/or (b) had undergone ventriculo-peritoneal shunting for posthemorrhagic hydrocephalus. Information relating to neonatal medical variables is provided in . By parent report, one of the preterm children had attention deficit/hyperactivity disorder (ADHD), two were experiencing difficulties in reading, spelling and/or math, and two had mild spastic diplegia.
Table 2 Medical Variables Describing the Preterm Sample.
Full-term children were recruited through elementary schools and the community, as well as through word-of-mouth. They had to have been born within two weeks of their expected due date, at an appropriate size for their gestational age, and without medical complication. Two parents reported that their children had been diagnosed with attention problems/ADHD, one noted a history of reading difficulties, and two reported that their children were receiving counseling or school psychology services related to anxiety.
General Procedure
Each child was tested individually. Before testing began, information about the procedures was presented and explained, consent for participation was obtained from a parent/guardian, and verbal assent was obtained from each child. Parents completed two questionnaires while their children completed a set of screening tests and the biological motion test battery (see below), along with several other tests used in a separate investigation. All testing was completed in a single test session. The Human Research Ethics Board at the University of Manitoba approved the research protocol.
Demographic and Screening Measures
General Information Questionnaire
A questionnaire regarding family demographics and the participating child’s early development was completed by a parent (or legal guardian). Information collected in this questionnaire included parental education and family income—two variables that have been linked to cognitive development in preterm children (Braid, Donohue, & Strobino, Citation2012; Sommerfelt, Ellertsen, & Markestad, Citation1995; Voss, Jungmann, Wachtendorf, & Neubauer, Citation2012).
Visual Screening
Three visual screening measures were administered to all participating children: (a) linear acuity was measured with the Lighthouse Distance Acuity Chart (Lighthouse International, New York, NY, USA), (b) binocular fusion was measured with the Worth 4 Dot Test (Richmond Products, Albuquerque, NM, USA), and (c) stereoacuity was measured with the Titmus Test of Stereoacuity (Stereo Optical Company, Chicago, IL, USA). Standard administration and scoring procedures were used (see Kniestedt & Stamper, Citation2003; Taylor et al., Citation2009).
Letter Cancellation Task (Geldmacher, Citation1996)
A letter cancellation task was used to provide a measure of visual attention and processing speed. In this task, the child was presented with an array of 100 letters printed on an 8.5” × 11” sheet of white paper and was asked to scan the letters and to cross out all 20 instances of the target letter “X” quickly and accurately. A processing efficiency score was computed by dividing the number of correctly identified targets by the time taken to complete the task (in seconds); higher values of this measure represent better processing efficiency.
Intellectual Screening
To estimate verbal intelligence we used the Peabody Picture Vocabulary Test, fourth edition (PPVT-4; Dunn & Dunn, Citation2007). Scores on this test are highly correlated with scores on the third edition of the task, which has been shown to be an excellent predictor of Full-Scale IQ (r = .90), Verbal IQ (r = .91), and Performance IQ (r =.82) as measured by the Wechsler Intelligence Scale for Children, third edition (Dunn & Dunn, Citation2007; Wechsler, Citation1991). On each trial of the PPVT-4, the child matches a word spoken by the examiner to one of four pictures. The test is comprised of sets of 12 words, with words in the test sets becoming progressively more difficult. Basal levels (one or no errors) and ceiling levels (eight or more errors) are found for each child, and performance is expressed as an age-corrected standard score (M = 100, SD = 15). The purpose of including this measure was to reduce the likelihood that any difficulties observed in our experimental tasks could be related to a general cognitive delay or to comprehension difficulties.
Autism Spectrum Quotient-Child Version (AQ)
The AQ is a parent-report measure of autistic-like traits in children ages 4–11 years (Auyeung, Baron-Cohen, Wheelwright, & Allison, Citation2008). It has shown good test-retest reliability (r = 0.85, p < .001) and a high Cronbach’s alpha of 0.97. It is comprised of 50 items relating to five broad areas associated with the broader autism phenotype: social skills, attention switching, attention to detail, communication, and imagination (10 questions each). A 4-point Likert scale is used to assess the extent to which the child displays each trait, with 0 representing definitely agree and 3 representing definitely disagree. A maximum total score of 150 represents full endorsement of all autistic traits sampled.
The traits described in the AQ are ones that are represented in the general population, but it is unusual for a neurotypical individual to display a large number of them. Indeed, cutoff scores have been identified that allow one to classify typically developing individuals and those with Asperger Syndrome/high-functioning autism (AS/HFA) with a high degree of accuracy, validating its use as a screening instrument (Auyeung et al., Citation2008). In the present investigation, we used the cut-score of 66 to identify children at risk; Auyeung et al. report that with this cut-score they were able to accurately identify 98.9% of children with AS/HFA and accurately screen out 90.3% of controls.
Biological Motion Perception Test Battery
Biological motion stimuli were presented on a PC computer, using custom-made software (http://www.biomotionlab.ca). The database includes motion-captured data from 50 male and 50 female walkers (for further information about the data acquisition and the creation of the stimuli, see Troje, Citation2002a). Four tasks were administered in a fixed order: SFM, local motion, action recognition, and identification. For full details on test construction, see Saunders (Citation2011).
Local Motion Task
This task (shown schematically in ) was designed to measure viewers’ sensitivity to local motion cues that signal the direction an animal is facing. On each trial, viewers saw a 15-dot point-light figure, with single dots representing the head, sternum, and center of the pelvis, along with both shoulders, elbows, wrists, hips, knees, and ankles. Observers were asked to indicate the direction into which the figure was facing. The figure had no discernable body structure that could have helped to reveal facing direction, as on each trial the positions of the dots were both horizontally scrambled (i.e., their locations were randomly reassigned, keeping the dot trajectory in the same vertical position but varying its position in the horizontal plane) and temporally scrambled (i.e., presented out-of-phase). The only cues that provide information about the walker’s facing direction are contained in the local motion of individual dots (Hirai, Chang, Saunders, & Troje, Citation2011).
Figure 1 Schematic depictions of the four tasks included in the biological motion perception battery: (a) local motion; (b) structure-from-motion; (c) action recognition; (d) person identification. Modified from Saunders (Citation2011) with permission.
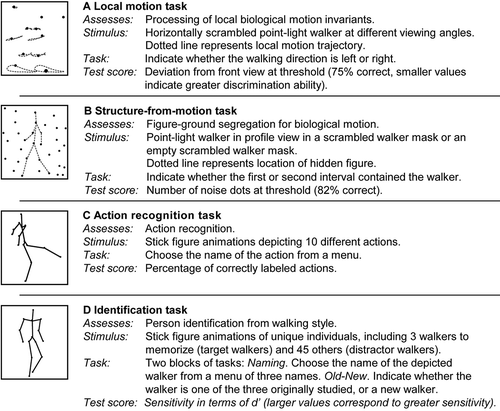
On each of 40 test trials, a scrambled figure was presented at the center of the screen for 1 s, and the child was asked to indicate its facing direction (left or right) quickly and accurately, using a key press. The next trial began 1 s later. Here and in the subsequent tasks, the target figure’s height was four degrees of visual angle, and it was presented on a black background using white dots that subtended 0.1 degrees of visual angle.
The direction the scrambled figure was facing varied between 0 degrees (frontal view) to ± 90 degrees (sagittal view) across trials, as determined by the QUEST method (Watson & Pelli, Citation1983). This is an established staircase procedure that allows one to estimate a perceptual threshold in a relatively small number of trials. This feature makes it useful when testing children and clinical populations, especially in cases where the viewer may have difficulty maintaining attention. In the present task, the threshold was defined as deviation from the frontal view required in order to achieve 75% correct direction discrimination. Figures presented in the sagittal view provide the strongest directional cues. Thus, smaller viewing angles at criterion are indicative of better sensitivity to directional information conveyed by local motion cues.
Structure-from-Motion Task
The SFM task (shown schematically in ) was designed to measure viewers’ ability to detect a point-light walker presented within a mask made from spatially scrambled dots derived from similarly oriented point-light figures. Because the local motion cues in the mask and the walker are identical, viewers must be able to extract motion cues that reveal global body structure in order to detect the target. In other words, good performance on this task requires intact SFM processing.
The target used for this task was an 11-dot point-light figure with single dots representing the head, one shoulder, one hip, and each of the two elbows, wrists, knees, and ankles. The target was shown facing either left or right and walking in place, as if on a treadmill. In each trial, two displays were presented sequentially for 1 s each, with an interstimulus interval of 0.5 s. One contained a masked walker, whose position was randomized within a 5 × 5 degree display window across trials. The other (nontarget) display contained the same number of dots as the target display, but in this case all dots were derived from spatially scrambled walkers. The display that contained the target (first or second), and the target’s facing direction (left or right), were randomized over a total of 40 trials. On each trial, the child’s task was to indicate (with a key press) which of the two displays contained the target; once a response was made, a 1 s interstimulus interval followed after which the next trial began.
The number of masking dots was varied trial by trial based on the QUEST procedure. Because we have previously noted that viewers find the SFM task easier than the local motion task, the threshold was defined as the number of masking dots that could be tolerated while maintaining an 82% correct detection rate (rather than the 75% correct criterion used in the local motion task). Higher dot counts at criterion represent better performance.
Action Recognition Task
The goal of this task (shown schematically in ) was to determine whether viewers could extract information about specific actions from degraded stimuli. In order to unconfound the task from effects of perceptual organization, we used moving stick figures instead of point-light displays. Stick figures displaying 10 different actions were presented, one at a time, in the center of the display. The actions depicted included catching a ball, climbing stairs, jumping, jumping jacks, kicking, lifting, running, sitting, throwing a ball, and walking. Each action was shown from three different viewing angles (0 degrees [frontal view], 30 degrees, and 90 degrees [sagittal view]), for a total of 30 randomly ordered trials. On each trial, the child chose the label that best described the action from a list of 10 descriptors. Stimuli were presented for 1 s, and responses were followed by a 1 s intertrial interval. Proportion correct was the dependent variable.
Identification Task
This test was designed to measure the child’s ability to recognize and name different individuals by their unique walking styles. The task involved a memorization phase, followed by two acquisition blocks, each of which consisted of learning trials (naming) and test trials (old-new discrimination). In the memorization phase, participants were shown three different stick figures, each associated with a unique name (Lee, Joe, Raj). Each was presented for 5 s at 0 degrees (frontal view) and at 45 degrees, for a total of six trials that were separated by intertrial intervals of 1 s. During the naming trials of each acquisition block, the child had to indicate (with a key press) which of the three walkers was being presented (see for a schematic representation of the task). The walker remained on the screen until a button was pressed. Corrective feedback was given on each trial, and the next trial began 1 s later. The child completed 18 naming trials. During each of the 45 old-new discrimination trials comprising each acquisition block, a figure was presented in the center of the screen and the child was asked to indicate (through a button press) if the walker currently being viewed was from the original (memorization) set or was new. Overall, during old-new discrimination testing, half of the walkers were new (with no repeats) and half were from the original set (with each walker presented an equal number of times from each viewing angle). No feedback was given during old-new discrimination testing. Hits and false alarms on the old-new discrimination trials were used to compute d-prime, which provided a measure of the child’s sensitivity to individuals’ idiosyncratic movement styles.
RESULTS
In order to explore age-related changes in performance on the biological motion test battery, we split each sample into subgroups of younger children (8–9 year olds) and older children (10–11 year olds). As can be seen in , the two preterm subgroups were comparable to one another in terms of birth weight, F(1, 31) = 0.28, p = .60, and gestational age, F(1, 31) = 0.001, p = .98. Within an age category, full-term and preterm children were matched for age: 8- to 9-year-olds: F(1, 29) = 0.32, p = .58; 10- to 11-year-olds: F(1,35) = 0.90, p = .35. The four subgroups of children were well matched on the remaining demographic and visual screening measures, χ2(3) = 4.88, p > .18 in all cases. Their mean PPVT-4 scores were also comparable, F(3, 64) = 1.94, p = .13; moreover, with the exception of 1 preterm child who obtained a score of 78, all participants scored within normal limits on this test (range = 89–141). The only screening measure in which significant group differences in performance were observed was the Cancellation task, F(3, 64) = 4.36, p = .007. Although preterm and full-term children within an age group obtained similar processing efficiency scores in this task, older full-term children showed significantly greater processing efficiency than younger full-term or preterm children (p < .05 for both pairwise comparisons). To control for individual differences in processing efficiency, this variable was included as a covariate in all subsequent analyses.
It is important to note that, in each sample, the majority of the children were right handed, came from families in the higher income brackets and had relatively well-educated parents. These factors precluded the possibility of carrying out supplementary analyses involving these variables and may limit the generalizability of some of the findings. In addition, preliminary analyses confirmed that neither verbal intelligence nor gender played a role in performance in any of the tasks in the biological motion test battery. For this reason, neither of these variables was included in subsequent analyses.
Biological Motion Perception Test Battery
As noted above, we computed thresholds for discerning facing direction in the local motion task (left/right deviation from the frontal view needed to achieve 75% correct discrimination of facing direction), thresholds for detecting biological motion in noise in the SFM task (number of masking dots to achieve 82% correct detection), accuracy (% correct) in the action identification task, and sensitivity for individual walkers (d-prime) in the old-new discrimination trials of the identification task. Data from each task were entered into a 2 (Group: Full-term, Preterm) × 2 (Age Category: 8–9 years old, 10–11 years old) analysis of covariance (ANCOVA), controlling for processing efficiency. Note that, due to a computer error, data for the identification task were not collected for one 10-year-old, full-term child.
One complication that arose was that children found the local motion task quite challenging. Indeed, only 53% of the study participants were able to achieve threshold levels of performance. Importantly, however, the proportion of children who were able to achieve this threshold was similar in all four subgroups: Full-term8–9 years = 53%; Full-term10–11 years = 50%; Preterm8–9 years = 50%; Preterm10–11years = 59%; χ2(3) = 0.36, p = .95. As all analyses involving this task were based only on data obtained from those children who met this performance criterion, we assessed whether these subgroups were well matched on the demographic and visual screening measures and in terms of estimated verbal intelligence, before conducting any further tests. This was indeed the case (all comparisons were nonsignificant; data not shown).
A significant main effect of Group was seen in the ANCOVAs carried out on the data from the local motion task, F(1, 31) = 4.41, p = .044, η2 = .124, the SFM task, F(1, 63) = 4.64, p = .035, η2 = .07, and the action recognition task, F(1, 63) = 5.07, p = .028, η2 = .075 (see -). Preterm children needed stronger directional cues than full-term children (i.e., larger deviation from frontal view) to discern the direction that scrambled figures were facing. They also had more difficulty than full-term peers detecting the presence of a masked point-light walker (i.e., they required lower levels of mask density in order to achieve the 82% correct detection threshold). Finally, they were less accurate than full-term controls at identifying the actions of moving stick figures. Together, these findings suggest problems with the analysis of local biological motion cues, with SFM processing, and with the ability to extract invariants from available structural and kinematic cues that specify particular actions. Neither the main effect of Age Category nor the Group × Age Category interaction were significant in any of these three analyses, suggesting that performance on these tasks did not improve with age in either preterm or full-term children.
Figure 2 Differences in the performance of preterm and full-term children on the four tasks included in the biological motion perception battery. Preterm children performed worse than full-term children on the local motion task (Panel A), the structure-from-motion task (SFM; Panel B), and the action recognition task (Panel C) (* p < .05 in all cases). Age-related improvement on these tasks was not evident in either group. As can be seen in Panel D, only full-term children showed age-related improvement on the identification task (** p = .002), resulting in a significant group difference in performance in the older age category (*** p = .001).
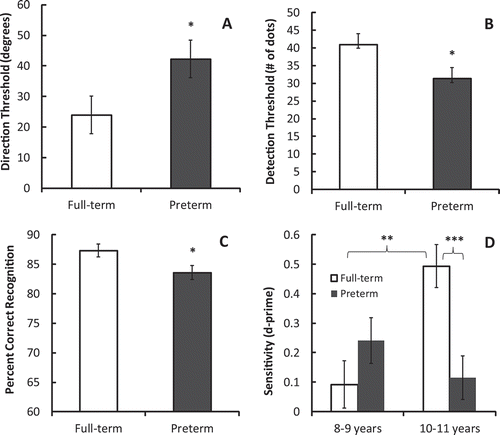
The only significant result in the ANCOVA conducted on data from the identification task was a significant Group × Age Category interaction, F(1, 62) = 12.31, p = .001, η2 = .17 (see ). Follow-up tests of simple main effects showed that only in the full-term samples did sensitivity to the identities of individual walkers show age-related improvement (p = .002). This resulted in a group difference in the older age category, with full-term children outperforming preterm children (p = .001). The partial correlation between age (in months) and sensitivity was significant in full-term children, r(31) = .66, p < .001, but not in preterm children, r(30) = −.10, p = .30; the difference in the strength of these two correlations was significant, Z = 3.49, p < .001.
Finally, we assessed intercorrelations between scores on the different tests in the battery, using a series of two-tailed Pearson correlation tests. Consistent with earlier findings from typical young adults (Saunders, Citation2011), none of the correlations were significant—either in the full sample, or in analyses performed on data from the preterm and full-term groups separately (p > .10 in all cases). These findings suggest that the tests measure relatively independent abilities.
Relationships between Performance on Tests of Biological Motion Perception and AQ Scores
As we have previously reported (Williamson & Jakobson, Citation2014a, Citation2014b), preterm children in our sample were rated by their parents as showing more autistic-like traits than full-term peers, F(1, 66) = 5.22, p = .03, η2 = .07; Mpreterm = 59.8, SEM = 2.2, range = 32–89; Mfull-term = 52.7, SEM = 2.1, range = 37–78. There was also a trend for more preterm than full-term children to score above the cutoff of 66 (11 vs. 5, respectively), Z = –1.84, p = .066. Despite this, the mean AQ total score for the preterm group was still well below the mean value reported by Auyeung et al. (Citation2008) for children with AS/HFA (M = 104.8, SEM = 0.84); this was true even in those preterm children who scored above the cut-score and might, therefore, be considered at risk (M = 75.9, SEM = 2.33).
To explore relationships between the presence of autistic-like traits and biological motion perception skills, we computed partial correlations between AQ total scores and performance on each of the four tests in our battery. Additional, exploratory analyses assessed the relationship between scores on each test of biological motion perception and scores on the fives subscales of the AQ (Social Skills, Attention Switching, Attention to Detail, Communication, and Imagination). In all cases, we controlled for processing efficiency. One-tailed tests were used, due to our expectation that the children who experienced problems with biological motion perception would also be those who showed a larger number of autistic-like traits. Due to the relatively small sample sizes and the number of correlations that were run, we urge caution in interpreting these findings.
As can be seen in , in both groups performance on the SFM task was related to the presence of autistic-like traits. In the full-term sample, detection thresholds on the SFM task were significantly correlated with scores on the Attention to Detail subscale of the AQ. As sensitivity to global SFM cues went down, parents were more likely to report that their children showed a strong interest in, and good memory for, details such as numbers and dates, and that their tendency to focus on details sometimes caused them to miss the “big picture.” In the preterm sample, performance on the SFM task was negatively correlated with AQ total scores (see , Panel A) and scores on the Communication subscale. Thus, as sensitivity went down, parents reported more autistic-like symptoms overall and more problems following conversational “rules” (taking turns, using verbal and nonverbal signals etc.) in particular.
Table 3 Correlations Between Performance on Tests of Biological Motion Perception and Autistic-Like Traits in Full-Term and Preterm Children.
Figure 3 Scatterplots showing the correlations between AQ Total Scores and detection thresholds on the SFM task (Panel A), and between AQ Total Scores and d-prime scores on the Identity task (Panel B), in preterm and full-term children.
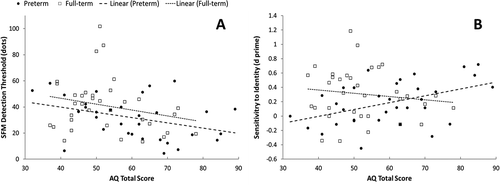
Performance in the identification task was also related to the presence of autistic-like traits, but only in the preterm sample. Unexpectedly, preterm children who were more sensitive to people’s idiosyncratic movement styles displayed a larger number of autistic-like traits overall (see , Panel B) and more problems on the Social Skills and Attention Switching subscales in particular. These associations remained significant even when we controlled for estimated intelligence (in addition to processing efficiency). High scores on the Social Skills scale reflect limited interest in/enjoyment of social situations, difficulties making new friends, and “reading” other people’s feelings or intentions. High scores on the Attention Switching scale reflect problems transitioning between activities, dealing with interruptions, or following conversations involving several people.
Given that, in the preterm sample, scores on the SFM and identification tasks were not correlated with one another but both were related to AQ total scores, we conducted a stepwise linear regression to determine which variable(s) explained the distribution of AQ total scores best. In the final model, Adjusted R2 = .299, F(1, 30) = 6.15, p = .019, both d-prime scores in the identification task (β = .46, p = .004) and detection thresholds in the SFM task (β = −.37, p = .019) were significant predictors, accounting for 20.8% and 13.5% of the variance in AQ total scores, respectively. Processing efficiency was not a significant predictor in this analysis.
Medical Variables Associated with Performance on Tests of Biological Motion Perception
To investigate relationships between medical risk and the ability to process biological motion in preterm children, we first computed correlations between performance measures on the biological motion tasks and each of the continuous variables extracted from the children’s medical records (birth weight, gestational age, Apgar score at 5 minutes, days on ventilation, days on supplemental oxygen, and length of hospital stay). All correlations were two tailed, and controlled for processing efficiency. Again, given the small sample size and the large number of analyses that were run, these results should be interpreted with caution. Having said that, as can be seen in , within this sample of very prematurely born children, sensitivity to SFM cues tended to be higher in children with heavier birth weights and in those who spent fewer days on oxygen (p < .10 in both cases). In the action recognition task, worse performance was seen in children who had spent more days on oxygen, r(30) = −.36, p < .05, and similar trends were seen for children who spent more days on ventilation and those who had longer hospital stays (p < .10 in both cases).
Table 4 Correlations Between Biological Motion Perception and Medical Variables in Preterm Children.
For two of the remaining medical variables (presence/absence of bronchopulmonary dysplasia at 28 days and retinopathy of prematurity), we compared scores on the biological motion tasks for children with and without complications using separate one-way ANCOVAs that controlled for processing efficiency. No significant results were found in these analyses. To explore the relationship between abnormalities detected in neonatal cranial ultrasound scans and task performance, we subdivided preterm children into three groups. Children in Group 1 (n = 16) had no discernible abnormality. Those in Group 2 (n = 10) had a subependymal cyst or an isolated intraventricular hemorrhage. Children in Group 3 (n = 7) showed signs of ventriculomegaly or periventricular echogenicity (with or without associated intraventricular hemorrhage) or were diagnosed with PVL. These groupings were chosen because, in preterm/low-birth-weight children, problems with both biological motion perception (Pavlova et al., Citation2003; see also Pavlova, Citation2012, and Pavlova & Krageloh-Mann, Citation2013) and the risk of screening positive for, or being diagnosed with, an autism spectrum disorder (Movsas et al., Citation2013) have been linked with white matter damage, specifically. We used Kruskall-Wallis tests to compare performance across the groups. On the SFM task, we observed a trend, χ2(2) = 5.05, p = .08, for children in Group 3 to perform worse than those in Groups 1 or 2 (MGroup 1 = 34.9 dots, SD = 13.9; MGroup 2 = 35.9 dots, SD = 16.3; MGroup 3 = 19.4 dots, SD = 14.8). In addition, both groups with atypical findings performed worse than children in Group 1 on the action recognition task, χ2(2) = 7.72, p = .02 (MGroup 1 = 86.8% correct, SD = 5.1; MGroup 2 = 79.3% correct, SD = 9.9; MGroup 3 = 81.4% correct, SD = 6.3). Group comparisons on the local motion and identification tasks were not significant.
DISCUSSION
In agreement with earlier observations (Pavlova et al., Citation2003, Citation2005; Pavlova, Sokolov, et al., Citation2006, Citation2008; Taylor et al., Citation2009), we found that children born prematurely at VLBW exhibited problems on tests of biological motion perception that require the extraction of motion-mediated body structure. We extended this finding by demonstrating a link between these problems and the presence of autistic-like traits. In addition, we went on to show that the difficulties preterm children experience with biological motion perception are not restricted to perceptually organizing motion cues but also affect the analysis of local motion cues and operations underlying action recognition and person identification. Evidence that these four operations are carried out relatively independently came from the observations that intercorrelations between scores on the different tests in the battery were not significant. The fact that the same preterm children tested here also show impairments on tasks requiring the analysis and interpretation of “animate motion” displays involving moving triangles (Williamson & Jakobson, Citation2014b) and emotions conveyed by nonverbal cues from face and body movements (Williamson & Jakobson, Citation2014a) supports the view that they experience generalized difficulties processing movements characteristic of living entities.
Although (by parent report) none of the preterm children had received an autism spectrum disorder diagnosis, one third of our relatively high-functioning sample scored above a cut-score that has shown excellent sensitivity and specificity for autism spectrum disorders. This is worth reporting as it suggests that they were showing symptoms that may be of clinical concern. The fact that even the mean score of this at-risk group was still below that of children with a formal diagnosis of AS/HFA fits with other suggestions that the “preterm autistic phenotype” represents a milder form of the disorder (Indredavik et al., Citation2008).
Our preterm and full-term samples were well matched on several key demographic variables (parental education, family income) and with respect to gender, handedness, and estimated intelligence, making it unlikely that the difficulties preterm children experienced on the tests of biological motion perception were related to these variables. Other work suggests that preterm children are at increased risk for problems related to reduced processing speed (e.g., Mulder, Pitchford, & Marlow, Citation2010), attention (e.g., Anderson et al., Citation2011), and visual, perceptual and spatial working memory (Saavalainen et al., Citation2007; Taylor, Klein, Hack, & Hack, Citation2000; Waber & McCormick, Citation1995). We feel fairly confident that the difficulties we observed in biological motion perception could not be fully accounted for by problems in these areas, given that the groups performed similarly on the cancellation task (which requires an individual to keep a target in mind while quickly searching for instances of it within a cluttered visual display), and our analyses controlled for individual differences in processing efficiency on this task. Additional support for the view that problems with SFM processing, at least, cannot be fully accounted for by attentional problems comes from work showing that very preterm children experience more difficulty than full-term peers detecting consecutive trials in which globally coherent, but not scrambled, walkers are presented—even though the attention demands are similar for both types of stimuli (Pavlova et al., Citation2005; Pavlova, Marconato, et al., Citation2006). In addition to the above, we should note that, although parents reported that 1 preterm child and 2 full-term children had an ADHD diagnosis, examination of the data confirmed that these children’s performance was not generally worse than that of their peers. Indeed, all 3 children performed within two standard deviations of the mean on all tests, with the exception of 1 full-term child who performed particularly well on the SFM task, and another full-term child who performed poorly on the action recognition task.
The problems with local motion perception we documented in preterm children are of interest given recent work showing that (a) full-term newborns prefer biological motion sequences to rigid nonbiological motion, even if the biological motion has been spatially scrambled (Bardi, Regolin, & Simion, Citation2011) and (b) visually naïve chicks reared and hatched in darkness prefer displays depicting both coherent and scrambled biological motion over those depicting rigidly rotating hens or randomly moving dots (Vallortigara, Regolin, & Marconato, Citation2005). The preference for scrambled biological motion in these studies suggests an innate sensitivity to invariants contained in the local motion. Such sensitivity makes sense from an evolutionary perspective, as it could allow us to orient towards and distinguish a moving animal from its environment quickly without undertaking a detailed analysis of its shape (Thompson et al., Citation2007; Troje & Chang, Citation2013; Troje & Westhoff, Citation2006). In future work, it would be interesting to assess sensitivity to these cues in preterm infants, as problems in this area could be an important early marker for problems with social attention and perception.
At this point, we cannot rule out that problems with figure-ground segregation may have contributed to preterm children’s poor performance on the SFM task, especially given previous reports of impairments in this ability in this population (Amicuzii et al., Citation2006; Davis, Burns, Wilkerson, & Steichen, Citation2005; Hård et al., Citation2000). However, it is important to note that figure-ground segregation was not required in the local motion, action recognition, or identification tasks, in which preterm children also experienced difficulties. Nor was it required in the one-back repetition task Pavlova, Marconato, et al. (Citation2006) used to explore preterm children’s ability to detect consecutive trials containing coherent point-light walkers. Together, these observations suggest that difficulties extracting motion-mediated cues to global body structure are more likely to account for problems on the SFM task than problems with figure-ground segregation.
Both local motion invariants and global (configural) motion cues convey semantic, socially relevant information that contributes to our ability to identify particular actions and stylistic differences between individuals. In the present study, a solitary (unmasked) stick figure was used in both the action recognition and the identification tasks. Observers were not required to perceptually organize the dots into a coherent articulated shape (as would be required with a point-light figure). The fact that preterm children showed impairments on these two tasks suggests they have difficulty detecting invariants that define particular actions, and performing the type of pattern recognition needed to retrieve specific information about the identity of the agent. However, while these skills are essential for successful performance in these tasks, the identification task also involves visual learning and memory. It is possible that problems in this area may have contributed to poor performance on this task above and beyond any problems associated with pattern recognition. Interestingly, Giménez et al. (Citation2005) have shown that, compared to controls, adolescents born very prematurely exhibit differential brain activation during the encoding phase of a face-name paired-associate learning task. Moreover, Narberhaus et al. (Citation2009) have found that, although both groups were matched in terms of task performance, adults born very prematurely and controls exhibited different patterns of brain activation during both the encoding and retrieval phases of a visual paired-associate learning task. Together these observations suggest that, in many children born very prematurely, disruptions to the development or functioning of systems normally supporting visual learning and memory may lead to neural compensation.
One question that remains is whether preterm children improve or, indeed, “catch-up” to their peers in their ability to process biological motion over time. It seems unlikely that this would be the case, as age-related improvement was not evident in the present sample of preterm children on any of the four tasks. This result complements findings from an earlier study from our laboratory (Downie et al., Citation2003) in which we showed that deficits in the perception of two-dimensional motion-defined forms persist at least into early adolescence in children born at extremely low birth weight (< 1000 g). As persisting impairments in biological motion perception may have important implications for the development of a range of higher order social skills, studying how preterm children and adolescents process and utilize these social cues in everyday situations may help to explain some of the social difficulties they are known to be at increased risk for (see also, Williamson & Jakobson, Citation2014a, Citation2014b). Indeed, preterm children in our sample who displayed more difficulties on the SFM task also tended to score higher on the AQ—a parent-report measure designed to quantify autistic traits in the general population, including those associated with impaired social functioning (see Koldewyn, Whitney, & Rivera, Citation2010, for a similar observation in children with autism). In the full-term sample, detection thresholds on this task were negatively correlated with scores on the Attention to Detail subscale, specifically; this subscale assesses traits linked to “weak central coherence”—a processing style that is biased towards local rather than global information processing (Happé, Citation1999; Happe & Frith, Citation1996).
Interestingly, performance on the identification task was a significant, independent predictor of AQ total scores in preterm children. The fact that preterm children who were more sensitive to people’s idiosyncratic movement styles displayed a larger number of autistic-like traits overall, and more problems on the Social Skills and Attention Switching subscales in particular, was unexpected. Given that the Social Skills subscale includes items that tap into children’s ability to “read” other people’s feelings or intentions, it is possible that the association we observed arose because, in our preterm sample, a relative strength in identity processing was often associated with a weakness in the ability to use nonverbal cues to extract information about an individual’s emotional state, or in other aspects of mentalizing. This seems unlikely given that, in typical adults, the ability to extract the identity of a point-light walker is not correlated with the ability to extract information about a walker’s emotional state (see Saunders, Citation2011). Nonetheless, it would be interesting to explore this possibility. Other research suggests that individuals on the autism spectrum have difficulty recognizing emotions in point-light displays, even when simple actions can be successfully identified (Atkinson, Citation2009; Hubert et al., Citation2007; Moore, Hobson, & Lee, Citation1997; Parron et al., Citation2008).
Another possible explanation for the relationship we observed between performance on the identification task and AQ scores is that it was mediated by emotional problems (such as anxiety or depression) or executive deficits that are not adequately sampled in the AQ but can be elevated in preterm children (e.g., Aarnoudse-Moens, Weisglas-Kuperus, van Goudoever, & Oosterlaan, Citation2009) and in individuals with high-functioning autism or Asperger Syndrome (e.g., Rinehart, Bradshaw, Brereton, & Tonge, Citation2002; Tantam, Citation2000). A substantial body of research suggests that mood can influence how we attend to (e.g., von Hecker & Meiser, Citation2005), process (e.g., Kuhbander et al., Citation2009), interpret (e.g., Bouhuys, Bloem, & Groothuis, Citation1995), and remember (e.g., Eich, Citation1995) different kinds of information. Moreover, recent work suggests links between mood disorders and certain aspects of biological motion perception, specifically (e.g., Kéri & Benedek, Citation2010; Kim et al., Citation2008; Loi, Vaidya, & Paradiso, Citation2013; Van de Cruys, Schouten, & Wagemans, Citation2013). While identity processing was not the focus of these latter studies, these observations do suggest that further research exploring possible relationships between performance on tests of biological motion perception and both emotional and behavioral outcomes in preterm children is warranted. It is possible that children who are socially anxious attend more closely to the movements of others than less-anxious peers; if so, this could influence their ability to encode and recall the characteristic movement styles (i.e., “movement signatures”) of particular individuals.
The results from the present study suggest that, even within a restricted sample of relatively high-functioning preterm children, those with more complicated medical histories are at increased risk for deficits in different aspects of biological motion perception and mild autistic-like symptoms. At this point, it is unclear how atypical neurodevelopment and/or early brain damage contribute to the difficulties we observed, although it seems likely that atypical development of white matter connections between regions in the social brain play an important role—particularly in processing SFM cues (see also Pavlova, Citation2012; Pavlova & Krageloh-Mann, Citation2013; Pavlova et al., Citation2003). How/if functional or structural problems in other parts of the social brain contribute to higher order problems with action recognition and with learning to recognize specific individuals on the basis of their unique movement styles remains to be seen. To address these questions, it will be important to incorporate more sophisticated brain-imagining techniques than those used here. For the majority of preterm children who participated in the present study, the available brain-imaging data consisted of results from routine, neonatal cranial ultrasound scans that are not detailed enough to reveal subtle disruptions in brain structure (Ramenghi et al., Citation2010) or capable of revealing functional impairments.
Past research has linked the ability to process biological motion with the ability to imitate seen movements (e.g., Bouquet, Gaurier, Shipley, Toussaint, & Blandin, Citation2007). Moreover, it has been suggested that the superior temporal sulcus—a region implicated in biological motion perception—plays an active role in “representing visuomotor correspondences between one’s own actions and the actions of others” (Molenberghs, Brander, Mattingley, & Cunnington, Citation2010, p. 1316). Exploring the effect(s) that problems with biological motion perception may have on the development of preterm children’s imitative skills (an important component of observational learning) would be an interesting avenue for future research.
Limitations
As noted earlier, our preterm sample was small and relatively homogeneous in the sense that most of the children were right handed, came from families in the higher income brackets and had relatively well-educated parents. They had also all escaped major intellectual disabilities. These factors may limit the generalizability of some of the findings.
A positive feature of our local motion task is that the stimuli were created using motion capture technology and, as such, the local motion cues are more naturalistic than those present in displays created using computer algorithms. Indeed, algorithms such as that originally employed by Cutting (Citation1978) have been shown to lack invariants in the motion of the feet that are present in real walkers (Saunders, Suchan, & Troje, Citation2009) and that supply critically important information for making facing judgments (Troje & Westhoff, Citation2006). Unfortunately, our local motion task proved to be too difficult for many of the participating children, making it impossible for them to achieve threshold levels of accuracy. By limiting our analyses to data from children who reached criterion, our ability to explore relationships between task performance and other measures was limited.
We did not incorporate measures that would allow us to determine if/how problems with executive function, which affect many children born prematurely (e.g., Aarnoudse-Moens et al., Citation2009) may have contributed to difficulties in task performance. Deficits in both executive function and social perception/cognition are often seen in individuals with autism spectrum disorders (e.g., Ozonoff, Pennington, & Rogers, Citation1991), but whether these deficits are linked or simply co-occur in this population is not clear. Interestingly, work with typical children suggests that performance on social attribution tasks is not related to performance on tests of executive function, after controlling for age and verbal IQ (Hu, Chan, & McAlonan, Citation2010).
We controlled for individual differences in visual attention and processing speed by including processing efficiency as a covariate in our analyses. The cancellation task we used to assess processing efficiency (from Geldmacher, Citation1996) had a target-to-distractor ratio of 1:4. In his work with adults, Geldmacher has found that performance (errors/s) was worse for displays with a 1:4 ratio than for those with a 1:9 ratio. It is possible that our results would have changed if processing efficiency had been assessed using a different measure, but it is interesting to note that, in the SFM task, the children we tested achieved 82% correct detection when the target-to-noise dot ratio was close to 1:4.
CONCLUSION
The present findings extend previous reports of vulnerability in systems involved in the processing of dynamic cues in children born prematurely at very low birth weight (e.g., Jakobson et al., Citation2006; MacKay et al., Citation2005; Taylor et al., Citation2009). In particular, they contribute to the literature by demonstrating that preterm birth and associated complications put children at risk for problems with multiple aspects of biological motion processing and that difficulties in this area of social perception are related to the presence of autistic-like traits. The fact that many of the preterm children in our relatively high-functioning sample were rated as showing social perceptual/cognitive problems that may be of clinical concern is important to note as it suggests the need for increased screening and follow-up of this high-risk group.
The ultimate goals of this research are to increase our understanding of the long-term consequences of prematurity and to gain new insights into the typical and atypical development of the social brain. These advances, in turn, may help to shape the development of more effective screening tools and clinical interventions for use with this and other clinical populations.
Additional information
Funding
REFERENCES
- Aarnoudse-Moens, C. S. H., Weisglas-Kuperus, N., van Goudoever, J. B., & Oosterlaan, J. (2009). Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics, 124, 717–728. doi:10.1542/peds.2008-2816
- Abell, F., Happé, F., & Frith, U. (2000). Do triangles play tricks? Attribution of mental states to animated shapes in normal and abnormal development. Cognitive Development, 15, 1–16. doi:10.1016/S0885-2014(00)00014-9
- Amicuzii, I., Stortini, M., Petrarca, M., Di Giulio, P., Di Rosa, G., Fariello, G., … Castelli, E. (2006). Visual recognition and visually guided action after early bilateral lesion of occipital cortex: A behavioral study of a 4.6-year-old girl. Neurocase, 12, 263–279. doi:10.1080/13554790601026106
- Anderson, P. J., De Luca, C. R., Hutchinson, E., Spencer-Smith, M. M., Roberts, G., Doyle, L. W., & Victorian Infant Collaborative Study Group. (2011). Attention problems in a representative sample of extremely preterm/extremely low birth weight children. Developmental Neuropsychology, 36, 57–73. doi:10.1080/87565641.2011.540538
- Apgar, V. (1953). A proposal for a new method of evaluation of the newborn infant. Current Research in Anesthesia and Analgesia, 32, 260–267.
- Atkinson, A. P. (2009). Impaired recognition of emotions from body movements is associated with elevated motion coherence thresholds in autism spectrum disorders. Neuropsychologia, 47, 3023–3029. doi:10.1016/j.neuropsychologia.2009.05.019
- Atkinson, J., & Braddick, O. (2007). Visual and visuocognitive development in children born very prematurely. Progress in Brain Research, 164, 123–149. doi:10.1016/S0079-6123(07)64007-2
- Atkinson, J., Braddick, O., Anker, S., Nardini, M., Birtles, D., Rutherford, M. A., … Cowan, F.M. (2008). Cortical vision, MRI and developmental outcome in preterm infants. Archives of Disease in Childhood – Fetal and Neonatal Edition, 93, F292–F297. doi:10.1136/adc.2007.116988
- Auyeung, B., Baron-Cohen, S., Wheelwright, S., & Allison, C. (2008). The autism spectrum quotient: Children’s version (AQ-Child). Journal of Autism and Developmental Disorders, 38, 1230–1240. doi:10.1007/s10803-007-0504-z
- Bardi, L., Regolin, L., & Simion, F. (2011). Biological motion preference in humans at birth: Role of dynamic and configural properties. Developmental Science, 14, 353–359. doi:10.1111/j.1467-7687.2010.00985.x
- Bouhuys, A. L., Bloem, G. M., & Groothuis, T. G. G. (1995). Induction of depressed and elated mood by music influences the perception of facial emotional expressions in healthy subjects. Journal of Affective Disorders, 33, 215–226. doi:10.1016/0165-0327(94)00092-N
- Bouquet, C. A., Gaurier, V., Shipley, T., Toussaint, L., & Blandin, Y. (2007). Influence of the perception of biological or non-biological motion on movement execution. Journal of Sports Sciences, 25, 519–530. doi:10.1080/02640410600946803
- Braid, S., Donohue, P. K., & Strobino, D. M. (2012). The impact of wealth on the cognitive development of children who were preterm infants. Advances in Neonatal Care, 12, 225–231. doi:10.1097/ANC.0b013e3182624636
- Cooke, R. W. I., Foulder-Hughes, L., Newsham, D., & Clarke, D. (2004). Ophthalmic impairment at 7 years of age in children born very preterm. Archives of Disease in Childhood-Fetal and Neonatal Edition, 89, F249–F253. doi:10.1136/adc.2002.023374
- Cutting, J. E. (1978). A program to generate synthetic walkers as dynamic point-light displays. Behavior Research Methods and Instrumentation, 10, 91–94. doi:10.3758/BF03205105
- Davis, D. W., Burns, B. M., Wilkerson, S. A., & Steichen, J. J. (2005). Visual perceptual skills in children born with very low birth weights. Journal of Pediatric Health Care, 19, 363–368. doi:10.1016/j.pedhc.2005.06.005
- Downie, A. L. S., Jakobson, L. S., Frisk, V., & Ushycky, I. (2003). Periventricular brain injury, visual motion processing, and reading and spelling abilities in children who were extremely-low-birthweight. Journal of the International Neuropsychological Society, 9, 440–449. doi:10.1017/S1355617703930098
- Dunn, L. M., & Dunn, D. M. (2007). Peabody picture vocabulary test (4th ed.). Bloomington, MN: Pearson Assessments.
- Eich, E. (1995). Searching for mood dependent memory. Psychological Science, 6, 67–75. doi:10.1111/j.1467-9280.1995.tb00309.x
- Geldmacher, D. S. (1996). Effects of stimulus number and target-to-distractor ratio on the performance of random array letter cancellation tasks. Brain and Cognition, 32, 405–415. doi:10.1006/brcg.1996.0073
- Giménez, M., Junqué, C., Vendrell, P., Caldú, X., Narberhaus, A., Bargalló, N., … Mercader, J. M. (2005). Hippocampal functional magnetic resonance imaging during a face-name learning task in adolescents with antecedents of prematurity. NeuroImage, 25, 561–569. doi:10.1016/j.neuroimage.2004.10.046
- Happé, F. (1999). Autism: Cognitive deficit or cognitive style? Trends in Cognitive Sciences, 3, 216–222. doi:10.1016/S1364-6613(99)01318-2
- Happe, F., & Frith, U. (1996). The neuropsychology of autism. Brain, 119, 1377–1400. doi:10.1093/brain/119.4.1377
- Hård, A. L., Niklasson, A., Svensson, E., & Hellström, A. (2000). Visual function in school-aged children born before 29 weeks of gestation: A population-based study. Developmental Medicine & Child Neurology, 42, 100–105. doi:10.1017/S0012162200000207
- Heider, F., & Simmel, M. (1944). An experimental study of apparent behavior. The American Journal of Psychology, 57, 243–259. doi:10.2307/1416950
- Hirai, M., Chang, D. H., Saunders, D. R., & Troje, N. F. (2011). Body configuration modulates the usage of local cues to direction in biological-motion perception. Psychological Science, 22, 1543–1549. doi:10.1177/0956797611417257
- Hu, Z., Chan, R. C., & McAlonan, G. M. (2010). Maturation of social attribution skills in typically developing children: An investigation using the social attribution task. Behavioral and Brain Functions, 6, 10–18. doi:10.1186/1744-9081-6-10
- Hubert, B., Wicker, B., Moore, D. G., Monfardini, E., Duverger, H., Fonséca, D., & Deruelle, C. (2007). Brief report: Recognition of emotional and non-emotional biological motion in individuals with autistic spectrum disorders. Journal of Autism and Developmental Disorders, 37, 1386–1392. doi:10.1007/s10803-006-0275-y
- Indredavik, M. S., Vik, T., Skranes, J., & Brubakk, A. M. (2008). Positive screening results for autism in ex-preterm infants. Pediatrics, 122, 222. doi:10.1542/peds.2008-1044
- Jakobson, L. S., Frisk, V., & Downie, A. L. (2006). Motion-defined form processing in extremely premature children. Neuropsychologia, 44, 1777–1786. doi:10.1016/j.neuropsychologia.2006.03.011
- Jakobson, L. S., Frisk, V., Knight, R. M., Downie, A. L., & Whyte, H. (2001). The relationship between periventricular brain injury and deficits in visual processing among extremely-low-birthweight (< 1000 g) children. Journal of Pediatric Psychology, 26, 503–512.
- Johansson, G. (1973). Visual perception of biological motion and a model for its analysis. Perception & Psychophysics, 14, 201–211. doi:10.3758/BF03212378
- Johansson, G. (1976). Spatio-temporal differentiation and integration in visual motion perception. Psychological Research, 38, 379–393. doi:10.1007/BF00309043
- Johnson, S., Hollis, C., Kochhar, P., Hennessy, E., Wolke, D., & Marlow, N. (2010). Autism spectrum disorders in extremely preterm children. The Journal of Pediatrics, 156, 525–531.e2. doi:10.1016/j.jpeds.2009.10.041
- Johnson, S., & Marlow, N. (2011). Preterm birth and childhood psychiatric disorders. Pediatric Research, 69, 11R–8R. doi:10.1203/PDR.0b013e318212faa0
- Kéri, S., & Benedek, G. (2010). The perception of biological and mechanical motion in female fragile X premutation carriers. Brain and Cognition, 72, 197–201. doi:10.1016/j.bandc.2009.08.010
- Kim, J., Blake, R., Park, S., Shin, Y. W., Kang, D. H., & Kwon, J. S. (2008). Selective impairment in visual perception of biological motion in obsessive-compulsive disorder. Depression and Anxiety, 25, E15–25. doi:10.1002/da.20402
- Kniestedt, C., & Stamper, R. L. (2003). Visual acuity and its measurement. Ophthalmology Clinics of North America, 16, 155–170. doi:10.1016/S0896-1549(03)00013-0
- Koldewyn, K., Whitney, D., & Rivera, S. M. (2010). The psychophysics of visual motion and global form processing in autism. Brain, 133, 599–610. doi:10.1093/brain/awp272
- Kuban, K. C. K., O’Shea, M., Allred, E. N., Tager-Flusberg, H., Goldstein, D. J., & Leviton, A. (2009). Positive screening on the modified checklist for autism in toddlers (M-CHAT) in extremely low gestational age newborns. The Journal of Pediatrics, 154, 535–540. doi:10.1016/j.jpeds.2008.10.011
- Kuhbander, C., Hanslmayr, S., Maier, M. A., Pekrun, R., Spitzer, B., Pastotter, B., & Bauml, K.-H. (2009). Effects of mood on the speed of conscious perception: Behavioral and electrophysiological evidence. Social Cognitive and Affective Neuroscience, 4, 278–285.
- Limperopoulos, C. (2009). Autism spectrum disorders in survivors of extreme prematurity. Clinics in Perinatology, 36, 791–805. doi:10.1016/j.clp.2009.07.010
- Limperopoulos, C., Bassan, H., Sullivan, N. R., Soul, J. S., Robertson, R. L., Moore, M., … du Plessis, A. J. (2008). Positive screening for autism in ex-preterm infants: Prevalence and risk factors. Pediatrics, 121, 758–765. doi:10.1542/peds.2007-2158
- Loi, F., Vaidya, J. G., & Paradiso, S. (2013). Recognition of emotion from body language among patients with unipolar depression. Psychiatry Research, 209, 40–49. doi:10.1016/j.psychres.2013.03.001
- MacKay, T. L., Jakobson, L. S., Ellemberg, D., Lewis, T. L., Maurer, D., & Casiro, O. (2005). Deficits in the processing of local and global motion in very low birthweight children. Neuropsychologia, 43, 1738–1748. doi:10.1016/j.neuropsychologia.2005.02.008
- Magill-Evans, J., Koning, C., Cameron-Sadava, A., & Manyk, K. (1995). The child and adolescent social perception measure. Journal of Nonverbal Behavior, 19, 151–169. doi:10.1007/BF02175502
- Molenberghs, P., Brander, C., Mattingley, J. B., & Cunnington, R. (2010). The role of the superior temporal sulcus and the mirror neuron system in imitation. Human Brain Mapping, 31, 1316–1326. doi:10.1002/hbm.20938
- Moore, D. G., Hobson, R. P., & Lee, A. (1997). Components of person perception: An investigation with autistic, non-autistic retarded and typically developing children and adolescents. British Journal of Developmental Psychology, 15, 401–423. doi:10.1111/j.2044-835X.1997.tb00738.x
- Moore, T., Johnson, S., Hennessy, E., & Marlow, N. (2012). Screening for autism in extremely preterm infants: Problems in interpretation. Developmental Medicine and Child Neurology, 54, 514–520. doi:10.1111/j.1469-8749.2012.04265.x
- Movsas, T. Z., Pinto-Martin, J. A., Whitaker, A. H., Feldman, J. F., Lorenz, J. M., Korzeniewski, S. J., … Paneth, N. (2013). Autism spectrum disorder is associated with ventricular enlargement in a low birth weight population. The Journal of Pediatrics, 163, 73–78. doi:10.1016/j.jpeds.2012.12.084
- Mulder, H., Pitchford, N. J., & Marlow, N. (2010). Processing speed and working memory underlie academic attainment in very preterm children. Archives of Disease in Childhood - Fetal and Neonatal Edition, 95, F267–F272. doi:10.1136/adc.2009.167965
- Narberhaus, A., Lawrence, E., Allin, M. P., Walshe, M., McGuire, P., Rifkin, L., … Nosarti, C. (2009). Neural substrates of visual paired associates in young adults with a history of very preterm birth: Alterations in fronto-parieto-occipital networks and caudate nucleus. NeuroImage, 47, 1884–1893. doi:10.1016/j.neuroimage.2009.04.036
- Ozonoff, S., Pennington, B. F., & Rogers, S. J. (1991). Executive function deficits in high-functioning autistic individuals: Relationship to theory of mind. Journal of Child Psychology and Psychiatry, 32, 1081–1105. doi:10.1111/j.1469-7610.1991.tb00351.x
- Parron, C., Da Fonseca, D., Santos, A., Moore, D. G., Monfardini, E., & Deruelle, C. (2008). Recognition of biological motion in children with autistic spectrum disorders. Autism, 12, 261–274. doi:10.1177/1362361307089520
- Pavlova, M., Bidet-Ildei, C., Sokolov, A. N., Braun, C., & Krägeloh-Mann, I. (2008). Neuromagnetic response to body motion and brain connectivity. Journal of Cognitive Neuroscience, 21, 837–846.
- Pavlova, M., Marconato, F., Sokolov, A., Braun, C., Birbaumer, N., & Krägeloh-Mann, I. (2006). Periventricular leukomalacia specifically affects cortical MEG response to biological motion. Annals of Neurology, 59, 415–419. doi:10.1002/ana.20762
- Pavlova, M., Sokolov, A., Birbaumer, N., & Krägeloh-Mann, I. (2006). Biological motion processing in adolescents with early periventricular brain damage. Neuropsychologia, 44, 586–593. doi:10.1016/j.neuropsychologia.2005.06.016
- Pavlova, M., Sokolov, A., Staudt, M., Marconato, F., Birbaumer, N., & Krägeloh-Mann, I. (2005). Recruitment of periventricular parietal regions in processing cluttered point-light biological motion. Cerebral Cortex, 15, 594–601. doi:10.1093/cercor/bhh162
- Pavlova, M., Staudt, M., Sokolov, A., Birbaumer, N., & Krägeloh-Mann, I. (2003). Perception and production of biological movement in patients with early periventricular brain lesions. Brain, 126, 692–701. doi:10.1093/brain/awg062
- Pavlova, M. A. (2012). Biological motion processing as a hallmark of social cognition. Cerebral Cortex, 22, 981–995. doi:10.1093/cercor/bhr156
- Pavlova, M. A., & Krageloh-Mann, I. (2013). Limitations on the developing preterm brain: Impact of periventricular white matter lesions on brain connectivity and cognition. Brain, 136, 998–1011. doi:10.1093/brain/aws334
- Pinto-Martin, J. A., Levy, S. E., Feldman, J. F., Lorenz, J. M., Paneth, N., & Whitaker, A. H. (2011). Prevalence of autism spectrum disorder in adolescents born weighing < 2000 grams. Pediatrics, 128, 883–891.
- Ramenghi, L. A., Ricci, D., Mercuri, E., Groppo, M., De Carli, A., Ometto, A., … Mosca, F. (2010). Visual performance and brain structures in the developing brain of pre-term infants. Early Human Development, 86, 73–75. doi:10.1016/j.earlhumdev.2010.01.010
- Rinehart, N. J., Bradshaw, J. L., Brereton, A. V., & Tonge, B. J. (2002). A clinical and neurobehavioural review of high-functioning autism and Asperger’s Disorder. Australian and New Zealand Journal of Psychiatry, 36, 762–770. doi:10.1046/j.1440-1614.2002.01097.x
- Rosch, E. (1988). Principles of categorization. In A. Collins & E. E. Smith (Eds.), Readings in cognitive science (pp. 312–322). San Mateo, CA: Morgan Kaufman.
- Saavalainen, P., Luoma, L., Bowler, D., Määttä, S., Kiviniemi, V., Laukkanen, E., & Herrgård, E. (2007). Spatial span in very prematurely born adolescents. Developmental Neuropsychology, 32, 769–785. doi:10.1080/87565640701539535
- Saunders, D. (2011). Components of biological motion perception (Unpublished dissertation). Queen’s University, Kingston, ON.
- Saunders, D., Suchan, J., & Troje, N. F. (2009). Off on the wrong foot: Local features in biological motion. Perception, 38, 522–532. doi:10.1068/p6140
- Sommerfelt, K., Ellertsen, B., & Markestad, T. (1995). Parental factors in cognitive outcome of non-handicapped low birthweight infants. Archives of Disease in Childhood - Fetal and Neonatal Edition, 73, F135–F142. doi:10.1136/fn.73.3.F135
- Tantam, D. (2000). Psychological disorder in adolescents and adults with Asperger syndrome. Autism, 4, 47–62. doi:10.1177/1362361300004001004
- Taylor, H. G., Klein, N., Minich, N. M., & Hack, M. (2000). Middle-school-age outcomes in children with very low birthweight. Child Development, 71, 1495–1511. doi:10.1111/1467-8624.00242
- Taylor, N. M., Jakobson, L. S., Lewis, D., & Maurer, T. L. (2009). Differential vulnerability of global motion, global form, and biological motion processing in full-term and preterm children. Neuropsychologia, 47, 2766–2778. doi:10.1016/j.neuropsychologia.2009.06.001
- Thompson, B., Hansen, B. C., Hess, R. F., & Troje, N. F. (2007). Peripheral vision: Good for biological motion, bad for signal noise segregation? Journal of Vision, 7(10), 1–7. doi:10.1167/7.10.12
- Troje, N. F. (2002a). Decomposing biological motion: A framework for analysis and synthesis of human gait patterns. Journal of Vision, 2, 371–387. doi:10.1167/2.5.2
- Troje, N. F. (2002b). The little difference: Fourier based synthesis of gender-specific biological motion. In R. Würtz & M. Lappe (Eds.), Dynamic perception (pp. 115–120). Berlin: Aka Press.
- Troje, N. F. (2008). Retrieving information from human movement patterns. In T. F. Shipley & J. M. Zacks (Ed.), Understanding events: From perception to action. Oxford series in visual cognition (pp. 308–334). New York, NY: Oxford University Press.
- Troje, N. F. (2013). What is biological motion? Definition, stimuli and paradigms. In M. D. Rutherford & V. A. Kuhlmeier (Eds.), Social perception: Detection and interpretation of animacy, agency, and intention. Cambridge, MA: MIT Press.
- Troje, N. F., & Chang, D. H. F. (2013). Shape-independent processes in biological motion perception. In K. L. Johnson & M. Shiffrar (Eds.), People watching: Social, perceptual, and neurophysiological studies of body perception (pp. 82–100). New York, NY: Oxford University Press.
- Troje, N. F., Sadr, J., Geyer, H., & Nakayama, K. (2006). Adaptation aftereffects in the perception of gender from biological motion. Journal of Vision, 6, 850–857. doi:10.1167/6.8.7
- Troje, N. F., & Westhoff, C. (2006). The inversion effect in biological motion perception: Evidence for a “life detector”? Current Biology, 16, 821–824. doi:10.1016/j.cub.2006.03.022
- Troje, N. F., Westhoff, C., & Lavrov, M. (2005). Person identification from biological motion: Effects of structural and kinematic cues. Perception & Psychophysics, 67, 667–675. doi:10.3758/BF03193523
- Vallortigara, G., Regolin, L., & Marconato, F. (2005). Visually inexperienced chicks exhibit spontaneous preference for biological motion patterns. Plos Biology, 3, 1312–1316.
- Van de Cruys, S., Schouten, B., & Wagemans, J. (2013). An anxiety-induced bias in the perception of a bistable point-light walker. Acta Psychologica (Amsterdam), 144, 548–553. doi:10.1016/j.actpsy.2013.09.010
- Volpe, J. J. (1995). Neurology of the newborn. Philadelphia, PA: W.B. Saunders Company.
- von Hecker, U., & Meiser, T. (2005). Defocused attention in depressed mood: Evidence from source monitoring. Emotion, 5, 456–463. doi:10.1037/1528-3542.5.4.456
- Voss, W., Jungmann, T., Wachtendorf, M., & Neubauer, A. (2012). Long-term cognitive outcomes of extremely low-birth-weight infants: The influence of the maternal educational background. Acta Paediatrica, 101, 569–573. doi:10.1111/j.1651-2227.2012.02601.x
- Waber, D. P., & McCormick, M. C. (1995). Late neuropsychological outcomes in preterm infants of normal IQ: Selective vulnerability of the visual system. Journal of Pediatric Psychology, 20, 721–735. doi:10.1093/jpepsy/20.6.721
- Watson, A. B., & Pelli, D. G. (1983). Quest: A Bayesian adaptive psychometric method. Perception & Psychophysics, 33, 113–120. doi:10.3758/BF03202828
- Wechsler, D. (1991). Wechsler intelligence scale for children (3rd ed.). San Antonio, TX: The Psychological Corporation.
- Westhoff, C., & Troje, N. F. (2007). Kinematic cues for person identification from biological motion. Perception & Psychophysics, 69, 241–253. doi:10.3758/BF03193746
- Williamson, K. E., & Jakobson, L. S. (2014a). Social perception in children born at very low birthweight and its relationship with social/behavioral outcomes. Journal of Child Psychology and Psychiatry. Advance online publication. doi:10.1111/jcpp.12210
- Williamson, K. E., & Jakobson, L. S. (2014b). Social attribution skills of children born preterm at very low birth weight. Development and Psychopathology. Advance online publication. http://dx.doi.org/10.1017/S0954579414000522
