Abstract
Previous research has provided inconclusive evidence regarding the neuropsychological difficulties of children born to mothers partaking in opioid or poly-drug use during pregnancy. Little is known about how these children fare as they get older. The present longitudinal study includes follow-up data on 45 children born to mothers who used heroin and poly-drugs and a group of 48 children without prenatal drug exposure. Most of the drug-exposed youths were placed in permanent foster or adoptive homes before one year of age. The youths (ages 17 to 21) were administered 10 neuropsychological tests. The drug-exposed youths had cognitive and fine motor functions within the normal range compared to population norms but performed significantly worse than the non-exposed group. There were indications of generally lower cognitive functions rather than specific problems with executive functioning. Lower mean birthweight in the risk group (619 grams mean difference, p < .001) only partially mediated the group differences in cognitive functioning. There was a tendency for youths who had few and early changes in their caregivers or who were born to mothers who had used the least number of different drugs during pregnancy to have the best cognitive scores. The study indicates that youths born to mothers who used multiple drugs during pregnancy are vulnerable relative to their peers within a wide range of cognitive functions. The vulnerability seems to be related not only to the mother’s drug use during pregnancy but also to factors such as birthweight and unstable parental care during infancy.
Previous studies have indicated an increased risk of neuropsychological difficulties for children born to mothers who use opioids or multiple illegal substances during pregnancy (Hunt, Tzioumi, Collins, & Jeffery, Citation2008; Lester & Lagasse, Citation2010; Moe, Siqveland, & Slinning, Citation2011; Ornoy et al., Citation2010). However, there is almost no knowledge concerning the fate of these children as they enter adolescence and young adulthood (Ornoy et al., Citation2010). This issue is of great concern because there is no reason to believe that prenatal and early childhood adversities are confined to early development. Rather, early life factors may likely affect an individual’s functioning across the entire lifespan (Walhovd, Fjell, & Espeseth, Citation2014). In the present study, we will investigate the cognitive functioning of youths born to mothers with poly-drug abuse during pregnancy. We will further expand the understanding of three possible mediation factors: prenatal heroin exposure, birthweight and early placement with stable foster or adoptive parents.
There have been many studies, including large longitudinal studies, of human prenatal exposure to alcohol (Flak et al., Citation2014) and cocaine (Ackerman, Riggins, & Black, Citation2010). It is well known that prenatal alcohol exposure (including moderate levels of consumption) can have detrimental effects on the child’s cognitive abilities (Flak et al., Citation2014). One of the larger studies of prenatal cocaine exposure is the Maternal Lifestyle Study, which includes more than 1100 children with cocaine exposure (Bauer et al., Citation2002), of whom several hundreds have been followed up to 15 years of age (Neonatal Research Network, Citation2013). These studies have found that prenatal cocaine exposure is related to problems with sustained attention and behavioral self-regulation even after controlling for covariates (Ackerman et al., Citation2010). However, there have been relatively few studies of opioid and poly-substance exposure (Lester & Lagasse, Citation2010; Logan, Brown, & Hayes, Citation2013).
The majority of children born to mothers who use opioids suffer from neonatal abstinence syndrome (Patrick et al., Citation2012). The few studies of young children indicate the likelihood of problems within fields related to executive control and attention (Hans, Citation1996; Hickey, Suess, Newlin, Spurgeon, & Porges, Citation1995; Melinder, Konijnenberg, & Sarfi, Citation2013; Ornoy, Segal, Bar-Hamburger, & Greenbaum, Citation2001; Slinning, Citation2004; Wahlsten & Sarman, Citation2013) and behavior regulation (de Cubas & Field, Citation1993; Hans, Citation1996; Sowder & Burt, Citation1980). Fine motor abilities, which are often related to executive control (Rigoli, Piek, Kane, & Oosterlaan, Citation2012), have also been found to be worse among young children born to mothers with opioid and poly-drug use during pregnancy than among other children (Bunikowski et al., Citation1998; Davis & Templer, Citation1988; Hans & Jeremy, Citation2001; Logan et al., Citation2011). Some studies find that motor abilities are the most affected functional area (Bernstein, Jeremy, Hans, & Marcus, Citation1984; Hans, Citation1989; Wahlsten & Sarman, Citation2013), whereas other studies have not found significant group differences in motor abilities (van Baar, Citation1990).
However, there are more divergent findings concerning general cognitive abilities. Some studies find that young children who have been exposed to opioids and multiple substances have greater impairments in general cognitive abilities than non-exposed children (Bunikowski et al., Citation1998; Hunt et al., Citation2008; Johnson, Diano, & Rosen, Citation1984; Logan et al., Citation2013; Moe, Citation2002a; Moe & Slinning, Citation2001; Salo et al., Citation2009; van Baar & de Graaff, Citation1994; Wahlsten & Sarman, Citation2013; Wilson, Citation1989; Wilson, McCreary, Kean, & Baxter, Citation1979), whereas others do not find such differences either before (de Cubas & Field, Citation1993; Kaltenbach & Finnegan, Citation1989; Melinder et al., Citation2013; Rosen & Johnson, Citation1985; Strauss, Lessen-Firestone, Chavez, & Stryker, Citation1979) or after controlling for covariates (Bauman & Levine, Citation1986; Lifschitz, Wilson, Smith, & Desmond, Citation1985; Messinger et al., Citation2004).
Thus, there are substantial differences between the results from the few existing studies of prenatally opioid- or poly-drug-exposed children. In addition, we have almost no knowledge of how these children fare as they get older. This issue is of concern because the few existing longitudinal studies indicate that drug-exposed children do not catch up with comparable children as they age (Crea, Barth, Guo, & Brooks, Citation2008; Hans & Jeremy, Citation2001; Messinger et al., Citation2004; Moe & Slinning, Citation2001; Ornoy et al., Citation2010; Strauss, Starr, Ostrea, Chavez, & Stryker, Citation1976; van Baar & de Graaff, Citation1994). The few studies of older children and youths indicate worse cognitive scores and more attentional problems for these youths than for comparable control groups (Davis & Templer, Citation1988; Ornoy et al., Citation2010). However, these studies are cross-sectional and include younger children (Davis & Templer, Citation1988), do not report on the youths’ cognitive functioning (Crea et al., Citation2008), or do not take into account perinatal factors (Ornoy et al., Citation2010).
The scarce data on the adult offspring of opioid-dependent parents indicate a high risk of criminal behavior, substance abuse and unemployment (Skinner, Haggerty, Fleming, & Catalano, Citation2009); these have also been found for people with fetal alcohol spectrum disorder (Dörrie, Föcker, Freunscht, & Hebebrand, Citation2014). Problems with executive functions, such as self-regulation and concentration, in young children may become more serious as they become adults. Executive functions normally continue to develop throughout the adolescent years and into young adulthood (Tamnes et al., Citation2010). It is possible that children’s earlier vulnerability within these fields becomes more serious along the developmental path as their environment places increasing demands on these complex executive functions. This may be especially true at the age when young adults normally move away from their parents and other support systems that have followed them through their upbringing. Thus, it is troubling that virtually no studies document the development of children into young adulthood who were exposed prenatally to opioids and multiple drugs.
Due to the heterogeneity of prenatal drug exposure and other risk factors for youths born to mothers with poly-drug abuse during pregnancy, there is a need for studies across samples with variations in risk and protective factors. The special features of the present sample enable us to expand knowledge concerning three such factors: prenatal opioid exposure, birthweight and early placement in foster or adoptive care (Moe & Slinning, Citation2002).
Animal studies have found that prenatal opioid exposure alters the myelin sheath in the developing brain (Sanchez, Bigbee, Fobbs, Robinson, & Sato-Bigbee, Citation2008). This may disrupt neuronal migration and/or cell survival (Harlan & Song, Citation1994), affect genetically programmed cell death in the hippocampus by influencing specific proteins in the apoptotic signal-transduction pathways (Wang & Han, Citation2009) and decrease dendrite length and branch numbers in pyramidal neurons in the somatosensory cortex (Lu, Liu, Long, & Ma, Citation2012). Many animal studies have also found that prenatal opioid exposure disrupts several important neurotransmitter systems (Konijnenberg & Melinder, Citation2011). Thus, it is likely that prenatal opioid exposure has a negative neurological effect on human fetuses. This is an area of concern because both maternal opioid use and neonatal abstinence syndrome have increased considerably (Manchikanti, Fellows, Ailinani, & Pampati, Citation2010; Patrick et al., Citation2012), and opioid maintenance treatment is the recommended treatment for opioid-addicted pregnant women (World Health Organization, Citation2009). There is, however, a lack of knowledge about how children and youths function following prenatal opioid exposure. Based on the discrepancies in previous studies, the American Academy of Pediatrics concluded in a recent extensive review that there is no consensus on the effects of prenatal opiate exposure on cognitive abilities (Behnke & Smith, Citation2013). Although human clinical studies, including the present study, cannot differentiate between different causal mechanisms, there is a need for knowledge on cognitive function of people who have been prenatally exposed to opioids as they age.
Children born to mothers partaking in opioid and poly-substance use during pregnancy often have lower birthweight than control groups (Creanga et al., Citation2012; Mactier, Shipton, Dryden, & Tappin, Citation2014). Low birthweight has been found to be a predictor of later cognitive abilities (Leitner et al., Citation2000), socio-emotional functioning (Hediger, Overpeck, Ruan, & Troendle, Citation2002), executive functioning, academic achievement (Aarnoudse-Moens, Weisglas-Kuperus, van Goudoever, & Oosterlaan, Citation2009) and neuroanatomical characteristics, even for normal birthweight variations (Walhovd et al., Citation2012). Birthweight is in part related to genetically determined body size, but also to prenatal environment variations such as maternal stress during pregnancy (Monk, Spicer, & Champagne, Citation2012), maternal food intake during pregnancy (Stein, Saenger, Susser, & Marolla, Citation1972) and maternal substance abuse during pregnancy, such as smoking (England et al., Citation2001) and alcohol use (Dörrie et al., Citation2014). However, it is also probable that maternal use of opioids or multiple substances influences birthweight (Creanga et al., Citation2012). Thus, birthweight may be a mediating factor for some of the negative consequences of prenatal drug exposure.
Some studies have indicated that part of the differences between opioid- or poly-substance-exposed and non-exposed children may be attributed to concordant factors, such as a non-optimal caregiving environment with drug-abusing parents (Hans & Jeremy, Citation2001; Lifschitz et al., Citation1985; Messinger et al., Citation2004; Moe & Slinning, Citation2002; Ornoy et al., Citation2001). It has been postulated that optimization of the postnatal environment may compensate for the biological vulnerabilities these children have (Mayes, Citation1999; van Baar & de Graaff, Citation1994). For example, a stable and nurturing home was found to be the most important protective factor to avoid secondary adversities in a large study of people with fetal alcohol syndrome (Streissguth et al., Citation2004). Thus, children with early placement in good foster or adoptive homes may have positive development over time (Julian, Citation2013). However, the few longitudinal studies of children exposed to opioids and multiple illegal drugs who were brought up in foster or adoptive homes do not indicate that the children catch up but instead that they have continuous problems (Crea et al., Citation2008; Moe & Slinning, Citation2001) or more clearly manifested symptoms throughout infancy, early childhood and adolescence (Ornoy et al., Citation2010).
The present study followed two groups of children from early infancy into their youth (17 to 22 years of age). One group of youths was heavily exposed to opioids (heroin) and multiple substances in utero. Most of these youths were moved into a stable foster or adoptive home at an early age. These youths were prospectively compared to a group of youths without any known prenatal drug exposure. Based on the literature mentioned above, our main hypothesis was that the drug-exposed youths would have lower cognitive functioning than the non-exposed group. We expected this difference to be particularly significant for executive functions and fine motor abilities. Our secondary hypothesis was that some of the group differences in cognitive and fine motor functions would be mediated through the risk effects of heroin exposure, lower birthweight and not being placed in stable foster or adoptive homes at an early age.
MATERIAL AND METHODS
Participants
The participants recruited for the longitudinal prospective study included infants born to mothers who partook in poly-drug use during pregnancy, with heroin as the most common main drug of choice. The initial sample was composed of 78 drug-exposed children and 58 non-exposed children. The initial sample, measures and test results at 1, 2, 3 and 4.5 years have been previously described in detail (Moe, Citation2002a; Moe & Slinning, Citation2001; Slinning, Citation2004). The drug-exposed children were recruited consecutively during the period 1992–1996 at an inpatient clinic for infants from 0 to 3 years of age, the Aline Infant and Family Center in Oslo. The majority (76.9%) of the birth mothers of the drug-exposed children were enrolled in the perinatal risk project at Ullevål Municipal Hospital by the second or third trimester of pregnancy. The rest of the children were born at other hospitals and enrolled in the risk project after the child’s birth. Because of the severity of the birth mothers’ drug abuse and general living conditions, the majority of the children were placed in permanent foster care within their first year of life, and many were adopted by the foster parents at a later stage. This natural experimental design gave us a unique opportunity to study drug-exposed children’s development under more normal rearing conditions. Thus, a group of non-exposed children and parents with similar socioeconomic status as the foster parents were recruited from a non-clinical setting of local maternal and child health centers in Norway, where biomedical vulnerability and social risk factors were minimal.
Originally, 88 mothers who partook in poly-drug use during pregnancy were contacted by the project. Of these, 8 mothers refused to participate, and 2 dropped out before the child was 1 year of age. Of the 67 volunteer families for the non-exposed group, 60 approximately matched the socioeconomic status of the caregivers in the risk group, of which 2 dropped out before the child was 1 year of age. Of the original 136 children who participated at 1 year of age, 11 were not invited to participate as youths because their caregiver had withdrawn consent to participate in the study at a previous time point. Of the 125 invited youths, 98 participated in the study. However, 5 children who were evaluated during their first year to have fetal alcohol syndrome or fetal alcohol spectrum disorder were excluded from the analyses in the present study. These 5 excluded children had an earlier gestational age, lower birthweight and smaller head circumference than the included participants. They also had worse cognitive scores on the assessments at 1, 2, 3, 4.5 and 8.5 years than the included participants (See Table A2 in Supplementary data). These 5 children were excluded to avoid some of the confounding aspects of severe prenatal alcohol exposure. Thus, the total number of participants in the present study consists of 45 drug-exposed (22 girls, 49%) and 48 non-exposed (16 girls, 33%) youths (chi-square = 2.3, p = .13). A flow chart depicting the study inclusion and drop out of the participants is presented in Figure B1 in Supplementary data.
Although all children in the non-exposed group lived with their biological families throughout the study period, the majority of the children in the drug-exposed group were either adopted or moved to permanent foster homes before the age of 6 months (n = 28, 62%), and almost all were moved by 1 year of age (n = 39, 87%). Only 5 children changed caregivers after 1 year of age, and 2 children in the drug-exposed group lived with their biological parent at the time of the last assessment, as 1 had moved back after living with other caregivers. The County Social Welfare Board made the decision concerning the custody of the child after the child protection services in Oslo had evaluated the mothers in the drug-exposed group for their ability to participate in a rehabilitation program for drug and alcohol addiction, as well as their ability to adequately care for their children.
Information concerning prenatal exposure was gathered by a combination of self-reports from the biological mothers and information from their medical and social records (Moe & Slinning, Citation2001). A limitation of many studies on prenatal substance exposure, including this study, is that regular toxicological test results for the mothers throughout their pregnancies are not available. Because the mothers were heavy heroin and poly-drug users, they often had trouble accounting for the amount, timing and frequency of drug use during their pregnancies. For these reasons, we have only included what may be the most reliable information: the women’s main drug of choice and information about what other substances they used. The biological mothers of the children in the drug-exposed group used a wide range of drugs. The most common main drug of choice besides tobacco was opiates (heroin) (n = 20, 44%), followed by benzodiazepines (n = 6, 13%), alcohol (n = 5, 11%) and psychopharmaca (n = 5, 11%). Heroin was also the drug reported to be used by most mothers (n = 24, 53%) (for further information about specific drugs, see Table A1 in Supplementary data). On average, the mothers had used 3.4 different drugs, including tobacco, during pregnancy (range 2–6). Most of the drug-exposed children (n = 35, 78%) had neonatal withdrawal symptoms, as recorded in their medical records. There is a risk of under-reporting from the mothers concerning their drug abuse because child protective services were involved and the biological mothers may have been afraid of losing custody of their children. Such under-reporting may partially explain why more children had withdrawal than mothers who reported heroin as their main drug of choice during the pregnancy.
The drug-exposed children had a significantly lower birthweight, gestational age and head circumference than the children in the non-exposed group (). None of the children in the non-exposed group and 9 (20%) of the children in the drug-exposed group had a low birthweight (<2500 grams). Similarly, 11 in the drug-exposed group and none in the non-exposed group were born before 38 weeks’ gestation. Only 2 (4%) of the youth in the drug-exposed group are left handed or had undecided hand preference compared to 8 (17%) in the non-exposed group (chi-square = 3.6, p = .06). A total of 16 (36%) of the youths in the risk group had previously been diagnosed with ADHD or ADD, as compared to 1 (2%) in the non-exposed group (chi-square = 17.9, p ≤ .001). There was also a higher risk of ongoing substance misuse or addiction in the risk group (n = 6, 13%) than in the non-exposed group (n = 1, 2%; chi-square = 4.2, p = .04).
Table 1 Descriptive Information about the Sample (n = 93).
As seen in , the non-exposed children were younger (range 17.3–18.9 years) than the drug-exposed children (range 17.6–21.9 years) at the time of assessment. Due to the continuing effects of age on both general cognitive abilities and some executive functions during youth, it would have been preferable to have an age-matched non-exposed group. However, we prioritized the advantage of having followed the non-exposed group from birth; thus, they were regularly assessed with the same measurements as the drug-exposed participants. Therefore, the study was performed on the original non-exposed group, and the age effects were controlled for statistically (see for further information about the sample).
Youths who were included in the analyses (n = 93) were significantly better off than the non-participants (n = 38) and the participants who were excluded due to fetal alcohol spectrum disorder (n = 5) on many measures (see Table A2 in Supplementary data). The analyzed participants included a smaller proportion of drug-exposed youths, had more often moved to another caregiver before 1 year of age, had less often had mothers with opiate (heroin) as their main drug of choice, had a higher birthweight and a larger head circumference at birth, and had higher general cognitive abilities in the previous assessments at 1, 2, 3, 4.5 and 8.5 years of age compared to non-participants. The included participants and non-participants did not differ by gender, neonatal abstinence or parental socioeconomic status at 1 year of age.
Measures
The Wechsler Abbreviated Scale of Intelligence (WASI) was used to measure general cognitive abilities. IQ was calculated based on the results from two subtests, matrix reasoning and vocabulary, with an expected mean of 100 and standard deviation of 15 based on United States (US) norms (Zhu, Citation1999). The mean standardized raw score was calculated by standardizing the raw scores on each of the two subtests for the present sample into Z-scores (M = 0, SD = 1) and producing the mean of these two Z-scores.
A Grooved Pegboard from Lafayette, model 32025, was used to measure fine motor abilities. In the test, the participants were timed on how quickly they could place 25 small pegs twice, first with their right and then with their left hand. US norms (Heaton, Grant, & Matthews, Citation1991) were used to calculate scale scores, with an expected mean of 10 and a standard deviation of 3 for the dominant and non-dominant hand separately. The scale scores for the dominant and non-dominant hand were highly related (r = .68) and were combined to a mean scale score for both hands.
The Rey Complex Figure Test (RCFT; Meyers & Meyers, Citation1995) was used to measure visual long-term memory. The RCFT has been found to be a valid measure of long-term memory with a high level of test-retest stability (r between .76 and .87; Meyers & Meyers, Citation1995). The participants copied an abstract drawing without any memory cues. Approximately 30 minutes later, they were asked to draw the figure again. Scoring was performed as suggested by Meyers and Meyers (Citation1995), with a possible range of 0–36 in raw scores that were transformed into t-scores based on their norms, with an expected mean of 50 and a standard deviation of 10.
The California Verbal Learning Test – Second Edition (CVLT-II; Delis, Kramer, Kaplan, & Ober, Citation2000) was used to measure verbal short- and long-term memory. Each participant had five trials to learn 16 words, which could be grouped into four logical groups. The participants were asked to remember the words for later recall. After a short delay while learning a list with other words, the participants were asked to recall the original list. They were then asked to recall the words from each of the four logical groups. After approximately 30 minutes, they were asked to recall the original list of words. The sum of the correct words during the five learning sessions was transformed into t-scores, and short-delay recall and recall after 30 minutes were transformed into scale scores based on US norms (Delis et al., Citation2000). The learning scores, short-delay scores and recall scores were highly related (r = .76 to .84) and were thus standardized (Z-scores) and combined into a mean verbal memory score.
The digit span subtest from the Wechsler Adult Intelligence Scale – Third Edition (WAIS-III; Wechsler, Citation2003) was used to measure short-term memory for numbers. Combined raw scores from forward and backward recalls were transformed into scale scores with an expected mean of 10 and a standard deviation of 3 based on Swedish norms (Wechsler, Citation2003).
In the present study, we used the theoretical perspectives of Miyake et al. (Citation2000) when choosing which tests of executive functions to include in the test battery. Miyake et al. found both theoretical and empirical evidence for a division of complex executive tasks into three factors: shifting, updating and inhibition. Thus, we included the tests that were considered by Miyake et al. to measure these features of executive control.
The Plus-Minus task (Miyake et al., Citation2000) was used to measure the function of number shifting. The task consisted of three lists with 30 random numbers (possible range 10–99) presented verbally. On the first list, the participants were asked to add 3 to each number and say the new number as quickly and correctly as possible. On the second list, they were instructed to subtract 3 from each number. On the third list, they were asked to alternate between adding and subtracting 3 by adding 3 to the first number, subtracting 3 from the second, adding 3 to the third, etc. Thus, whereas the two first tasks measured mathematical abilities, the third task also measured the ability to shift between tasks. The completion times were measured with a stopwatch. The cost of shifting was calculated as the time spent on the third task divided by the mean time spent on the two first tasks.
The Color-Word interference test from the D-KEFS test battery (Delis, Kaplan, & Kramer, Citation2001a) was used as a Stroop task to measure the ability to inhibit an overlearned verbal response. The two first tasks measured the baseline ability to name color patches (three colors and 50 trials) and basic reading of the color words. The third task was a traditional Stroop task in which the words were presented in a dissonant color and the participant had to name the printed color and inhibit reading. The time spent on each task was measured with a stopwatch, and the raw scores of time spent were recalculated to scale scores with an expected mean of 10 and a standard deviation of 3 based on US norms. The Color-Word interference test has been found to have acceptable levels of internal consistency (.75 to .82) and test-retest reliability (.49 to .90) for the age groups presented (Delis, Kaplan, & Kramer, Citation2001b). Inhibition ability was measured as the difference in the scale score on the third task (inhibition) and on the first task (color naming) with the difference recalculated to scale scores.
Antisaccade (Miyake et al., Citation2000) was used to measure the ability of visual inhibition. In each trial, a visual cue (black square) was presented on one side of a computer screen for 225 ms, followed by a target stimulus (an arrow inside an open square) on the opposite side for 120 ms before being masked by gray cross-hatching. The participants’ task was to indicate the direction (left, up, right) of the arrow on buttons on a serial response box. Between each trial, a fixation point was presented in the middle of the computer screen. Because the arrow was presented for such a short time, the participants had to inhibit the reflexive response of looking at the initial cue to maximize the ability to correctly identify the direction of the arrow on the other side of the screen. The participants had 18 learning trials before performing 132 target trials, except for 5 participants, who performed 72 target trials. There were almost no differences (mean = 0.79 vs 0.80, respectively, p = .86) in the proportion of correct replies between those with 132 and 72 target trials; thus, no exclusions or corrections were performed due to the use of two different versions. The proportion of correct answers versus the total number of answers in the target trials served as the dependent measure.
The letter memory task (Miyake et al., Citation2000) was used to measure the ability to constantly monitor and update information in working memory. A list of letters of varying length was presented on a computer screen for 2000 ms per letter. The task was to recall and write down the 4 last letters after the list of letters was completed. After practicing for 2 trials with 5 and 7 letters, 12 trials with 5, 7, 9 or 11 letters (varied randomly) were presented. The score was the number of correct letters recalled (possible range 0–48).
An N-back task was used to measure the ability to update temporal sequence and monitor visual stimuli (Miyake et al., Citation2000). The participants were presented with stylistic black and white drawings of common objects for 2500 ms. On all pictures, they had to reply “yes” or “no” on a response box to indicate whether the current figure was the same as the one just presented. There were three difficulty levels: 1-back, in which the participant should reply “yes” if the picture was the same as the last one; 2-back for two pictures earlier; and 3-back for three pictures earlier. The participants had 50 learning trials divided between the three difficulty levels before performing three rounds of 30 trials of each difficulty level for a total of 270 test trials. The rounds were semi-randomly distributed. Performance was assessed in terms of reaction times (hits only) and accuracy (proportion of hits plus false alarms) serving as dependent variables (Snodgrass & Corwin, Citation1988). N-back tasks have shown increasing reliability with higher (2- or 3-back) difficulty levels (Jaeggi, Buschkuehl, Perrig, & Meier, Citation2010). In the present sample, there appears to have been a ceiling effect on the simplest tasks, with a mean correct response rate of 96.6% on 1-back, 94.7% on 2-back and 90.5% on 3-back. Thus, the results are only presented for the 3-back difficulty level when investigating group differences.
The Antisaccade, letter memory and N-back tasks were performed on a personal computer with a 19-inch screen with 1920×1080 resolution using E-prime 2 software (Psychology Software Tools). The participants used a PST serial response box (Psychology Software Tools) with millisecond accuracy for responses on the Antisaccade and N-back tasks.
Caregiver education was based on caregivers’ reports of their own education on the adult self-report (18–59; Achenbach & Rescorla, Citation2003) from the Achenbach System of Empirical Based Assessment questionnaire battery at approximately the same time the youth were tested. The caregivers had 10 response alternatives. The responses were regrouped into 4 alternatives representing the highest education the parent had finished: no upper secondary education (0), upper secondary education (1), short tertiary education (2) and tertiary education of four years or more (3) (Statistics Norway, Citation2014). Mean caregiver education was used to control for parental socioeconomic status. Mean caregiver education was based on the responses from either one (n = 21) or two caregivers (n = 71), depending on whether or not both parents had responded. In one case, neither of the caregivers participated.
Statistics
The Pearson’s chi-square test, Student’s t-test and Mann-Whitney U test were used for bivariate analyses of group differences for grouped, normal and skewed background variables, respectively. There were only marginal differences when using parametric versus non-parametric tests on the skewed distributed background variables.
General linear models (GLMs) were used for analyzing group differences in cognitive and fine motor abilities. All measures of cognitive and fine motor abilities were approximately normally distributed, with the exception of the proportion of correct responses on the N-back task. There were similar results using both parametric and non-parametric statistics when analyzing the proportion of correct responses on the N-back task. To facilitate comparability across analyses, parametric tests were used for all analyses of group differences in cognitive and fine motor abilities. Some measures have age-adjusted norms, but the specificity of age groups varies across the different tests. To facilitate comparability across the analyses and avoid double correction for age, all bivariate and multiple analyses of cognitive and fine motor abilities used raw data adjusted to Z-values (mean = 0, SD = 1) based on the present sample. To balance the pros of statistical control of covariates and the cons of systematically regressing out effects of variables that may be of interest, we chose to perform separate GLM analyses for bivariate relations and then to control for gender, gender and age, and gender, age and caregiver education. The reason for this strategy was that with a small sample size, very few cases can have undue influences on the results. Therefore, we want the reader to be able to evaluate the effects in different circumstances. All participants started the Grooved Pegboard test with their right hand; thus, hand preference was always controlled for in the analysis of this test.
All three perinatal variables were highly related (birthweight correlated .71 with gestational age and .82 with head circumference, and gestational age correlated .65 with head circumference). Due to the high covariation, only birthweight, which had the highest correlation with the two other perinatal factors and was the most reliable perinatal measure, was used as the mediating factor between group belonging and cognitive scores in the analyses in which perinatal factors were assessed. The mediation analyses were performed using the process computational tool for SPSS (Hayes, Citation2012a). This method uses bootstrapping to calculate the indirect mediation effect, thus avoiding unrealistic assumptions about the sample distribution of the indirect effect. The model investigated whether birthweight mediated the group effect on cognitive scores, with gender, age at testing and caregiver education as covariates. Hand preference was controlled for when the Grooved Pegboard test was the dependent variable. The covariates were not allowed to influence the mediator in the model, and the analyses used model 4 from Hayes (Citation2012b) and 10,000 bootstraps for calculating the indirect mediation effect.
The possible effects of heroin, the number of drugs and a change in caregiver could not be analyzed across the groups because none of the participants in the non-exposed group had any of these experiences. Thus, instead of mediation analyses, general linear regression analyses were performed separately for the drug-exposed group to analyze the relation between these risk and protective factors and the drug-exposed youths’ functioning.
All analyses were performed with IBM SPSS Statistics, version 22 and used a 95% confidence level.
RESULTS
Group Differences in Cognitive and Fine Motor Abilities
shows the means and variations on tests of cognitive and fine motor abilities for the drug-exposed and non-exposed group separately. Both groups had results within one standard deviation of population-based norms on all tests that have such norms: general mental abilities, fine motor abilities, visual long-term memory, short-term memory for numbers and inhibition on color naming. Furthermore, the results on the verbal memory task (CVLT) were within one standard deviation of the population means for both groups. For example, the mean t-scores for verbal learning on the CVLT were 51.7 (SD = 10.1) for the drug-exposed group and 56.6 (SD = 10.0) for the non-exposed group. On the short-delay task from CVLT, the drug-exposed group had a mean of 0.1 (SD = 1.2) and the non-exposed group had a mean of 0.6 (SD = 0.9). Similarly, the results of the long-delay verbal memory task were M = 0.1, SD = 0.9 and M = 0.4, SD = 1.0 for the drug-exposed and non-exposed groups, respectively (expected M = 0 and expected SD = 1 for both short-delay and long-delay verbal memory tasks).
Table 2 Cognitive Levels and Fine Motor Abilities among Drug-exposed and Non-exposed Youth (n = 93).
depicts the results from regression analyses of the group differences, both without taking any other factors into account and when taking demographical covariates into account. There were statistically significant group differences on most measures, and all significant group differences were to the disadvantage of the drug-exposed group. Most of the significant group differences were statistically significant both with and without controls for the demographic variables of gender, age at testing and caregiver education.
Table 3 Significance Test of Differences in Cognitive and Fine Motor Abilities between Drug-exposed and Non-exposed Youth (n = 93).
The drug-exposed group had lower general cognitive abilities on the WASI test than the non-exposed group, independent of demographic variables (). They had also worse fine motor abilities on the Grooved Pegboard task both before and after controlling for demographic variables.
The drug-exposed youths had, in general, worse memory functions than the non-exposed youths on all tests: visual long-term memory (after 30 minutes), verbal memory (learning, 5-minute delay and 30-minute delay combined) and short-term memory for numbers (). However, the group difference in verbal memory was not significant when controlling for the caregiver education.
The drug-exposed group spent significantly longer on the third Plus-Minus subtest, the shifting task (M = 136.0 seconds, SD = 52.9), than the non-exposed group (M = 101.0 seconds, SD = 32.5), even after accounting for demographic covariates (b = 27.1 seconds, p = .02). However, because the shifting score is corrected for mathematical abilities, as reported by time spent on the simpler non-shifting plus and minus tasks (), the group differences in shifting became insignificant when also controlling for age ().
The only test without a significant group difference was the inhibition of color naming (D-KEFS). Although the drug-exposed group performed significantly worse than the non-exposed group on the third inhibition subtest on the D-KEFS task (M = 59.0 seconds, SD = 13.5 vs M = 49.8 seconds, SD = 8.5, respectively) even after controlling for gender, age and caregiver education (b = 8.2 seconds, p = .007), this group difference was not significant when adjusting for their score on the first subtest of naming the colors ().
The non-exposed youths had a higher proportion of correct responses than the drug-exposed youths on the computerized Antisaccade test, indicating better inhibition abilities for the youths without prenatal risk factors (), which were also present when controlling for gender and age. However, the group difference was no longer significant when accounting for caregiver education.
The youths with prenatal drug exposure performed significantly worse than the non-exposed group on measures of their ability to continuously monitor and update information in working memory on both the computerized tests of letters (Letter memory task) and pictures (N-back). The non-exposed group remembered more letters and had a higher proportion of correct responses on the N-back task, and spent more time before responding on the picture-updating memory task. The group differences remained significant after controlling for demographic variables ().
Heroin vs Another Main Drug of Choice and the Number of Drugs
There were no significant differences in any of the cognitive or fine motor scores between youths born to mothers who used heroin as their main drug of choice and youths born to mothers who used another main drug of choice in bivariate analyses or when controlling for demographic covariates (see Table A3 in Supplementary data). Approximately half of the regression coefficients were negative. Thus, there was no indication that participants born to mothers who reported using heroin as their main drug of choice had worse functioning than the non-exposed group.
General linear models found that a lower number of drugs used by the mothers during pregnancy was significantly related to better scores on the Letter memory task, both bivariate (b = 0.35, p = .01) and when controlling for gender, age and caregiver education (b = 0.38, p = .009), within the risk group. A lower number of drugs was also significantly related to better short-term memory for numbers on the WAIS when controlling for the demographic covariates (b = 0.22, p = .05) but not in the bivariate analysis. None of the other measures of functioning were significantly related to the number of drugs used by the mother during pregnancy (see Table A3 in Supplementary data).
Mediation Effect of Birthweight on Group Differences
Perinatal factors can be both an indication of prenatal conditions (Creanga et al., Citation2012; England et al., Citation2001; Monk et al., Citation2012) and a risk factor for later cognitive development (Aarnoudse-Moens et al., Citation2009; Hediger et al., Citation2002; Leitner et al., Citation2000; Walhovd et al., Citation2012). Thus, some of the group differences found above () may have occurred because the prenatally drug-exposed children were born earlier, with a lower birthweight and a smaller head circumference. Therefore, we performed mediation analyses to determine whether such perinatal factors could explain the group differences in cognitive and fine motor abilities. The perinatal factors were highly interrelated (see analysis above and Table A4 in Supplementary data). There are some uncertainties regarding the gestational age at which the children were born. This is partly due to the unstable life situation for many of the opioid and poly-drug-using mothers before they knew they were pregnant. Studies have found that head circumference measurements vary by approximately 0.1 to 0.4 cm across clinicians (Ismail et al., Citation2013), making the birthweight registered in the child’s health record the most reliable perinatal measure. The drug-exposed youths had an average lower birthweight of 619.1 grams compared to the non-exposed youths (). The difference in birthweight between the groups was on the border of significance after taking into account that the drug-exposed children also had, on average, an earlier gestational age (b = 206.8 grams; F = 3.6; p = .06).
Because of the clear timeline between the variables, it is logical that gender may have directly influenced birthweight and may have moderated the mediation effect of birthweight on cognitive and fine motor abilities. It is also possible that gender may have moderated the direct effects between the groups and their cognitive and fine motor abilities. Thus, a combined sub-model in which gender was included as both a moderator and covariate was tested, similar to model 59 from Hayes (Citation2012b). All interaction effects were highly insignificant on a model with general cognitive abilities from the WASI as an independent variable (p = .94 for group × gender on birthweight; p = .99 for birthweight × gender on IQ from WASI; p = .66 for group × gender on IQ from WASI), with nearly identical indirect mediation effects of birthweight on group differences in general cognitive abilities for girls (b = 0.15, 95% CI = −0.14 to 0.48) and boys (b = 0.15, 95% CI = −0.08 to 0.47). Due to the lack of any moderating effect of gender in the model that predicts general cognitive abilities, we present a simpler model in which gender is only included as a covariate.
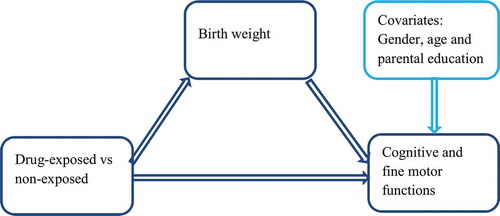
Our conceptual mediation model on which our analyses are based is similar to model 4 from Hayes (Citation2012b) and is presented in . The model splits the effect of the group on cognitive and fine motor abilities into two parts, one direct effect and one indirect effect mediated by birthweight. The model takes into account gender, age at testing and caregiver education as covariates. With one exception, the indirect mediation effects of birthweight on group differences in cognitive and fine motor abilities were insignificant (). The only significant mediation effect of birthweight was on group differences in short-term memory for numbers on the WAIS. Thus, in general, the observed group differences in cognitive scores () cannot be significantly explained by lower birthweight in the drug-exposed group. However, most of the mediation effects of birthweight were in the expected direction; thus, it is possible that some part of the group differences in cognitive scores can be explained by lower birthweight.
Table 4 Birthweight as a Mediating Factor for Group Differences (n = 92.)
Moving to a Stable Caregiver
Because only one drug-exposed youth was raised by the child’s biological mother, we could not analyze whether living with the biological mother or alternative caregivers was preferable. We could also not conduct a mediation analysis of whether changing caregivers mediated the differences between the drug-exposed and non-exposed groups because none of the non-exposed youths had moved to alternate caregivers. We could, however, investigate whether the change in caregivers was related to cognitive functioning within the group of drug-exposed youths.
Only four children had more than two changes in caregivers after leaving the hospital after birth. With one exception, there was no significant difference in any of the cognitive or fine motor abilities between the drug-exposed youths who changed caregivers one or zero times (n = 21, 47%) versus those with two or more changes (n = 24, 53%). The exception was in short-term memory for numbers (WAIS), in which the drug-exposed youths with the least number of changes in caregivers had better memory than those with two or more changes in caregivers after correction for demographic covariates ().
Table 5 Relationship between the Change in Caregiver and Cognitive and Fine Motor Functioning for Youths Born to Mothers Who Partook in Poly-drug Use During Pregnancy (n = 44).
Even though most children moved early in life to stable foster or adoptive parents, there was some variation in the time this occurred. The age at which the youths had their last change in caregiver was skewed; thus, a ranked age for last change was used in the analyses. Most of the cognitive and fine motor abilities were not significantly related to the age at the last change in caregiver. However, there were significant relationships between earlier age at last change in caregiver and better scores on verbal memory (CVLT) and short-term memory for numbers (WAIS) (). However, only the relationship for short-term memory for numbers was significant after controlling for gender, age and caregivers’ education.
DISCUSSION
Overview
Both groups had mean cognitive and fine motor functioning within the normal range (plus or minus one standard deviation) compared to population norms. However, the youths with prenatal drug exposure performed significantly worse than the non-exposed group on most measures of such functioning, which was also true after controlling for demographic factors such as gender, age and caregiver education. The group differences were not substantially higher on tests of fine motor abilities and executive functions than on other neuropsychological tests. There were no significant differences between those born to mothers who used heroin as their main drug of choice compared to those who used another main drug. However, the results indicated that being born to mothers who used a higher number of different drugs during pregnancy were somewhat related to worse cognitive functioning. Although the drug-exposed group had a much worse starting point as measured by perinatal factors, the group differences in cognitive and fine motor abilities at youth could not be solely explained by a possible mediating effect of perinatal factors such as birthweight. Cognitive scores within the drug-exposed group were partially related to the number of changes in caregivers and the age at which the last change in caregiver occurred. However, these relationships were only significant for short-term memory.
Cognitive Scores and Group Differences
In general, the drug-exposed group did not obtain substantially worse results than those expected in the general population. Although some of these findings can be related to old norms and a Flynn effect (Trahan, Stuebing, Fletcher, & Hiscock, Citation2014), for example on the RCFT with normative data from 1995, the drug-exposed group performed quite well compared to our original hypothesis. These positive results are especially remarkable when considering that these youths have a combination of several risk factors that are not limited to prenatal drug exposure. It is likely that the mothers’ drug abuse coexisted with other unhealthy habits during pregnancy. Environmental factors that are known to influence the fetus include maternal stress (Monk et al., Citation2012), smoking (England et al., Citation2001) and poor nurturing (Stein et al., Citation1972), all factors that the mothers of these youths may have experienced more than the average population. In addition, it is likely that the mothers had genetic factors that may have influenced their addiction to poly-drugs, and in turn may have genetically influenced their children’s cognitive and fine motor functioning. Thus, it is possible that the youths of mothers partaking in poly-drug use had an unfavorable genetic starting point compared to the rest of the population. One reason for the positive findings may be that environmental factors, such as stable, sensitive and supportive parenting and adaptations to individual needs in day care and school, promoted positive cognitive development in these high-risk children. We used a naturalistic design in which most of the drug-exposed children were placed very early in specially selected permanent foster and adoptive homes. Their caregivers had a relatively high socioeconomic status compared to the norm for foster parents and the general population in Norway (Moe & Slinning, Citation2001; Statistics Norway, Citation2014). The foster and adoptive parents were trained to take care of children with special needs (Moe, Citation2002b; Slinning, Citation2003), and most caregivers in the risk group had support for 2 to 3 years after birth from the Aline Infant and Family Center or the research group. Unfortunately, it is not possible to separate these probable causal factors in clinical studies, including the present study.
However, the youths in the risk group had statistically significantly worse cognitive and fine motor functioning compared to the non-exposed group, even after accounting for the differences in age and caregiver education. The significant effect sizes were in the range of approximately 0.5 to 0.9 standard deviations. The combination of cognitive functions within the normal range but significantly worse than a non-exposed group is in accordance with previous studies of young children with prenatal opioid or poly-drug exposure (Bauman & Levine, Citation1986; Bunikowski et al., Citation1998; Hans & Jeremy, Citation2001; Hunt et al., Citation2008; Johnson et al., Citation1984; Logan et al., Citation2011; van Baar & de Graaff, Citation1994; Wilson, Citation1989; Wilson et al., Citation1979) and youths with such prenatal exposure (Davis & Templer, Citation1988; Ornoy et al., Citation2010).
However, there are earlier studies that did not find significant group differences in the cognitive abilities of drug-exposed children compared to non-exposed children (Bernstein et al., Citation1984; de Cubas & Field, Citation1993; Hans, Citation1989; Kaltenbach & Finnegan, Citation1989; Lifschitz et al., Citation1985; Melinder et al., Citation2013; Messinger et al., Citation2004; Rosen & Johnson, Citation1985; Salo et al., Citation2009). The present study is, to our knowledge, the first such study of vulnerable youths that does not include younger children and investigates the mediating effects of perinatal factors. It is possible that cognitive problems increase over time for this vulnerable group, as described in one of the few previous longitudinal studies of youths who were prenatally exposed to opioids and multiple substances (Ornoy et al., Citation2010). The findings of clearer group differences in the cognitive abilities of youth compared to earlier studies of the same sample at up to 4.5 years of age indicate that the children’s problems did not decline (Moe, Citation2002a; Moe & Slinning, Citation2001; Slinning, Citation2004); rather, there seemed to be an increase in problems up to 8 years of age (Nygaard, Moe, Slinning, & Walhovd, Citation2015). It is possible that the lack of findings in some studies may be because most studies have investigated much younger children than the present study. Another difference may be the use of control groups that were exposed to more risk factors than in the present study. For example, both the drug-exposed children and the control group in the Hans (Citation1989) study lived with their biological parents and had a low socioeconomic status. Thus, possible group differences may have been partially hidden within the context of higher levels of accumulated risk (Tronick & Beeghly, Citation1999).
In addition to age, there may be other reasons for the findings of significant group differences. One strength of this study is the prospective method, which avoids selection bias. Most studies have either a cross-sectional design or do not include children before birth. Most of the participants in the present study were included before birth or immediately after birth. Most of the children born to mothers who were addicted to illegal drugs, who needed help and who were located within a geographical area were included in the study (Moe & Slinning, Citation2001). Although there was a drop-out bias of children with worse outcomes than indicated by the presented results, it is possible that the present study included a larger proportion of children with exposure to multiple drugs and other risk factors than is common for studies of children born to mothers who have partaken in opioid and poly-drug use during pregnancy. The quite high incidence of early placement in foster or adoptive families also indicates a high level of early risk factors among the participants.
Non-specific Problems with Executive Functions and Fine Motor Abilities
Many studies have found that prenatal drug exposure, including nicotine, alcohol, marijuana and cocaine, is related to problems with executive functions and related behaviors (Behnke & Smith, Citation2013). For example, a review found that prenatal cocaine exposure is related to many different functions but is most clearly related to sustained attention and behavioral self-regulation when controlling for covariates (Ackerman et al., Citation2010). There is also a relationship between neuroanatomical features, such as the thickness of the corpus callosum, and specific problems with executive functions and motor performance in people with fetal alcohol exposure (Bookstein, Streissguth, Sampson, Connor, & Barr, Citation2002). It is thought that such exposure may have specific consequences for basic abilities related to attention (Lane et al., Citation2014) and fine motor abilities (Doney et al., Citation2014). Although there is much less research concerning prenatal exposure to opioids and multiple drugs, there seems to be a growing consensus related to problems of attention and emotional and behavior regulation than cognitive problems (Behnke & Smith, Citation2013). For example, a neurocognitive study found that children who were prenatally exposed to opioids and multiple drugs have a thinner right lateral orbitofrontal cortex and right anterior cingulate cortex, areas that are highly related to attentional and social problems (Walhovd et al., Citation2007). Worse motor function has also been found in infants who were prenatally exposed to methadone without worse cognitive behaviors in these infants (Bernstein et al., Citation1984). Thus, we predicted more group differences on tests related to executive functions than on other tests.
However, we found group differences on tests of general cognitive abilities and short- and long-term memory that were just as large as on tests of specific executive functions. The only factor of executive control with significant group differences after controlling for demographic features was the ability to monitor and update working memory, and the effect size was approximately the same as for general mental abilities and visual long-term memory (). Moreover, the group differences on tests of the specific executive functions of shifting and inhibition were not significant when controlling for all demographic variables. It is difficult to determine whether the lack of specific problems with executive functions is due to methodological differences between the tests or because those with prenatal drug exposure have general rather than specific problems. A test with low reliability or validity would have less chance of showing significant group differences than a test with higher reliability. However, this does not seem to be the reason for the present findings. For example, Miyake et al. (Citation2000) found the Antisaccade test to have a reliability of .77, whereas the reliability of the Letter memory task was only .42. In spite of this, we found significant group differences on the Letter memory task, whereas the group differences on the Antisaccade test were not significant after controlling for demographic features. Furthermore, the D-KEFS (Delis et al., Citation2001b) and Plus-Minus (Miyake et al., Citation2000) tests, which were used to measure inhibition and shifting, have similar acceptable levels of reliability and validity and thus should have had a similar likelihood of showing group differences as the tests of short- and long-term memory. It is possible that the correction within these tests of results on prior subtests (mathematical abilities on Plus-Minus and color naming on D-KEFS) may have overcorrected, and thus underestimated the shifting and inhibition problems in the drug-exposed group. However, our findings do not support the hypothesis that youth who were prenatally exposed to opioids and multiple drugs have specific problems with shifting and inhibition. Rather, it seems that they have problems within a broad spectrum of abilities.
As found in previous studies (Bernstein et al., Citation1984; Bunikowski et al., Citation1998; Davis & Templer, Citation1988; Hans, Citation1989; Hans & Jeremy, Citation2001; Logan et al., Citation2011; Wahlsten & Sarman, Citation2013), the drug-exposed group had significantly worse fine motor functioning than the non-exposed group. However, the results were within the normal range, and the differences between the groups were not larger than the differences in general cognitive abilities. Problems with fine motor abilities are often related to problems with executive functions (Rigoli et al., Citation2012). Thus, the lack of specific difficulties with fine motor abilities may support a lack of specific problems with executive functioning within the present sample.
Opiates and Number of Drugs
Clinical studies such as the present one cannot differentiate between the effects of the different drugs of poly-drug use during pregnancy. Because poly-drug use is a common feature of opioid use and is common for people on opioid maintenance treatment (Delano, Gareri, & Koren, Citation2013), most clinical studies of prenatal opioid exposure include persons who use a wide variety of drugs.
Although animal studies indicate that opiates may have neurotoxicological effects (Konijnenberg & Melinder, Citation2011), the present study did not find any significant differences between children born to mothers who used heroin as their main drug of choice compared to other drugs. It is possible that more mothers used heroin than was self-reported, as the mothers were in a situation in which the authorities would evaluate whether the child should be moved to another caretaker. The mothers may have deduced that it was preferable to report other drugs with less prejudice and remain silent about their heroin abuse. Neither nicotine, alcohol, marijuana nor cocaine is thought to give withdrawal symptoms (Behnke & Smith, Citation2013). Other studies have found that approximately 60–80% of newborns exposed to heroin or methadone in utero have symptoms of withdrawal (Patrick et al., Citation2012). Thus, the finding in the present study that 78% of the drug-exposed children experienced neonatal withdrawal symptoms whereas only 53% of the mothers reported using heroin indicates an underreporting of opioid use.
There were indications of a relationship between the number of different drugs and cognitive abilities. The number of drugs to which the youths were prenatally exposed may have increasing or synergistic toxicological effects, but it may also be an indication of the severity of the mothers’ general situation and functioning.
Birthweight
It is well known that children born to mothers who used opioids and multiple drugs during pregnancy are often smaller and born earlier than children without such risk factors (Creanga et al., Citation2012), even after allowing for gestation age and cigarette smoking (Mactier et al., Citation2014). It is thought that perinatal factors such as birthweight may be a result of the prenatal environment, such as drug exposure (Creanga et al., Citation2012; England et al., Citation2001). It has also been found that perinatal factors such as birthweight may be predictors of later brain volume (Walhovd et al., Citation2012) and cognitive abilities (Leitner et al., Citation2000). Therefore, it is possible that the group differences in cognitive abilities in the present study could have been mediated by perinatal factors such as birthweight. However, the mediation analyses indicated that such possible mediation effects were, in general, not significant, and thus could not explain the group differences in cognitive abilities. There were some variations; the only significant mediation effect was that birthweight partially mediated group differences in short-term memory for numbers on the WAIS. We presented 11 test results; the test showed the mediation effect may be coincidental when using a significance level of 5%. We will therefore be cautious in interpreting why the short-term memory for the numbers test and not any other test showed a significant mediating effect of birthweight. However, it is worth noting that most of the mediation effects trended in same direction, which may explain a possible path of causality for some of the observed vulnerabilities in the drug-exposed group.
Change in Caregiver
The analyses of changes in caregiver indicated a positive effect of undergoing fewer changes in caregivers and moving earlier rather than later to an alternative caregiver. Because most youths were brought up outside their biological family, we could not directly analyze whether there was a positive effect of the change in caregiver. However, it is probable that the children who moved at an early age from their biological mothers had a worse starting point than those who moved later; thus, the opposite findings should be expected. The more serious the problems the mothers had, the earlier it was likely to become clear for the County Social Welfare Board and child protective services that the mothers were unable to participate in rehabilitation programs or take adequate care of their children; thus, more rapid changes in custody were likely. The finding that an earlier rather than later change was related to a positive cognitive outcome indicates a positive effect of this change in caregivers that partially counteracts the likely more negative starting point. Most (87%) of the drug-exposed children moved to another final caregiver before they were 1 year of age, a period that is important for the child’s establishment of attachment (Bowlby, Citation1982; Sroufe, Egeland, Carlson, & Collins, Citation2005). The early age of placement and intense follow up by the Aline Infant and Family Center before moving should have minimized the effect of an early postnatal detrimental environment. The adoptive and foster parents in the present study were stable, specially selected to care for children at risk, and had a relatively high socioeconomic status compared to what was common for foster parents and the general population in Norway at the time (Moe & Slinning, Citation2001; Statistics Norway, Citation2014). Thus, there are indications that the children who moved to foster and adoptive homes were brought up in normal, stable, caring family environments and that the earlier this was done, the better. Although there were indications of a relationship between a change in caregiver and cognitive scores, the relationship was not strong. It is possible that there are larger protective effects of caregiving variables on more secondary disabilities from prenatal exposure, such as behavioral problems or mental health problems, than the more primary conditions of prenatal drug exposure on cognitive and fine motor functions (Streissguth, Barr, Kogan, & Bookstein, Citation1996; Streissguth et al., Citation2004).
We do not believe that it is possible to provide a simple explanation for why the drug-exposed group showed lower cognitive abilities than the non-exposed group. As suggested by Sameroff (Citation2010), there are transactional processes between nature and nurture features over time that facilitate how people develop. For example, the child’s brain is influenced by both heritable factors (Rimol et al., Citation2010) and environmental factors both before (Dörrie et al., Citation2014) and after (Zatorre, Fields, & Johansen-Berg, Citation2012) birth. Children are in constant interaction with their environment and are thus both influenced by and have an influence on, for example, their caretaking environment. In some cases, extended family is also important; grandparents were alternative caregivers for some of the vulnerable children in the present sample. It is also well known that the way that schools take care of vulnerable children may influence their development, and that the children’s behavior may influence the teacher’s reactions toward them. In addition, we believe that communities’ attitudes toward children with special needs and the thoughts of political and health authorities on how to take care of pregnant mothers who use opioids and multiple drugs are important for the development of the children. It is impossible in the present clinical study to statistically test such realistic and complex models of development.
Other Strengths and Limitations
A limitation of the present study, as for most studies of such vulnerable samples, is the low number of participants, which increases the chance of random effects and minimizes the possibility to control the results of covariate factors. We have therefore included results from all the cognitive tests performed on the present sample of youth to avoid presenter bias. We have also analyzed general findings across the different tests and have been careful when interpreting findings on a single test that were not supported by findings of other tests, such as the finding of significant mediation effects of birthweight on only 1 of the 11 tests. A major strength of the study is its prospective features: we followed the same drug-exposed group and non-exposed group from birth, reducing the possibility of selection bias and ensuring vital information about the drop-out group.
As is normal for prospective longitudinal studies, there was a skewed drop out over the 20-year period. The sample that participated in youth may have had better functioning than the original sample, as indicated by more drop outs among children who stayed with their biological parents or who had parents who used heroin as their main drug of choice. The analyzed participants also had better perinatal features and better cognitive abilities at earlier assessments than the drop outs (see Table A2 in Supplementary data). We also excluded five children with symptoms of fetal alcohol spectrum disorder, who performed worse on earlier tests of cognitive abilities. Thus, it is probable that the results for all children with prenatal drug exposure who were originally included in the study at infancy would be worse than what has been presented here for the subsample of youth participants.
Conclusions
The present study is, to our knowledge, the first study of youths and young adults (aged 17 to 22 years) who were prenatally exposed to opioids and multiple drugs. Although the drug-exposed youths functioned within the normal range, the current study indicates that the cognitive problems that were found in previous investigations (ages 1, 2, 3 and 4.5 years) in the same sample (Moe, Citation2002a; Moe & Slinning, Citation2001; Slinning, Citation2004) do not disappear over time. In youth, the results of neuropsychological tests did not indicate specific problems with executive functions but rather a mild general feature. The problems identified cannot be explained by perinatal factors, even though it is probable that birthweight and gestational age are important for this group of vulnerable children. The number of drugs may be a risk factor. Several changes of caregivers instead of an early change to stable foster or adoptive care may also be related to worse outcomes in youth. It is difficult to untangle the more direct causal effects in a longitudinal clinical study, but it is likely that a transaction process during the life span that involves both environmental postnatal factors and factors at birth is important (Sameroff, Citation2010). However, the knowledge that these youths have worse outcomes on basic cognitive abilities tests than other comparable youths should, in itself, be important knowledge for policymakers and health or school personnel who come into contact with these vulnerable youths.
Supplementary Materials
Download MS Word (75.6 KB)We are grateful to the youths and their caregivers for generously devoting their time to this project. We thank Tore Wentzel-Larsen for statistical advice and Unni Rosenkilde for the help with recruiting the participants.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Supplementary data
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Aarnoudse-Moens, C. S. H., Weisglas-Kuperus, N., van Goudoever, J. B., & Oosterlaan, J. (2009). Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics, 124, 717–728. doi:10.1542/peds.2008-2816
- Achenbach, T. M., & Rescorla, L. A. (2003). Manual for the ASEBA adult forms & profiles. Burlington: University of Vermont, Research Center for Children, Youth, & Families.
- Ackerman, J. P., Riggins, T., & Black, M. M. (2010). A review of the effects of prenatal cocaine exposure among school-aged children. Pediatrics, 125, 554–565. doi:10.1542/peds.2009-0637
- Bauer, C. R., Shankaran, S., Bada, H. S., Lester, B., Wright, L. L., Krause-Steinrauf, H., … Verter, J. (2002). The maternal lifestyle study: Drug exposure during pregnancy and short-term maternal outcomes. American Journal of Obstetrics and Gynecology, 186, 487–495. doi:10.1067/mob.2002.121073
- Bauman, P. S., & Levine, S. A. (1986). The development of children of drug-addicts. International Journal of the Addictions, 21, 849–863. Retrieved from http://www.tandfonline.com/action/journalInformation?journalCode=isum20#.VgU5QP7otaR
- Behnke, M., Smith, V. C., & Committee on Substance Abuse and Committee on Fetus and Newborn. (2013). Prenatal substance abuse: Short- and long-term effects on the exposed fetus. Pediatrics, 131, e1009–e1024. doi:10.1542/peds.2012-3931
- Bernstein, V., Jeremy, R. J., Hans, S. L., & Marcus, J. (1984). A longitudinal study of offspring born to methadone-maintained women. II. Dyadic interaction and infant behavior at 4 months. The American Journal of Drug and Alcohol Abuse, 10, 161–193. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6475886
- Bookstein, F. L., Streissguth, A. P., Sampson, P. D., Connor, P. D., & Barr, H. M. (2002). Corpus callosum shape and neuropsychological deficits in adult males with heavy fetal alcohol exposure. Neuroimage, 15, 233–251. doi:10.1006/nimg.2001.0977
- Bowlby, J. (1982). Attachment and loss: Retrospect and prospect. The American Journal of Orthopsychiatry, 52, 664–678. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7148988
- Bunikowski, R., Grimmer, I., Heiser, A., Metze, B., Schafer, A., & Obladen, M. (1998). Neurodevelopmental outcome after prenatal exposure to opiates. European Journal of Pediatrics, 157, 724–730. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9776530
- Creanga, A. A., Sabel, J. C., Ko, J. Y., Wasserman, C. R., Shapiro-Mendoza, C. K., Taylor, P., … Paulozzi, L. J. (2012). Maternal drug use and its effect on neonates: A population-based study in Washington State. Obstetrics & Gynecology, 119, 924–933. doi:10.1097/AOG.0b013e31824ea276
- Crea, T. M., Barth, R. P., Guo, S., & Brooks, D. (2008). Behavioral outcomes for substance-exposed adopted children: Fourteen years postadoption. The American Journal of Orthopsychiatry, 78, 11–19. doi:10.1037/0002-9432.78.1.11
- Davis, D. D., & Templer, D. I. (1988). Neurobehavioral functioning in children exposed to narcotics in utero. Addictive Behaviors, 13, 275–283. Retrieved from http://www.journals.elsevier.com/addictive-behaviors/
- de Cubas, M. M., & Field, T. (1993). Children of methadone-dependent women: Developmental outcomes. The American Journal of Orthopsychiatry, 63, 266–276. Retrieved from http://www.apa.org/pubs/journals/ort/
- Delano, K., Gareri, J., & Koren, G. (2013). Rates of fetal polydrug exposures in methadone-maintained pregnancies from a high-risk population. PLoS ONE, 8, e82647. doi10.1371/journal.pone.0082647
- Delis, D. C., Kaplan, E., & Kramer, J. H. (2001a). Delis-Kaplan Executive Function System. Examiners manual. San Antonio, TX: The Psychological Corporation.
- Delis, D. C., Kaplan, E., & Kramer, J. H. (2001b). Delis-Kaplan Executive Function System. Technical manual. San Antonio, TX: The Psychological Corporation.
- Delis, D. C., Kramer, J. H., Kaplan, E., & Ober, B. A. (2000). California verbal learning test – Second edition (CVLT-II). Adult version. Manual. San Antonio, TX: The Psychological Corporation.
- Doney, R., Lucas, B. R., Jones, T., Howat, P., Sauer, K., & Elliott, E. J. (2014). Fine motor skills in children with prenatal alcohol exposure or fetal alcohol spectrum disorder. Journal of Developmental and Behavioral Pediatrics, 35, 598–609. doi:10.1097/DBP.0000000000000107
- Dörrie, N., Föcker, M., Freunscht, I., & Hebebrand, J. (2014). Fetal alcohol spectrum disorders. European Child & Adolescent Psychiatry, 23, 863–875. doi:10.1007/s00787-014-0571-6
- England, L. J., Kendrick, J. S., Wilson, H. G., Merritt, R. K., Gargiullo, P. M., & Zahniser, S. C. (2001). Effects of smoking reduction during pregnancy on the birth weight of term infants. American Journal of Epidemiology, 154, 694–701. doi:10.1093/aje/154.8.694
- Flak, A. L., Su, S., Bertrand, J., Denny, C. H., Kesmodel, U. S., & Cogswell, M. E. (2014). The association of mild, moderate, and binge prenatal alcohol exposure and child neuropsychological outcomes: A meta-analysis. Alcoholism-Clinical and Experimental Research, 38, 214–226. doi:10.1111/Acer.12214
- Hans, S. L. (1989). Developmental consequences of prenatal exposure to methadone. Annals of the New York Academy of Sciences, 562, 195–207. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2742277
- Hans, S. L. (1996). Prenatal drug exposure: Behavioral functioning in late childhood and adolescence. NIDA Research Monograph, 164, 261–276. Retrieved from http://archives.drugabuse.gov/pdf/monographs/
- Hans, S. L., & Jeremy, R. J. (2001). Postneonatal mental and motor development of infants exposed in utero to opioid drugs. Infant Mental Health Journal, 22, 300–315. doi:10.1002/imhj.1003
- Harlan, R. E., & Song, D. D. (1994). Prenatal morphine treatment and the development of the striatum. Regulatory Peptides, 54, 117–118. doi:10.1016/0167-0115(94)90417-0
- Hayes, A. F. (2012a). PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling. Retrieved from http://www.afhayes.com/public/process2012.pdf
- Hayes, A. F. (2012b). SPSS PROCESS documentation. Retrieved from http://www.processmacro.org/
- Heaton, R. K., Grant, I., & Matthews, C. G. (1991). Comprehensive norms for an expanded Halstead-Reitan battery. Odessa: Psychological Assessment Resources.
- Hediger, M. L., Overpeck, M. D., Ruan, W. J., & Troendle, J. F. (2002). Birthweight and gestational age effects on motor and social development. Paediatric and Perinatal Epidemiology, 16, 33–46. doi:10.1046/j.1365-3016.2002.00393.x
- Hickey, J. E., Suess, P. E., Newlin, D. B., Spurgeon, L., & Porges, S. W. (1995). Vagal tone regulation during sustained attention in boys exposed to opiates in utero. Addictive Behaviors, 20, 43–59. Retrieved from http://www.journals.elsevier.com/addictive-behaviors/
- Hunt, R. W., Tzioumi, D., Collins, E., & Jeffery, H. E. (2008). Adverse neurodevelopmental outcome of infants exposed to opiate in-utero. Early Human Development, 84, 29–35. doi:10.1016/j.earlhumdev.2007.01.013
- Ismail, L. C., Knight, H. E., Ohuma, E. O., Hoch, L., Chumlea, W. C., & Consortiu, I. F. N. G. (2013). Anthropometric standardisation and quality control protocols for the construction of new, international, fetal and newborn growth standards: The INTERGROWTH-21st Project. Bjog: An International Journal of Obstetrics and Gynaecology, 120, 48–55. doi:10.1111/1471-0528.12127
- Jaeggi, S. M., Buschkuehl, M., Perrig, W. J., & Meier, B. (2010). The concurrent validity of the N-back task as a working memory measure. Memory, 18, 394–412. doi:10.1080/09658211003702171
- Johnson, H. L., Diano, A., & Rosen, T. S. (1984). 24-Month neuro-behavioral follow-up of children of methadone-maintained mothers. Infant Behavior & Development, 7, 115–123. doi:10.1016/S0163-6383(84)80027-2
- Julian, M. M. (2013). Age at adoption from institutional care as a window into the lasting effects of early experiences. Clinical Child and Family Psychology Review, 16, 101–145. doi:10.1007/s10567-013-0130-6
- Kaltenbach, K., & Finnegan, L. P. (1989). Children exposed to methadone inutero – Assessment of developmental and cognitive-ability. Annals of the New York Academy of Sciences, 562, 360–362. doi:10.1111/j.1749-6632.1989.tb21039.x
- Konijnenberg, C., & Melinder, A. (2011). Prenatal exposure to methadone and buprenorphine: A review of the potential effects on cognitive development. Child Neuropsychology, 17, 495–519. doi:10.1080/09297049.2011.553591
- Lane, K. A., Stewart, J., Fernandes, T., Russo, N., Enns, J. T., & Burack, J. A. (2014). Complexities in understanding attentional functioning among children with fetal alcohol spectrum disorder. Frontiers in Human Neuroscience, 8. doi:10.3389/Fnhum.2014.00119
- Leitner, Y., Fattal-Valevski, A., Geva, R., Bassan, H., Posner, E., Kutai, M., … Harel, S. (2000). Six-year follow-up of children with intrauterine growth retardation: Long-term, prospective study. Journal of Child Neurology, 15, 781–786. doi:10.1177/088307380001501202
- Lester, B. M., & Lagasse, L. L. (2010). Children of addicted women. Journal of Addictive Diseases, 29, 259–276. doi:10.1080/10550881003684921
- Lifschitz, M. H., Wilson, G. S., Smith, E. O., & Desmond, M. M. (1985). Factors affecting head growth and intellectual function in children of drug addicts. Pediatrics, 75, 269–274. Retrieved from http://pediatrics.aappublications.org/
- Logan, B. A., Brown, M. S., & Hayes, M. J. (2013). Neonatal abstinence syndrome: Treatment and PEDIATRIC OUTCOMES. Clinical Obstetrics and Gynecology, 56, 186–192. doi:10.1097/GRF.0b013e31827feea4
- Logan, B. A., Heller, N. A., Paul, J. A., Morrison, D. G., Brown, M., Krishnan, R., & Hayes, M. J. (2011). Longitudinal developmental outcomes in the first year in opiate-exposed infants: Role of prenatal alcohol exposure. Alcoholism-Clinical and Experimental Research, 35, 40a–40a. doi:10.1111/j.1530-0277.2011.01497.x
- Lu, R., Liu, X., Long, H., & Ma, L. (2012). Effects of prenatal cocaine and heroin exposure on neuronal dendrite morphogenesis and spatial recognition memory in mice. Neuroscience Letters, 522, 128–133. doi:10.1016/j.neulet.2012.06.023
- Mactier, H., Shipton, D., Dryden, C., & Tappin, D. M. (2014). Reduced fetal growth in methadone-maintained pregnancies is not fully explained by smoking or socio-economic deprivation. Addiction, 109, 482–488. doi:10.1111/Add.12400
- Manchikanti, L., Fellows, B., Ailinani, H., & Pampati, V. (2010). Therapeutic use, abuse, and nonmedical use of opioids: A ten-year perspective. Pain Physician, 13, 401–435. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20859312
- Mayes, L. C. (1999). Developing brain and in utero cocaine exposure: Effects on neural ontogeny. Development and Psychopathology, 11, 685–714. Retrieved from http://journals.cambridge.org/action/displayJournal?jid=DPP
- Melinder, A., Konijnenberg, C., & Sarfi, M. (2013). Deviant smooth pursuit in preschool children exposed prenatally to methadone or buprenorphine and tobacco affects integrative visuomotor capabilities. Addiction, 108, 2175–2182. doi:10.1111/Add.12267
- Messinger, D. S., Bauer, C. R., Das, A., Seifer, R., Lester, B. M., Lagasse, L. L., … Poole, W. K. (2004). The maternal lifestyle study: Cognitive, motor, and behavioral outcomes of cocaine-exposed and opiate-exposed infants through three years of age. Pediatrics, 113, 1677–1685. Retrieved from http://pediatrics.aappublications.org/
- Meyers, J. E., & Meyers, K. R. (1995). Rey complex figures test and recognition trail: Professional manual. Odessa: Psychological Assessment Resources.
- Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., & Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cognitive Psychology, 41, 49–100. doi:10.1006/cogp.1999.0734
- Moe, V. (2002a). Foster-placed and adopted children exposed in utero to opiates and other substances: Prediction and outcome at four and a half years. Journal of Developmental and Behavioral Pediatrics, 23, 330–339. Retrieved from http://journals.lww.com/jrnldbp/Pages/default.aspx
- Moe, V. (2002b). A prospective, longitudinal study of children prenatally exposed to drugs. Prediction and developmental outcome at 4 1/2 years ( PhD). University of Oslo, Oslo. (ISBN 82-569-2027-0).
- Moe, V., Siqveland, T., & Slinning, K. (2011). Children of parents with substance abuse and mental health problems. In H. E. FitzGerald, K. Puura, M. Tomlinson, & C. Paul (Eds.), International perspectives on children and mental health, Vols 1 and 2: Development and context, prevention and treatment (pp. 155–177). Santa Barbara, CA: Praeger/ABC-CLIO.
- Moe, V., & Slinning, K. (2001). Children prenatally exposed to substances: Gender-related differences in outcome from infancy to 3 years of age. Infant Mental Health Journal, 22, 334–350. doi:10.1002/imhj.1005
- Moe, V., & Slinning, K. (2002). Prenatal drug exposure and the conceptualization of long-term effects. Scandinavian Journal of Psychology, 43, 41–47. Retrieved from http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1467-9450
- Monk, C., Spicer, J., & Champagne, F. A. (2012). Linking prenatal maternal adversity to developmental outcomes in infants: The role of epigenetic pathways. Development and Psychopathology, 24, 1361–1376. doi:10.1017/S0954579412000764
- Neonatal Research Network. (2013). The Maternal Lifestyle Study (MLS). Retrieved November 25, 2014, from https://neonatal.rti.org/about/mls_background.cfm
- Nygaard, E., Moe, V., Slinning, K., & Walhovd, K. B. (2015). Longitudinal cognitive development of children born to mothers with opioid and polysubstance use. Pediatric Research, 78, 330–335. doi:10.1038/pr.2015.95
- Ornoy, A., Daka, L., Goldzweig, G., Gil, Y., Mjen, L., Levit, S., … Greenbaum, C. W. (2010). Neurodevelopmental and psychological assessment of adolescents born to drug-addicted parents: Effects of SES and adoption. Child Abuse & Neglect, 34, 354–368. doi:10.1016/j.chiabu.2009.09.012
- Ornoy, A., Segal, J., Bar-Hamburger, R., & Greenbaum, C. (2001). Developmental outcome of school-age children born to mothers with heroin dependency: Importance of environmental factors. Developmental Medicine and Child Neurology, 43, 668–675. Retrieved from http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1469-8749
- Patrick, S. W., Schumacher, R. E., Benneyworth, B. D., Krans, E. E., McAllister, J. M., & Davis, M. M. (2012). Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA, 307, 1934–1940. doi:10.1001/jama.2012.3951
- Rigoli, D., Piek, J. P., Kane, R., & Oosterlaan, J. (2012). An examination of the relationship between motor coordination and executive functions in adolescents. Developmental Medicine and Child Neurology, 54, 1025–1031. doi:10.1111/j.1469-8749.2012.04403.x
- Rimol, L. M., Panizzon, M. S., Fennema-Notestine, C., Eyler, L. T., Fischl, B., Franz, C. E., … Dale, A. M. (2010). Cortical thickness is influenced by regionally specific genetic factors. Biological Psychiatry, 67, 493–499. doi:10.1016/j.biopsych.2009.09.032
- Rosen, T. S., & Johnson, H. L. (1985). Long-term effects of prenatal methadone maintenance. In T. Pinkert (Ed.), Current research on the consequences of maternal drug abuse (pp. 73–83). Rockville, MD: National Institute on Drug Abuse.
- Salo, S., Kivistö, K., Korja, R., Biringen, Z., Tupola, S., Kahila, H., & Kivitie-Kallio, S. (2009). Emotional availability, parental self-efficacy beliefs, and child development in caregiver-child relationships with buprenorphine-exposed 3-year-olds. Parenting-Science and Practice, 9, 244–259. doi:10.1080/15295190902844563
- Sameroff, A. (2010). A unified theory of development: A dialectic integration of nature and nurture. Child Development, 81, 6–22. doi:10.1111/j.1467-8624.2009.01378.x
- Sanchez, E., Bigbee, J., Fobbs, W., Robinson, S., & Sato-Bigbee, C. (2008). Opioid addiction and pregnancy: Perinatal exposure to buprenorphine affects myelination in the developing brain. Glia, 56, 1017–1027. doi:10.1002/glia.20675
- Skinner, M. L., Haggerty, K. P., Fleming, C. B., & Catalano, R. F. (2009). Predicting functional resilience among young-adult children of opiate-dependent parents. The Journal of Adolescent Health, 44, 283–290. doi:10.1016/j.jadohealth.2008.07.020
- Slinning, K. (2003). A prospective, longitudinal study of children prenatally exposed to substances: With special emphasis on attention and self-regulation ( PhD). University of Oslo, Oslo. (ISBN 82-569-2149-8).
- Slinning, K. (2004). Foster placed children prenatally exposed to poly-substances–attention-related problems at ages 2 and 4 1/2. European Child & Adolescent Psychiatry, 13, 19–27. doi:10.1007/s00787-004-0350-x
- Snodgrass, J. G., & Corwin, J. (1988). Pragmatics of measuring recognition memory – Applications to dementia and amnesia. Journal of Experimental Psychology-General, 117, 34–50. doi:10.1037//0096-3445.117.1.34
- Sowder, B., & Burt, M. (1980). Children of heroin addicts: An assessment of health, learning, behaviroal, and adjustment problems. New York, NY: Praeger.
- Sroufe, L. A., Egeland, B., Carlson, E., & Collins, W. A. (2005). Placing early attachment experiences in developmental context: The Minnesota longitudinal study. In K. E. Grossmann, K. Grossmann, & E. Waters (Eds.), Attachment from infancy to adulthood: The major longitudinal studies (pp. 48–70). New York, NY: Guilford Publications.
- Statistics Norway. (2014). Population’s level of education, 1 October 2013. Retrieved November 18, 2014, from http://www.ssb.no/en/utdanning/statistikker/utniv/aar/2014-06-19
- Stein, Z., Saenger, G., Susser, M., & Marolla, F. (1972). Nutrition and mental performance. Science, 178, 708–713. doi:10.1126/science.178.4062.708
- Strauss, M. E., Lessen-Firestone, J. K., Chavez, C. J., & Stryker, J. C. (1979). Children of methadone-treated women at five years of age. Pharmacology, Biochemistry, and Behavior, 11(Suppl.), 3–6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/550133
- Strauss, M. E., Starr, R. H., Ostrea, E. M., Chavez, C. J., & Stryker, J. C. (1976). Behavioural concomitants of prenatal addiction to narcotics. The Journal of Pediatrics, 89, 842–846. Retrieved from http://www.jpeds.com/
- Streissguth, A. P., Barr, H. M., Kogan, J., & Bookstein, F. L. (1996). Understanding the occurrence of secondary disabilities in clients with fetal alcohol syndrome (FAS) and fetal alcohol effects (FAE). Final report to the centers of disease control and prevention (CDC). Seattle: University of Washington.
- Streissguth, A. P., Bookstein, F. L., Barr, H. M., Sampson, P. D., O’Malley, K., & Young, J. K. (2004). Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. Journal of Developmental and Behavioral Pediatrics, 25, 228–238. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15308923
- Tamnes, C. K., Ostby, Y., Walhovd, K. B., Westlye, L. T., Due-Tonnessen, P., & Fjell, A. M. (2010). Neuroanatomical correlates of executive functions in children and adolescents: A magnetic resonance imaging (MRI) study of cortical thickness. Neuropsychologia, 48, 2496–2508. doi:10.1016/j.neuropsychologia.2010.04.024
- Trahan, L. H., Stuebing, K. K., Fletcher, J. M., & Hiscock, M. (2014). The Flynn effect: A meta-analysis. Psychological Bulletin, 140, 1332–1360. doi:10.1037/A0037173
- Tronick, E. Z., & Beeghly, M. (1999). Prenatal cocaine exposure, child development, and the compromising effects of cumulative risk. Clinics in Perinatology, 26, 151–171. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10214547
- van Baar, A. (1990). Development of infants of drug dependent mothers. Journal of Child Psychology and Psychiatry and Allied Disciplines, 31, 911–920. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2246341
- van Baar, A., & de Graaff, B. M. (1994). Cognitive development at preschool-age of infants of drug-dependent mothers. Developmental Medicine and Child Neurology, 36, 1063–1075. Retrieved from http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1469-8749
- Wahlsten, V. S., & Sarman, I. (2013). Neurobehavioural development of preschool-age children born to addicted mothers given opiate maintenance treatment with buprenorphine during pregnancy. Acta Paediatrica, 102, 544–549. doi:10.1111/Apa.12210
- Walhovd, K. B., Fjell, A. M., Brown, T. T., Kuperman, J. M., Chung, Y., Hagler, D. J., Jr., … Pediactric Imaging, Neurocognition, and Genetics Study. (2012). Long-term influence of normal variation in neonatal characteristics on human brain development. Proceedings of the National Academy of Sciences of the United States of America, 109, 20089–20094. doi:10.1073/pnas.1208180109
- Walhovd, K. B., Fjell, A. M., & Espeseth, T. (2014). Cognitive decline and brain pathology in aging–need for a dimensional, lifespan and systems vulnerability view. Scandinavian Journal of Psychology, 55, 244–254. doi:10.1111/sjop.12120
- Walhovd, K. B., Moe, V., Slinning, K., Due-Tønnessen, P., Bjørnerud, A., Dale, A. M., … Fischl, B. (2007). Volumetric cerebral characteristics of children exposed to opiates and other substances in utero. Neuroimage, 36, 1331–1344. doi:10.1016/j.neuroimage.2007.03.070
- Wang, Y., & Han, T. Z. (2009). Prenatal exposure to heroin in mice elicits memory deficits that can be attributed to neuronal apoptosis. Neuroscience, 160, 330–338. doi:10.1016/j.neuroscience.2009.02.058
- Wechsler, D. (2003). Wechsler Adult Intelligence Scale – Third Edition, Norsk version. Stockholm: Psykologiförlaget AB.
- Wilson, G. S. (1989). Clinical studies of infants and children exposed prenatally to heroin. Annals of the New York Academy of Sciences, 562, 183–194. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2742276
- Wilson, G. S., McCreary, R., Kean, J., & Baxter, J. C. (1979). The development of preschool children of heroin-addicted mothers: A controlled study. Pediatrics, 63, 135–141. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/86983
- World Health Organization. (2009). Guidelines for the psychosocially assisted pharmacological treatment of opioid dependence. Geneva: Author.
- Zatorre, R. J., Fields, R. D., & Johansen-Berg, H. (2012). Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nature Neuroscience, 15, 528–536. doi:10.1038/Nn.3045
- Zhu, J. (1999). WASI Wechsler abbreviated scale of intelligence manual. San Antonio, TX: NCS Pearson.