ABSTRACT
Interference control refers to the ability to selectively attend to certain information while ignoring distracting information. This ability can vary as a function of distractor relevance. Distractors that are particularly relevant to an individual may attract more attention than less relevant distractors. This is referred to as attention bias. Weak interference control and altered reward sensitivity are both important features of attention deficit hyperactivity disorder (ADHD). However, interference control is typically studied in isolation. This study integrates both. Youths (aged 9 to 17 years) with ADHD (n = 37, 25 boys) and typically-developing controls (n = 38, 20 boys) completed a Stroop task using appetitive words and matched neutral words to assess whether appetitive distractors diminished interference control more in youths with ADHD than controls. In order to test for specificity, aversive words were also included. As expected, appetitive words disrupted interference control but this effect was not stronger for youths with ADHD than the controls. Aversive words, on the other hand, facilitated interference control. Dimensional analyses revealed that this facilitation effect increased substantially as a function of ADHD symptom severity. Possible mechanisms for this effect include up-regulation of interference control as a function of induced negative mood, or as a function of increased effort. In conclusion, appetitive words do not lead to worse interference control in youths with ADHD compared with controls. Interference control was modulated in a valence-specific manner, concurrent with mood-induced effects on cognitive control.
Attention deficit hyperactivity disorder (ADHD) is a prevalent developmental disorder which affects about 5% of school-aged children worldwide (Polanczyk & Rohde, Citation2007). It is characterized by high age-inappropriate levels of inattention and/or hyperactivity/impulsivity (American Psychiatric Association, Citation2000, Citation2013) and associated with impaired cognitive, social, and academic functioning (Barkley, Anastopoulos, Guevremont, & Fletcher, Citation1991; Becker et al. Citation2013; Dopheide & Pliszka, Citation2009).
Impaired inhibitory control has been suggested to be one of the key cognitive characteristics associated with ADHD (Barkley, Citation1997). Interference control, a form of inhibition, refers to the ability to suppress distraction by stimuli that are irrelevant to the task at hand. Interference control is typically assessed with Stroop color-word tasks (Stroop, Citation1935). Meta-analyses of this task show that interference control is indeed compromised in ADHD, yet the effect sizes are small (Lansbergen, Kenemans, & van Engeland, Citation2007; van Mourik, Oosterlaan, & Sergeant, Citation2005). This may be unexpected, because age-inappropriate high distractibility is one of the main criteria for ADHD according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR and DSM-V) (American Psychiatric Association, Citation2000, Citation2013).
One potential explanation is the fact that the standard Stroop task uses “neutral” distractors (color words), which do not carry extra relevance to individuals with ADHD. In contrast, the well-established attentional bias Stroop task requires participants to name the font colors while ignoring the semantics of disorder-relevant distractors (words). Specifically, words referring to connotations relevant to the individual have been demonstrated to cause more interference than neutral words (Mathews & MacLeod, Citation1985). This concept has been widely applied to the investigation of psychiatric disorders (for reviews, see Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & Van Ijzendoorn, Citation2007; Williams, Mathews, & MacLeod, Citation1996). These studies confirm that in anxiety and mood disorders or alcohol dependence, disorder-related words caused more interference in patients than in healthy control groups. This is typically interpreted as an attentional bias towards disorder-relevant cues (e.g., the word “alcohol” attracts more attention than the word “table” from someone who is alcohol dependent than from someone who is not). Attentional biases in psychiatric disorders are thought to play an important role in the persistence of the disorder (for a review and a meta-analysis, see Field & Cox, Citation2008; Field, Munafò, & Franken, Citation2009).
In addition to relatively weak inhibitory control, altered motivation has been theorized to play a key role in ADHD (e.g., Barkley, Citation1997; Haenlein & Caul, Citation1987; Sagvolden, Johansen, Aase, & Russell, Citation2005; Sonuga-Barke, Citation2005; for empirical reviews, see Luman, Oosterlaan, & Sergeant, Citation2005; Luman, Tripp, & Scheres, Citation2010). A recent meta-analysis on experimental studies demonstrated that children with ADHD show impaired inhibitory control during non-reinforced conditions, and that reinforcing correct inhibition leads to the normalization of inhibition to the baseline level of the performance of typically-developing controls in children and adolescents with ADHD (Ma, van Duijvenvoorde, & Scheres, Citation2016). In addition, Geurts, Luman, and Van Meel (Citation2008) indicated larger positive effects of social motivation on interference control in children with ADHD compared with controls. Together, these studies suggest that inhibition and interference control deficits in youths with ADHD may be modulated by motivation.
However, interference control deficits in individuals with ADHD are only small to moderate when measured with Stroop tasks (containing abstract, neutral distractors such as color words; e.g., Lansbergen et al., Citation2007; Van Mourik et al., Citation2005). One factor which potentially contributes to this modest effect size is the use of neutral distractors, as multiple studies have shown that healthy individuals are less successful at ignoring or inhibiting their response to a stimulus when that stimulus has appetitive properties compared with when it has neutral properties (e.g., Krebs, Boehler, & Woldorff, Citation2010; Mogg, Bradley, Hyare, & Lee, Citation1998; Somerville, Hare, & Casey, Citation2011). Individuals who show reduced cognitive control may be specifically susceptible to responding to appetitive stimuli. For example, individuals with low cognitive control show more unhealthy food intake when hungry compared with individuals with higher cognitive control (Nederkoorn, Guerrieri, Havermans, Roefs, & Jansen, Citation2009). The question arises as to whether or not the interference control deficit in individuals with ADHD can be exacerbated when appetitive distractors are used. Because of the relationship between ADHD and altered motivation, this study investigated whether motivational words (appetitive and aversive) are more distracting/detrimental to interference control in children and adolescents with ADHD compared with controls. The use of such disorder-related distractors in a Stroop task increases task validity and thus it was hypothesized that it would enhance the ability to discriminate between ADHD and control groups. Due to the altered reward-sensitivity that is associated with ADHD, it was expected that using appetitive words as distractors would increase distractibility compared with neutral words, especially in individuals with ADHD. In order to examine valence specificity, aversive words are additionally compared with neutral words. While, in absolute terms, classic Stroop color-word tasks lead to more interference than attentional bias Stroop tasks, which include non-color-words, this study investigates whether appetitive/aversive distractor words in the Stroop task better distinguish between ADHD and control groups than neutral distractor words.
As there is increasing awareness that the field of psychiatry benefits from using dimensional approaches (e.g., Hyman, Citation2007; Robbins, Gillan, Smith, de Wit, & Ersche, Citation2012), this study additionally examined the relationship between ADHD symptom dimensions (inattention and hyperactivity/impulsivity) and the effects of appetitive/aversive distractors on interference control. An additional reason for using this approach is that it makes it possible to examine the effects of ADHD symptom dimensions separately. This is relevant because altered motivation in ADHD may be more strongly associated with hyperactivity/impulsivity than inattention (e.g., Castellanos, Sonuga-Barke, Milham, & Tannock, Citation2006; Scheres, Tontsch, Thoeny, & Kaczkurkin, Citation2010).
It was hypothesized that (1) individuals with ADHD would demonstrate weaker interference control than controls, (2) attentional bias to motivational cues (appetitive words) would be detrimental to interference control, and (3) this effect would be exacerbated in individuals with ADHD and higher levels of hyperactivity/impulsivity.
Method
Participants and Selection Procedure
Children and adolescents with and without ADHD between 9 and 17 years of age were recruited. Participants with ADHD were recruited via the Child and Adolescent Psychiatry Department at Radboud UMC in Nijmegen, The Netherlands. The control participants were recruited via local advertisements and schools.
Clinical assessment was conducted as follows. All participants with ADHD had been previously diagnosed by accredited clinical psychologists/psychiatrists who had based their diagnostic decision on a combination of observations, interviews, and questionnaires obtained from parents and teachers. The diagnosis of ADHD was then reconfirmed at the time of the study using a structured parent interview: the Diagnostic Interview Schedule for Children – Fourth Edition (DISC–IV; Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, Citation2000). The Dutch version of the Child Behavior Checklist (CBCL; Achenbach & Edelbrock, Citation1991) was included as an additional screening instrument. Participants with ADHD who met psychiatric disorder criteria other than ADHD on the DISC-IV or CBCL were not included, with the exception of participants with ADHD with comorbid oppositional defiant disorder (ODD, n = 9; comorbid conduct disorder [CD] did not occur in this sample) because of the high comorbidity rate. The Disruptive Behavior Disorders Rating Scale (DBDRS; Oosterlaan, Scheres, Antrop, Roeyers, & Sergeant, Citation2000) was used as descriptive instrument (). Children with ADHD who used psychotropic medication other than psychostimulants were excluded from participation, and those who were using psychostimulants (n = 20) discontinued their medication 24 hours prior to testing (Greenhill, Citation1998).
Table 1. Participant Characteristics.
Controls were excluded if they met criteria for psychiatric disorders on the DISC-IV, the DBDRS or the CBCL. Participants in both groups were required to have an estimated IQ>75 based on the vocabulary and block design of the Dutch version of the Wechsler Intelligence Scale for Children – Third Edition (WISC-III; Kort et al., Citation2002). This estimation has an acceptable reliability and correlates highly with full-scale IQ (r= 0.86; Sattler & Saklofske, Citation2001). A total of 16 participants with ADHD and 3 controls were excluded because they met the abovementioned exclusion criteria. The final sample consisted of 75 children and adolescents (ADHD group, n = 37; control group, n = 38). The groups did not differ on gender and age ().
The Stroop Tasks
Comparable to the original Stroop task (Stroop, Citation1935), the experiment began by naming the colors of 100 solid rectangles (baseline color card). The color card was administered once and served as a manipulation check to assess if completion times on the interference cards were indeed slower than just naming colors without any interference. After the color card, participants read aloud 100 words written in black (word cards). Next, they received an interference card with the same words as on the word card, but printed in different font colors (for an overview of the stimuli, see ). For the interference card, participants were instructed to name the font color (red, yellow, blue, or green) as quickly as possible while ignoring the semantic meaning, and to make as few errors as possible. The word card was always administered prior to the interference card and served to prime the semantics of the words. Stimuli (Calibri font, size 16) were presented on white A4-sized landscape format cards in a randomized order. Each row contained 10 stimuli.
Figure 1. Overview of cards and stimuli.
Note. The presented words were translated from the original Dutch used in the study.
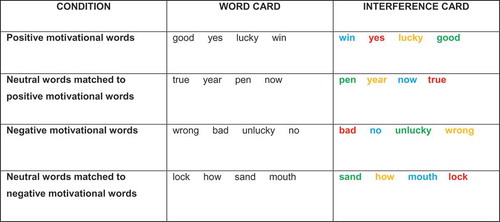
Participants were instructed to perform this task under four conditions: the appetitive words condition, the aversive words condition, and two neutral conditions, one for each motivational condition (appetitive/aversive). Importantly, words on the neutral cards were matched to the words on the motivational cards in word length and frequency in the Dutch language, as these have been shown to influence word recognition (Larsen, Mercer, & Balota, Citation2006). The order of the conditions was counterbalanced using a Latin square design and was equal for each group, χ2(1, 7) = 4.58, p = .845.
The card completion time (in seconds) and the number of errors were recorded for each card. Participants with invalid scores due to skipping or doubling a word row were excluded from analyses. This group composition change did not lead to differences in the results for IQ, gender or age.
Procedure
This study was approved by the local medical ethics committee (CMO 2011/402) as conforming to the Dutch Act on Medical Research Involving Human Subjects. Informed consent was obtained from all participants and their parents. The study consisted of three consecutive test sessions. During the first session, participants completed the WISC-III and the Stroop task while parents were administered the DISC-IV, the DBDRS and the CBCL.
During subsequent sessions, the participants performed the tests that are reported elsewhere. Each session was 1.5 to 2.0 hours in duration, and the sessions were scheduled approximately two weeks apart. The tests were conducted in the behavioral labs of the Behavioural Science Institute of the Radboud University Nijmegen, which provide a quiet, controlled environment devoid of window views and other distractions. Families received €30 for the completion of all sessions.
Data Analyses
Interference Check and Baseline Color Card Performance
To first confirm that each of the four interference cards indeed showed an interference effect, repeated-measures analyses of covariance (ANCOVAs) with age as a covariate were used to compare the baseline color card (naming the colors of 100 solid rectangles) with each interference card. This was carried out for completion times (CT) and/or number of errors. Interference effects were expected for all four interference cards, including the neutral cards. In addition, group differences on the baseline color card were examined. Errors and CTs on the baseline color card were therefore submitted to a univariate ANCOVA with group as an independent variable and age as a covariate.
Second, group differences in interference control were assessed using CTs and errors on neutral cards independent of valence to avoid interaction effects. The advice of Lansbergen et al. (Citation2007) was followed by quantifying interference control as ratio scores. Ratios were used because ADHD groups are typically slowerindependent of interference control compared with controls, and ratios avoid transformation-dependent results (Lansbergen et al., Citation2007). The ratios were calculated as:
and were conducted separately for CTs and errors for both neutral cards (matched to appetitive card, and matched to aversive card). Note that a ratio score of 1 indicates that there was no difference between the two cards. The ratio scores were log transformed to make sure that ratios > 1 are comparable to ratios < 1, and that the ratios were normally distributed. Univariate ANCOVAs with age as covariate were used to assess the group differences. Only neutral cards were used for this comparison in order to avoid interaction effects with appetitive and aversive cards.
The effects of ADHD symptom dimensions were explored, in addition to the group comparisons. Univariate ANCOVAs were used to examine the effects of the ADHD symptom dimensionsFootnote1 of inattention and hyperactivity/impulsivity on baseline color card performance and interference control (ratio scores), while controlling for age.
Stroop Task Main Analyses
First, tests were conducted to establish whether or not the valence manipulation had worked as intended. It was expected that appetitive and aversive words would result in more interference than neutral words. Repeated measures ANCOVAs including age as a covariate were used to examine the differences between the valence interference cards and their matched neutral interference cards. These tests primarily served as task manipulation checks and were conducted both across and within groups. Second, the main research question was examined, namely whether or not the groups differ on the extent to which appetitive and aversive words led to interference compared to neutral words. Therefore, the ratio scores for both valences were computed as follows:
These ratio scores were log transformed and submitted to an ANCOVA, with group (ADHD and control) as the between-subjects factor, age as a covariate, and the age × group interaction as an additional factor. Age was included in these analyses because it might interact with group on interference control, for example as a result of delayed (prefrontal) cortical brain maturation in ADHD (e.g., Shaw et al., Citation2007).
Unrestricted by categorical boundaries, dimensional analyses assessed the relationship between ratio scores and ADHD symptom dimensions (inattention and hyperactivity/impulsivity) across all individuals.Footnote2 To this end, ANCOVAs were conducted with the inattention or hyperactivity/impulsivity DBDRS scores as the predictor, age as a covariate including its interaction with the predictor, and ratio score as the dependent variable.
Results
Interference Check and Baseline Color Card Performance
The descriptive statistics of the CTs and errors on the interference cards are presented for each group in the supplementary materials Table S1. All interference cards (neutral words, appetitive, and aversive words) showed slower CTs when compared with the baseline color card, with substantial effect sizes. This demonstrates that even words that are not color names lead to interference. In addition, older participants were faster on all cards (color cards and interference cards, all ps < .01, all ηp2s > .37) and showed less difference in CTs for the interference cards in comparison with the baseline color card (all ps < .01, all ηp2s > .12). No significant age effects were found on errors.
The ADHD group was slower than the control group on the baseline color card, (F(1, 69) = 5.95, p = .01, ηp2 = .08), but did not make more errors, (F(1, 69) = .75, p = .39, ηp2 = .01). There was no interaction between age and group on baseline color card CTs, (F(1, 69) = 1.76, p = .19, ηp2 = .03), or errors, (F(1, 69) = 2.41, p = .13, ηp2 = .03).
The dimensional analyses revealed that there was an effect of symptom dimension on baseline color card CTs of hyperactivity/impulsivity, (F(1, 60) = 4.80, p = .03, ηp2 = .07), but a weaker relation with inattention, (F(1, 60) = 2.86, p = .09, ηp2 = .05). Interference control ratio scores (baseline color card divided by neutral cards) did not show significant associations with ADHD dimensions (all ps > .09).
The Stroop Task
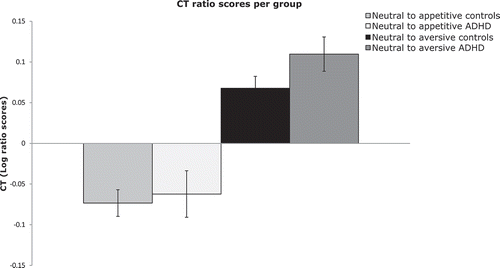
After data inspection, one extreme outlier (more than three interquartile ranges above the median) for the CT ratio scores of appetitive words (a control participant) was excluded, along with two extreme outliers for error ratio scores of appetitive words (both control participants). The descriptive statistics of log ratio scores are presented in . As expected, the appetitive words showed more interference than the neutral words on CTs, (F(1, 67) = 12.75, p = .01, ηp2 = .16), but not on errors, (F(1, 66) = 3.58, p = .06, ηp2 = .05). Unexpectedly, aversive words showed less interference than neutral words for CTs, (F(1, 68) = 29.36, p < .01, ηp2 = .30), and errors, (F(1, 68) = 5.21, p = .03, ηp2 = .07). The interference was present in both groups.
Table 2. Descriptive Statistics of Log Ratio Scores per Condition per Group.
Contrary to the hypothesis, the ANCOVAs showed no effect of group on the appetitive or aversive word ratios compared to the neutral condition: appetitive CT, (F(1, 65) = 0.35, p = .56, ηp2 = .01), appetitive errors, (F(1, 64) = 1.59, p = .21, ηp2 = .02), aversive CT, (F(1, 66) = 3.93, p = .05, ηp2 = .06), aversive errors, (F(1, 66) = 1.18, p = .28, ηp2 = .02). This suggests that the interfering effects of appetitive compared to neutral words were not significantly different between groups ().
Figure 2. Bar graphs depicting the completion time (CT) log ratio between interference cards and the baseline color card for each group.
Note. Log ratio scores >0 indicate slower CT on the neutral card than on the baseline color card (interference effect), while log ratio scores <0 indicate the opposite effect (facilitation).
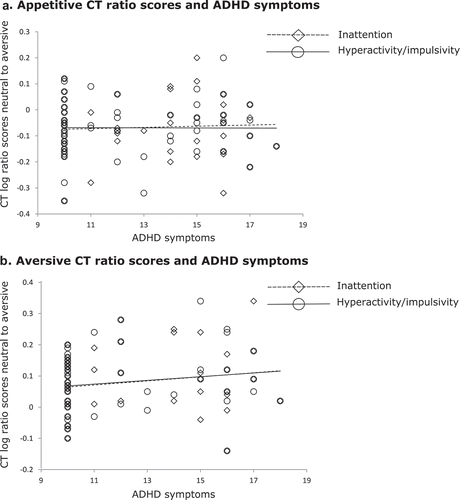
No main effects of age or interactions between age and group were found for ratio scores for CTs and errors in both the appetitive and aversive word conditions (all ps > .18, all ηp2s < .028).
Dimensional Analyses
Inattention and hyperactivity/impulsivity symptoms correlated highly with one another, r = .86, p < .01. For CT ratio scores in the aversive condition there was a main effect of inattention, (F(1, 58) = 8.15, p = .01, ηp2 = .12), and hyperactivity/impulsivity, (F(1, 58) = 11.52, p = .01, ηp2 = .17), indicating that the aversive words had a facilitating effect on the CT with increasing levels of ADHD symptoms (). No significant associations were found in the appetitive condition for inattention, (F(1, 57) = 1.00, p = .32, ηp2 = .02), or hyperactivity/impulsivity, (F(1, 57) = .56, p = .46, ηp2 = .01). No significant effects of inattention and hyperactivity/impulsivity were found on error ratio scores for appetitive words—inattention, (F(1, 57) = .21, p = .65, ηp2 = .01), hyperactivity/impulsivity, (F(1, 57) = 0.01, p = .91, ηp2 < .01)—or aversive words—inattention, (F(1, 58) = 1.62, p = .21, ηp2 = .03), hyperactivity/impulsivity, (F(1, 58) = 0.29, p = .60, ηp2 = .01). Again, no significant effects of age or interactions between age and symptom dimension were found (all ps > .24, all ηp2s < .02).
Discussion
This study aimed to investigate whether interference control in children and adolescents with ADHD was exacerbated by appetitive distracting words. It was hypothesized that (1) appetitive words would cause more interference as compared to neutral words for all participants, and (2) that this detrimental effect of appetitive words on interference control would be stronger in children and adolescents with ADHD than in controls. To investigate whether the effect of appetitive words was valence-specific, the effects of aversive words were also examined.
First of all, performance (CTs and errors) on all interference cards—including appetitive, aversive, and neutral words—differed from performance on the baseline color card (simply naming the colors of rectangles), with substantial effect sizes. This concurs with early research demonstrating that any common word, not only color words, produces interference (Klein, Citation1964). Importantly, this result confirms that the Stroop task indeed measured interference control.
Second, as expected, appetitive words had detrimental effects on interference control, reflected in slower CTs and more errors. This finding indicates that the task manipulation of appetitive words was effective. However, the primary hypothesis has not been confirmed; the detrimental effects of appetitive words on interference control were not exacerbated in the ADHD group. The ADHD group also did not show an interference control deficit, as measured with ratio scores between the interference cards with neutral words and the baseline color card. Lansbergen et al. (Citation2007) showed in a meta-analysis that, when using ratio scores as with the present study, interference control deficits were present in individuals with ADHD. However, the present findings do not support this, as this study found that the ADHD group was overall slower than the control group but that there were no specific interference control deficits. This is in accordance with the findings of van Mourik et al. (Citation2005), who reported that general slow performance in ADHD groups showed a larger effect size than interference control deficits. This suggests that general slowness in information processing may be a more robust characteristic of those with ADHD than difficulty with interference control (however, see Willcutt, Sonuga-Barke, Nigg, & Sergeant, Citation2008). This general slowness is thought to be due to non-optimal energetic states (i.e., arousal, activation) associated with ADHD, or an inability to maintain a stable reaction time over trials (Sergeant, Oosterlaan, & van der Meere, Citation1999; Tamm et al., Citation2012). The current data suggest that using appetitive, distracting words—as measured with a Stroop color-word task—can hamper interference control, but that this is not a more sensitive approach for distinguishing children and adolescents with ADHD from TD individuals.
Unexpectedly, aversive words had facilitating effects on interference control, reflected as faster CTs (but not reflected by a change in errors). This finding contradicts the attention bias theory, which suggests that meaningful words draw attention and may therefore interfere with the task at hand. Furthermore, using a dimensional approach, it became apparent that the facilitating effect of aversive words was stronger with increasing ADHD symptom scores (medium effect sizes). Interpreted within the attentional bias account, this facilitation effect suggests that aversive words are even less relevant for those with more ADHD symptoms than for individuals with fewer ADHD symptoms. There are, however, several alternative theories that could account for both the appetitive as well as aversive effects in the current study. A few accounts are discussed below, and a section on how these accounts may explain the relatively strong facilitating effects of aversive words with higher levels of ADHD symptoms is presented, as this may guide future studies.
The present findings match the idea that frequent, uninterrupted exposure to stimuli of one type of valence may induce mood effects (Ben-Haim, Mama, Icht, & Algom, Citation2014; Richards, French, Johnson, Naparstek, & Williams, Citation1992). The “mood as information theory” (Clore, Schwarz, & Conway, Citation1994; Schwarz & Clore, Citation1983) proposes that mood states inform the individual of environmental demands to alter the current situation: a positive mood signals that no change is required, leading to a down-regulation of cognitive control, while a negative mood signals a need for adaptation, and therefore cognitive control is up-regulated to do so. There is a large body of work of mood effects on cognitive control confirming this effect (e.g., Dreisbach & Fischer, Citation2012; Mitchell & Phillips, Citation2007; van Steenbergen, Band, & Hommel, Citation2009, 2010). Mechanistically, negative mood reduces the range of action tendencies and enhances focused processing, while positive mood induces global processing (Fredrickson & Branigan, Citation2005). Applied to the current study, the global processing of a stimulus could lead to relatively more processing of the irrelevant dimension (words) and thus diminish interference control in the appetitive condition, while aversive stimuli enhance focus on the relevant dimension, thereby facilitating interference control.
An alternative explanation for the differential effects of appetitive versus aversive words on interference control is that the unpleasantness of the aversive semantics could have induced more effort recruitment for the active suppression of the words (while the pleasantness of the appetitive semantics could have induced less effort recruitment for active suppression; Mathews & Sebastian, Citation1993; Williams et al., Citation1996). In other words, by increasing the effort expended in naming the color, attentional bias can be overridden (see the connectionist model of Cohen, Dunbar, & McClelland, Citation1990). It is also possible that individuals benefit from aversive words because these words are stimulating rather than distracting per se, in line with the optimal stimulation theory (Zentall & Zentall, Citation1983) and the cognitive energetic model of ADHD (Sergeant, Citation2005). Perhaps aversive words—but not appetitive words—lead to a more optimal arousal level, which in turn leads to better performance. Another alternative interpretation is an approach versus avoidance tendency. Appetitive words may have induced approach behavior, thereby attracting attention to the distracting words while increasing the demand on task performance (color naming) and resulting in poorer interference control. Aversive words may have induced avoidance behavior, thereby decreasing the demand on color naming and resulting in better interference control (Hare, Tottenham, Davidson, Glover, & Casey, Citation2005; Koch, Holland, & van Knippenberg, Citation2008; Puschmann & Sommer, Citation2011).
In addition to these alternative accounts, the mood-as-information theory would suggest that the mood effects evoked by negative words are stronger as ADHD symptoms increase, i.e., those with higher levels of ADHD symptoms increase their cognitive control to a larger extent in response to negative stimuli than those with lower levels of ADHD symptoms. Alternative interpretations include: (1) higher levels of hyperactivity/impulsivity and inattention were associated with a larger increase in applied effort in the context of aversive words; (2) higher levels of hyperactivity/impulsivity and inattention were associated with a larger increase in arousal or activation in response to aversive words (in other words, these individuals may have benefited more from aversive words because it led to a more optimal energetic state for performing the task at hand); and (3) higher levels of hyperactivity/impulsivity and inattention were associated with stronger voidance of aversive words, in turn facilitating color naming. Skin conductance or pupil dilation measurements may help to investigate the involvement of arousal in future studies.
The lack of evidence for a deviant attentional bias toward appetitive/aversive words in the ADHD group was contrary to the formulated hypotheses, but may be in line with recent ADHD studies which assessed the attentional bias effect using positive and negative emotion words, designed to relate to the emotional lability that is associated with ADHD. Specifically, both Posner et al. (Citation2011) and Passaroti et al. (Citation2010) reported no attentional bias to emotionally relevant words in individuals with ADHD compared to TD controls, despite group differences in brain activation patterns. These findings can be supported by recent studies which demonstrated that cognitive control deficits and altered reward sensitivity are independent characteristics in those with ADHD, which do not often co-occur within the same individuals (Coghill, Seth, & Matthews, Citation2014; de Zeeuw, Weusten, van Dijk, van Belle, & Durston, Citation2012; Fair, Bathula, Nikolas, & Nigg, Citation2012; Sjöwall, Roth, Lindqvist, & Thorell, 2013; Solanto et al., Citation2001; Sonuga-Barke, Bitsakou, & Thompson, Citation2010). The current study distinguishes itself—and adds to this knowledge—through valence-specific effects of distractor words on interference control in ADHD groups compared with controls. The interpretation of the facilitating effect of aversive words remains speculative, and future research is needed to replicate these findings and examine which mechanisms (mood effects, effort/arousal effects, avoidance effects) underlie this facilitation effect. The present observation that individuals with higher levels of hyperactivity/impulsivity and inattention show larger improvements in interference control in certain contexts suggests that inhibitory control in these individuals is flexible, and that there is room for improvement.
Conclusion
In conclusion, the present findings reveal that interference control in children and adolescents with ADHD is not exacerbated by appetitive distracting words. Specifically, the appetitive words were not significantly more distracting for the individuals with ADHD than for the controls. The aversive words were found to be facilitating rather than distracting, and this effect of aversive words appears to be stronger in individuals with more ADHD symptoms. This pattern of results cannot be fully accounted for by the attention bias view, but is supported by other frameworks such as the mood-as-information theory.
Table S1
Download PDF (172.1 KB)Acknowledgements
We thank the participating families, Meta Freriks, Merel de Groot, and Hannah Ferenzi for assistance with data collection, and Leon Kenemans for helpful discussion.
Disclosure statement
Jan K Buitelaar has been in the past 3 years a consultant to/member of advisory board of/and/or speaker for Janssen Cilag BV, Eli Lilly, Lundbeck, Shire, Roche, Medice, Novartis, and Servier. He has received research support from Roche and Vifor. He is not an employee of any of these companies, and not a stock shareholder of any of these companies. He has no other financial or material support, including expert testimony, patents, royalties. No potential conflict of interest was reported by the other authors.
Supplemental material
The supplemental data for this article can be accessed here.
Additional information
Funding
Notes
1 For these dimensional analyses there was incomplete data for 8 participants (n = 4 ADHD). Scores on the DBDRS were used to quantify ADHD symptoms.
2 See footnote 1.
References
- Achenbach, T. M., & Edelbrock, C. (1991). Child behavior checklist. Burlington (Vt), 7.
- American Psychiatric Association. (2000). DSM-IV-TR: Diagnostic and statistical manual of mental disorders, text revision. Washington, DC: Author.
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: Author.
- Bar-Haim, Y., Lamy, D., Pergamin, L., Bakermans-Kranenburg, M. J., & Van Ijzendoorn, M. H. (2007). Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin, 133(1), 1–24. doi:10.1037/0033-2909.133.1.1
- Barkley, R. A. (1997). Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin, 121(1), 65–94. doi:10.1037/0033-2909.121.1.65
- Barkley, R. A., Anastopoulos, A. D., Guevremont, D. C., & Fletcher, K. E. (1991). Adolescents with ADHD: Patterns of behavioral adjustment, academic functioning, and treatment utilization. Journal of the American Academy of Child and Adolescent Psychiatry, 30(5), 752–761.
- Becker, S. P., Fite, P. J., Garner, A. A., Greening, L., Stoppelbein, L., & Luebbe, A. M. (2013). Reward and punishment sensitivity are differentially associated with ADHD and sluggish cognitive tempo symptoms in children. Journal of Research in Personality, 47(6), 719–727. doi:10.1016/j.jrp.2013.07.001
- Ben-Haim, M. S., Mama, Y., Icht, M., & Algom, D. (2014). Is the emotional Stroop task a special case of mood induction? Evidence from sustained effects of attention under emotion. Attention, Perception, & Psychophysics, 76(1), 81–97. doi:10.3758/s13414-013-0545-7
- Castellanos, F. X., Sonuga-Barke, E. J., Milham, M. P., & Tannock, R. (2006). Characterizing cognition in ADHD: Beyond executive dysfunction. Trends in Cognitive Sciences, 10(3), 117–123. doi:10.1016/j.tics.2006.01.011
- Clore, G. L., Schwarz, N., & Conway, M. (1994). Affective causes and consequences of social information processing. Handbook of Social Cognition, 1, 323–417.
- Coghill, D., Seth, S., & Matthews, K. (2014). A comprehensive assessment of memory, delay aversion, timing, inhibition, decision making and variability in attention deficit hyperactivity disorder: Advancing beyond the three-pathway models. Psychological Medicine, 44(09), 1989–2001. doi:10.1017/S0033291713002547
- Cohen, J. D., Dunbar, K., & McClelland, J. L. (1990). On the control of automatic processes: A parallel distributed processing account of the stroop effect. Psychological Review, 97(3), 332.
- de Zeeuw, P., Weusten, J., van Dijk, S., van Belle, J., & Durston, S. (2012). Deficits in cognitive control, timing and reward sensitivity appear to be dissociable in ADHD. PLoS One, 7(12), e51416. doi:10.1371/journal.pone.0051416
- Dopheide, J. A., & Pliszka, S. R. (2009). Attention-deficit-hyperactivity disorder: An update. Pharmacotherapy, 29(6), 656–679. doi:10.1592/phco.29.6.656
- Dreisbach, G., & Fischer, R. (2012). The role of affect and reward in the conflict-triggered adjustment of cognitive control. Frontiers in Human Neuroscience, 6. doi:10.3389/fnhum.2012.00342
- Fair, D. A., Bathula, D., Nikolas, M. A., & Nigg, J. T. (2012). Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proceedings of the National Academy of Sciences, 109(17), 6769–6774. doi:10.1073/pnas.1115365109
- Field, M., & Cox, W. M. (2008). Attentional bias in addictive behaviors: A review of its development, causes, and consequences. Drug and Alcohol Dependence, 97(1), 1–20. doi:10.1016/j.drugalcdep.2008.03.030
- Field, M., Munafò, M. R., & Franken, I. H. (2009). A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychological Bulletin, 135(4), 589. doi:10.1037/a0015843
- Fredrickson, B. L., & Branigan, C. (2005). Positive emotions broaden the scope of attention and thought‐action repertoires. Cognition & Emotion, 19(3), 313–332. doi:10.1080/02699930441000238
- Geurts, H. M., Luman, M., & Van Meel, C. S. (2008). What’s in a game: The effect of social motivation on interference control in boys with ADHD and autism spectrum disorders. Journal of Child Psychology and Psychiatry, 49(8), 848–857. doi:10.1111/j.1469-7610.2008.01916.x
- Greenhill, L. L. (1998). The use of psychotropic medication in preschoolers: Indications, safety, and efficacy. The Canadian Journal of Psychiatry, 43(6), 576–581. doi:10.1177/070674379804300604
- Haenlein, M., & Caul, W. F. (1987). Attention deficit disorder with hyperactivity: A specific hypothesis of reward dysfunction. Journal of the American Academy of Child & Adolescent Psychiatry, 26(3), 356-362.
- Hare, T. A., Tottenham, N., Davidson, M. C., Glover, G. H., & Casey, B. J. (2005). Contributions of amygdala and striatal activity in emotion regulation. Biological Psychiatry, 57(6), 624–655. doi:10.1016/j.biopsych.2004.12.038
- Hyman, S. E. (2007). Can neuroscience be integrated into the DSM-V? Nature Reviews Neuroscience, 8(9), 725–732. doi:10.1038/nrn2218
- Klein, G. S. (1964). Semantic power measured through the interference of words with color-naming. The American Journal of Psychology, 77(4), 576–588. doi:10.2307/1420768
- Koch, S., Holland, R. W., & van Knippenberg, A. (2008). Regulating cognitive control through approach-avoidance motor actions. Cognition, 109(1), 133–142. doi:10.1016/j.cognition.2008.07.014
- Kort, D. W., Compaan, E., Bleichrodt, N., Resing, W., Schittekatte, M., Bosmans, M., … Verhaeghe, P. (2002). WISC-III NL. Handleiding. London, UK: The Psychological Corporation.
- Krebs, R. M., Boehler, C. N., & Woldorff, M. G. (2010). The influence of reward associations on conflict processing in the Stroop task. Cognition, 117(3), 341–347. doi:10.1016/j.cognition.2010.08.018
- Lansbergen, M. M., Kenemans, J. L., & van Engeland, H. (2007). Stroop interference and attention-deficit/hyperactivity disorder: A review and meta-analysis. Neuropsychology, 21(2), 251–262. doi:10.1037/0894-4105.21.2.251
- Larsen, R. J., Mercer, K. A., & Balota, D. A. (2006). Lexical characteristics of words used in emotional Stroop experiments. Emotion, 6(1), 62–72. doi:10.1037/1528-3542.6.1.62
- Luman, M., Oosterlaan, J., & Sergeant, J. A. (2005). The impact of reinforcement contingencies on AD/HD: A review and theoretical appraisal. Clinical Psychology Review, 25(2), 183–213.
- Luman, M., Tripp, G., & Scheres, A. (2010). Identifying the neurobiology of altered reinforcement sensitivity in ADHD a review and research agenda. neuroscience & biobehavioral reviews. 34(5), 744-754.
- Ma, I., van Duijvenvoorde, A., & Scheres, A. (2016). The interaction between reinforcement and inhibitory control in ADHD: A review and research guidelines. Clinical Psychology Review, 44, 94–111. doi:10.1016/j.cpr.2016.01.001
- Mathews, A., & MacLeod, C. (1985). Selective processing of threat cues in anxiety states. Behaviour Research and Therapy, 23(5), 563–569. doi:10.1016/0005-7967(85)90104-4
- Mathews, A., & Sebastian, S. (1993). Suppression of emotional Stroop effects by fear-arousal. Cognition & Emotion, 7(6), 517–530. doi:10.1080/02699939308409203
- Mitchell, R. L., & Phillips, L. H. (2007). The psychological, neurochemical and functional neuroanatomical mediators of the effects of positive and negative mood on executive functions. Neuropsychologia, 45(4), 617–629. doi:10.1016/j.neuropsychologia.2006.06.030
- Mogg, K., Bradley, B. P., Hyare, H., & Lee, S. (1998). Selective attention to food-related stimuli in hunger: Are attentional biases specific to emotional and psychopathological states, or are they also found in normal drive states? Behaviour Research and Therapy, 36(2), 227–237. doi:10.1016/S0005-7967(97)00062-4
- Nederkoorn, C., Guerrieri, R., Havermans, R. C., Roefs, A., & Jansen, A. (2009). The interactive effect of hunger and impulsivity on food intake and purchase in a virtual supermarket. International Journal of Obesity, 33(8), 905–912. doi:10.1038/ijo.2009.98
- Oosterlaan, J., Scheres, A., Antrop, I., Roeyers, H., & Sergeant, J. A. (2000). Handleiding bij de Vragenlijst voor Gedragsproblemen bij Kinderen VvGK. In Nederlandse bewerking van de DBD Rating Scale [Dutch translation of the DBD Rating Scale]. Lisse: Swets Test Publishers.
- Passarotti, A. M., Sweeney, J. A., & Pavuluri, M. N. (2010). Differential engagement of cognitive and affective neural systems in pediatric bipolar disorder and attention deficit hyperactivity disorder. Journal of the International Neuropsychological Society, 16(1), 106–117. doi:10.1017/S1355617709991019
- Polanczyk, G., & Rohde, L. A. (2007). Epidemiology of attention-deficit/hyperactivity disorder Across the lifespan. Current Opinion in Psychiatry, 20(4), 386–392. doi:10.1097/YCO.0b013e3281568d7a
- Posner, J., Maia, T. V., Fair, D., Peterson, B. S., Sonuga-Barke, E. J., & Nagel, B. J. (2011). The attenuation of dysfunctional emotional processing with stimulant medication: An fMRI study of adolescents with ADHD. Psychiatry Research, 193(3), 151–160. doi:10.1016/j.pscychresns.2011.02.005
- Puschmann, A.-K., & Sommer, C. (2011). Hypervigilance or avoidance of trigger related cues in migraineurs?-A case-control study using the emotional stroop task. BMC Neurology, 11(1), 141. doi:10.1186/1471-2377-11-141
- Richards, A., French, C. C., Johnson, W., Naparstek, J., & Williams, J. (1992). Effects of mood manipulation and anxiety on performance of an emotional Stroop task. British Journal of Psychology, 83(4), 479–491. doi:10.1111/bjop.1992.83.issue-4
- Robbins, T. W., Gillan, C. M., Smith, D. G., de Wit, S., & Ersche, K. D. (2012). Neurocognitive endophenotypes of impulsivity and compulsivity: Towards dimensional psychiatry. Trends in Cognitive Sciences, 16(1), 81–91. doi:10.1016/j.tics.2011.11.009
- Sagvolden, T., Johansen, E. B., Aase, H., & Russell, V. A. (2005). A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behavioral and Brain Sciences, 28(3), 397–418. doi:10.1017/S0140525X05000075
- Sattler, J., & Saklofske, D. (2001). Wechsler Intelligence Scale for Children (WISC-III): Description. In Sattler, J. M. (Ed.), Assessment of Children: Cognitive Applications (4th ed., pp. 220–265). San Diego: Author.
- Scheres, A., Tontsch, C., Thoeny, A. L., & Kaczkurkin, A. (2010). Temporal reward discounting in attention-deficit/hyperactivity disorder: The Contribution of symptom domains, reward magnitude, and session length. Biological Psychiatry, 67(7), 641–648. doi:10.1016/j.biopsych.2009.10.033
- Schwarz, N., & Clore, G. L. (1983). Mood, misattribution, and judgments of well-being: Informative and directive functions of affective states. Journal of Personality and Social Psychology, 45(3), 513–523. doi:10.1037/0022-3514.45.3.513
- Sergeant, J. A. (2005). Modeling attention-deficit/hyperactivity disorder: A critical appraisal of the cognitive-energetic model. Biological Psychiatry, 57(11), 1248–1255. doi:10.1016/j.biopsych.2004.09.010
- Sergeant, J. A., Oosterlaan, J., & van der Meere, J. (1999). Information processing and energetic factors in attention-deficit/hyperactivity disorder. In Quay, H. C., & Hogan, A. E. (Eds.), Handbook of disruptive behavior disorders (pp. 75–104). New York: Kluwer/Plenum.
- Shaffer, D., Fisher, P., Lucas, C. P., Dulcan, M. K., & Schwab-Stone, M. E. (2000). NIMH diagnostic interview schedule for children version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry, 39(1), 28–38. doi:10.1097/00004583-200001000-00014
- Shaw, P., Eckstrand, K., Sharp, W., Blumenthal, J., Lerch, J., Greenstein, D., … Rapoport, J. (2007). Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences, 104(49), 19649–19654. doi:10.1073/pnas.0707741104
- Sjöwall, D., Roth, L., Lindqvist, S., & Thorell, L. B. (2013). Multiple deficits in ADHD: Executive dysfunction, delay aversion, reaction time variability, and emotional deficits. Journal of Child Psychology and Psychiatry, 54(6), 619–627. doi:10.1111/jcpp.12006
- Solanto, M. V., Abikoff, H., Sonuga-Barke, E., Schachar, R., Logan, G. D., Wigal, T., … Turkel, E. (2001). The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: A supplement to the NIMH multimodal treatment study of AD/HD. Journal of Abnormal Child Psychology, 29(3), 215–228. doi:10.1023/A:1010329714819
- Somerville, L. H., Hare, T., & Casey, B. J. (2011). Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience, 23(9), 2123–2134. doi:10.1162/jocn.2010.21572
- Sonuga-Barke, E. J. (2005). Causal models of attention-deficit/hyperactivity disorder: From common simple deficits to multiple developmental pathways. Biological Psychiatry, 57(11), 1231–1238.
- Sonuga-Barke, E., Bitsakou, P., & Thompson, M. (2010). Beyond the dual pathway model: Evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 49(4), 345–355.
- Stroop, J. R. (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18(6), 643–662. doi:10.1037/h0054651
- Tamm, L., Narad, M. E., Antonini, T. N., O’Brien, K. M., Hawk, L. W., Jr, & Epstein, J. N. (2012). Reaction time variability in ADHD: A review. Neurotherapeutics, 9(3), 500–508. doi:10.1007/s13311-012-0138-5
- van Mourik, R., Oosterlaan, J., & Sergeant, J. A. (2005). The Stroop revisited: A meta-analysis of interference control in AD/HD. Journal of Child Psychology and Psychiatry, 46(2), 150–165. doi:10.1111/j.1469-7610.2004.00345.x
- van Steenbergen, H., Band, G., & Hommel, B. (2009). Reward counteracts conflict adaptation: Evidence for a role of affect in executive control. Psychological Science, 20(12), 1473–1477. doi:10.1111/psci.2009.20.issue-12
- van Steenbergen, H., Band, G., & Hommel, B. (2010). In the mood for adaptation: How affect regulates conflict-driven control. Psychological Science, 21(11), 1629–1634. doi:10.1177/0956797610385951
- Willcutt, E., Sonuga-Barke, E., Nigg, J., & Sergeant, J. (2008). Recent developments in neuropsychological models of childhood psychiatric disorders. Biological Child Psychiatry, 24, 195–226. doi:10.1159/000118526
- Williams, J. M. G., Mathews, A., & MacLeod, C. (1996). The emotional Stroop task and psychopathology. Psychological Bulletin, 120(1), 3. doi:10.1037/0033-2909.120.1.3
- Zentall, S. S., & Zentall, T. R. (1983). Optimal stimulation: A model of disordered activity and performance in normal and deviant children. Psychological Bulletin, 94(3), 446–471. doi:10.1037/0033-2909.94.3.446