ABSTRACT
Data on the long-term neurodevelopmental outcomes of children exposed to hematological maternal cancer with or without treatment during pregnancy are lacking. A total of 57 children, of whom 33 males and 24 females, prenatally exposed to hematological malignancies and its treatment, were invited for neuropsychological and physical examinations at 18 months, 36 months, 6, 9, 12, 15 and 18 years of age. Oncological, obstetrical, neonatal and follow-up data of these children were collected. Parents were asked to complete questionnaires on their child’s general health, school performances, social situation, behavioral development, executive functioning, and if their child receives supportive care. Non-Hodgkin lymphoma was diagnosed in 35.1%, Hodgkin lymphoma in 28.1%, acute myeloid leukemia in 15.8%, chronic myeloid leukemia in 12.3%, and acute lymphoblastic leukemia in 8.8%. Cognitive development at a median age of 10.7 years was within the normal range. In subgroup analyses of children in early childhood, the gestational age at birth was correlated with the cognitive outcome at a median age of 1.7 years. Scores for language development, intelligence, attention, memory and behavior, as well as clinical neurological and general pediatric examinations were within normal ranges. In subgroup analyses, the need for supportive care in the child was associated with the loss of the mother. Prenatal exposure to hematological maternal malignancies with or without treatment did not affect the neurodevelopment of the child in the long term. Yet, caution is indicated and surveillance of the emotional development of the child is needed, especially when the mother is deceased to cancer.
Cancer is diagnosed during 1 in 1000 pregnancies with a cancer type distribution that is similar to non-pregnant women. After breast cancer, thyroid cancer and skin cancer (including melanoma), lymphoma is the fourth most common cancer type diagnosed during pregnancy, with an estimated prevalence of 1 in 6000 pregnancies (Brenner et al., Citation2012; Parazzini et al., Citation2017). In contrast, leukemia during pregnancy is very rare, 1 in 75000 to 100000 pregnancies (Brenner et al., Citation2012). Typical symptoms of hematological malignancies can be falsely attributed to pregnancy such as fatigue, nausea and abdominal pain, leading to a delay in diagnosis. A maternal malignancy, most frequently hematological malignancies, can also be detected by a discordant result of the noninvasive prenatal testing (NIPT) (Lenaerts et al., Citation2019). The use of NIPT is increasing likely to result in more cancer in pregnancy diagnoses.
Chemotherapy from the second trimester onwards is considered to be relatively safe since no more or other congenital malformations are found after chemotherapy compared to the normal population. Increasing awareness of the feasibility of antenatal chemotherapy has resulted in more pregnant patients receiving treatment over the past few years (De Haan et al., Citation2018). The general health, the neurocognitive and cardiac outcome of children prenatally exposed to maternal cancer in age ranges of 18 months until 18 years were previously published by the International Network of Cancer, Infertility and Pregnancy (INCIP) (Amant et al., Citation2012, Citation2015; Vandenbroucke et al., Citation2020). Cognitive and cardiac outcomes were within normal ranges, but subtle differences in development were found. In early childhood, preterm birth was a predictor of worse neurodevelopmental outcome; however, this association was independent of cancer treatment during pregnancy (Amant et al., Citation2015). This study resulted in the recommendation to aim for a term delivery (after 37 weeks of gestation) and currently the overall frequency of preterm births in pregnancies complicated by cancer is decreasing. At 6 years, children prenatally exposed to maternal malignancy were at risk for lower verbal intelligence, visuospatial long-term memory scores, and higher diastolic blood pressure (Vandenbroucke et al., Citation2020). In addition, verbal intelligence was more affected in children whose mothers died than those with surviving mothers.
In 2020, a comprehensive literature review on long-term neurodevelopmental outcome was published (Korakiti et al., Citation2020). Based on 17 cohort studies, no major cognitive abnormalities were reported; however, it was concluded that more thorough follow-up of the children is required. In addition, results from most of these studies made no distinction between the type of malignancy and the associated therapy. Hence, the study heterogeneity may mask significant differences in smaller subgroups and underscores the need for further research to identify whether specific subgroups of children born to women with a cancer diagnosis during pregnancy are at higher risk of developmental problems. Important in these subgroups is defining the specific impact of type of malignancy on child development. In addition, the first 1000 days after conception are a crucial period when the foundations of neurodevelopment are established (Roseboom, Citation2018). Hematological malignancies and comorbidities such as stress, attachment issues, and malnutrition can weaken these foundations. Unfortunately, to date, there is still a lack of knowledge about how the diagnosis and treatment in pregnancy may affect the neurodevelopment in the offspring.
Therefore, this study aims to describe the specific impact of maternal hematological malignancy and its treatment during pregnancy on the perinatal outcome, health status, and neurocognitive development of the offspring.
Materials and methods
Study design and participants
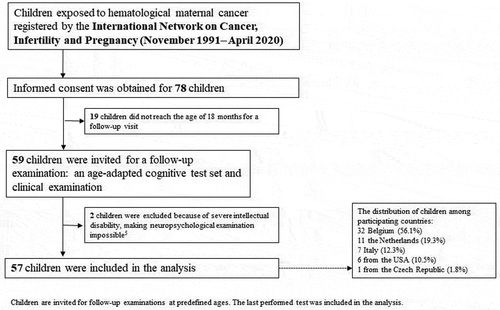
This is a multicenter prospective cohort study, with data obtained from the long-term follow-up study of INCIP. Data were obtained from seven referral centers in five countries (Belgium, Czech Republic, Italy, the Netherlands, and the United States). summarizes the study design and recruitment. Two children with a severe neurodevelopmental delay, which made it impossible to perform the neuropsychological examination, were excluded and previously published (Amant et al., Citation2012). The ethical committee of each participating institution approved the study and written (parental) informed consent was obtained for each child. The full study protocol is available at www.cancerinpregnancy.org/study-protocols and the study is registered at ClinicalTrials.gov, NCT00330447.
Data collection and instruments
For each mother-child pair oncological, obstetrical and neonatal data were collected from the INCIP database that contains data of patients diagnosed with any pregnancy-associated malignancy. Furthermore, the children were invited for neuropsychological and physical examination at predefined ages of 18 months, 36 months, 6, 9, 12, 15 and 18 years. Neuropsychological examination consisted of an age-adapted cognitive test set. For the purpose of the present study, we focused on developmental indexes such as intelligence, attention, and verbal and non-verbal memory. During each visit, the children underwent a clinical neurological and general pediatric examination, and the parents were asked to fill out questionnaires on general health status, executive functioning, and behavior of their child. An overview of the age-adapted tests is provided in .
Table 1. An overview of the age-adapted test sets
Cognitive development
Cognitive development was tested at 18 months and 36 months using the Bayley Scales of Infant and Toddler Development III (BSID-III) (Barker et al., Citation1993; Bayley, Citation2005). At 36 months, the language scale of the BSID-III was also obtained. From the age of 6 years onwards, intelligence was tested using the Wechsler Preschool and Primary Scale of Intelligence Revised or III (WPPSI-R or WPPSI-II I Wechsler, Citation2002, Citation1989), the Wechsler Intelligence Scale for Children (WISC-III, WISC-IV, and WISC-V Wechsler, Citation2014; Citation2012, Citation2003), or the Wechsler Adult Intelligence Scale III or IV (WAIS-III or WAIS-IV Wechsler, Citation1997, Citation2008). The scores of these tests were referred to developmental index scores (at 18 months and 36 months) or Intelligence Quotient (IQ) (from 6 years of age). Different intelligence tests were used as this is a multicenter internal study and the currently used edition or revision of the Wechsler test was not always the same in all participating countries during the inclusion period.
Attention
Five subtasks from the Amsterdam Neuropsychological Tasks (ANT, De Sonneville, Citation2014) were used to evaluate different aspects of attention. ANT is a computerized program which enables to measure not only the accuracy of responses but also the reaction times. We used data obtained from the subtasks: “Baseline Speed,” “GoNoGo,” “Memory Search Objects 2 keys,” “Focused Attention Objects 2 keys,” and “Shifting Attentional Set Visual.”
Verbal and non-verbal memory
To evaluate the learning and memory function for verbal material, and to track changes in memory function over time, the Auditory Verbal Learning Test (AVLT, Forrester & Geffen, Citation1991) was used.
The Children’s Memory Scale (CMS, Cohen, Citation1997) includes a series of tasks on verbal and non-verbal memory. CMS Numbers were used to evaluate verbal memory span and working memory. CMS Pictures assessed the memory span for visuospatial material. Learning and memory of non-verbal visuospatial material was assessed using CMS Dots.
Questionnaires on general health, behavior and executive functioning
Parents were asked to fill out a questionnaire which addressed general information, such as their child’s general health, school performance (at school ages), receiving supportive care, non-academic interests, social situation, and important life-events. This questionnaire also addressed child’s growth and any developmental or medical problems (Van Gerwen et al., Citation2020). Furthermore, parents were asked to fill out a questionnaire on the incidence of internalizing and externalizing behavior problems (Child Behavior Checklist [CBCL], Achenbach & Rescorla, Citation2001). Higher scores indicate more behavior problems.
To assess executive functioning, parents have filled out the preschool version (BRIEF-P, Garon et al., Citation2016), or the Behavior Rating Inventory of Executive Function (BRIEF, Smidts & Huizinga, Citation2010). As outcome variables, we used the Inhibitory-Selfcontrol Index, the Flexibility scale, the Emergent-Metacognition Index, and the Global Executive Composite for the BRIEF-P. Outcome variables for the BRIEF were the Behavioral-Regulation Index, the Metacognition Index, and the Global Executive Composite.
Data management and analyses
Descriptive statistics were used to review maternal oncological data, demographic characteristics of the mothers and children, results of the health questionnaire, and clinical neurologic evaluations. For all children, raw scores of the neuropsychological tests were converted to standardized scores using normative data for the specific age-group provided by the manual of the respective test. We used the last available neuropsychological assessment for each child. Descriptive statistics were used to describe the outcome of the neuropsychological assessment. The relationship between cognitive outcome and gestational age was investigated using Pearson correlations. A Pearson’s chi-square test of contingencies (with α = 0.05) was used to evaluate whether the death of the mother was related to receiving supportive care. For data analyses and reporting, a Statistical Package for Social Sciences Version 25.0 (SPSS 25.0) was used.
Results
Characteristics of the study children and the mothers
This interim analysis was performed with a data cutoff in April 2020. In total, 57 children (born 1991–2017) were eligible for analysis of which 33 male (57.9%) and 24 female (42.1%). Thirty-eight children (66.7%) were exposed to chemotherapy in utero, 4 children (7.0%) were exposed to targeted therapy, 3 children (5.3%) were exposed to radiotherapy (with or without surgery), 10 children (17.5%) were not exposed to any treatment, and of 2 children (3.5%) data on oncological treatment during pregnancy were missing. Non-Hodgkin lymphoma was diagnosed in 20 mothers (35.1%), Hodgkin lymphoma in 16 mothers (28.1%), acute myeloid leukemia in 9 mothers (15.8%), chronic myeloid leukemia in 7 mothers (12.3%), and acute lymphoblastic leukemia in 5 mothers (8.8%). Additional information about the (timing of) diagnosis and specific treatments is provided in Table S1.
Perinatal outcome
The median gestational age at delivery was 36.4 weeks (range: 26.4–41.3) and the median birth weight was 2590 g (range: 720–4423). Sub-categories of preterm births based on gestational age are provided in . Twenty-one children born preterm and two born full term (40.4%) were admitted to the neonatal intensive care unit. Six children (10.5%; median gestational age 33.8 weeks) were born with congenital malformations, wherefrom 1 child had contractures of limbs, 1 child had congenital laryngomalacia, 2 children had plagiocephaly, and 2 children had partial syndactyly (Table S2).
Table 2. Classification of delivery according to gestational age
General health
Clinical neurological examination (n = 40) did not show any focal neurological abnormalities; however, two children had a delay in motor development (gestational age of 34.1 and 38.6 weeks).
Data from a health questionnaire addressing different types of medical problems reported by parents were available for 44 children (response rate; 57%) (). One child used the drug risperidone for explosive and aggressive behavior. No other children used psychotropic medication.
Table 3. Overview of the different types of medical problems
Fourteen (24.6%) children needed a surgery in their lives (Table S3). Five children (8.8%) received supportive psychosocial care: two children (3.5%) received support for social-emotional development, two children (3.5%) received support at school because of hypersensitivity and dyslexia, and one child (1.8%) received support for performance anxiety. Learning disabilities, as reported by parents on this questionnaire, were reported in eight children (14%) ().
Table 4. Overview of reported cognitive problems and learning disabilities in eight patients (median gestational age: 36.3 weeks)
Neurodevelopment
The median follow-up period at the time of the last neuropsychological assessment of the children was 6.1 years (range: 1.4–18.8). Total scores for the children were within normal ranges, overall the median score was 100 (n = 57; 95% CI = 96.9–104.6).
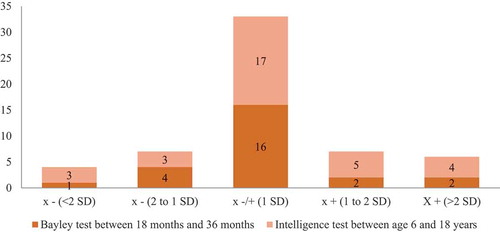
shows the distribution of the results for the last performed developmental or intelligence test (Bayley test [n = 25, median assessment age of 1.70 years], Wechsler intelligence test [n = 32; median assessment age of 10.7 years]). In early childhood, the median score for the cognitive development scale assessed by the Bayley test was 95.0 (n = 25; 95% CI = 93.4–104.3) and related to the gestational age at birth (Rs = 0.459, P = 0.021). In the older age group, the median score for total IQ of the Wechsler intelligence test was 101.5 (n = 32; 95% CI = 96.6–107.8) and not related to the gestational age at birth (Rs = 0.115, P = 0.531). We recorded normal language development at the age of 36 months and the secondary outcomes of the Wechsler tests at the age of 10 years were within normal ranges. In both groups, the scores were not related to the duration of chemotherapy exposure during pregnancy (Rs = 0.153, P = 0.410).
The results on the different subtasks of attention, verbal and non-verbal memory were within normal ranges. and S1 in the supplementary appendix give a detailed overview and distribution of the results on attention and memory. Median scores on internalizing problems, externalizing problems and the total number of problems on the Child Behavior Checklist (1.5–5 years and 6–18 years) were for both groups within normal ranges. Data from the BRIEF-P and BRIEF were also within the normal ranges for both groups.
Death of mother
Ten mothers (17.5%) died of their disease with a median time period of 1.4 years (range: 0.5–5.2) after diagnosis. The median age of the children at maternal decease was 1.4 years (0.4–13.3). Of the five children whose mothers died, three children received supportive psychosocial care. The association between receiving supportive care and the death of the mother was considered as a medium-sized effect, Φ = .31, although the chi-square test was not statistically significant χ2 (1, N = 43) = 4.69, p = 0.08.
Discussion
In this multicenter prospective cohort study, neurodevelopment, perinatal outcome, and general health was reported of 57 children born to mothers diagnosed with hematological malignancies during pregnancy. Although the incidence of preterm delivery in these children was high (52.7%), the cognitive development at a median age of 10.7 years was normal. In subgroup analyses, the cognitive development of 25 children at median age of 1.7 years was related to their gestational age at birth. This finding confirms the negative prognostic effect of preterm birth on early cognitive development, which is highlighted in previous studies (Amant et al., Citation2012, Citation2015). This relationship is no longer present in the older age group, which can be explained by the fact that mild developmental delay in premature children will catch up with increasing age (Odd et al., Citation2012).
In general, the outcomes for language development, intelligence, attention and memory were reassuring. All scores were within normal ranges. Developmental index scores and Full Scale intelligence scores were not related to the duration of chemotherapy exposure during pregnancy. Additionally, reassuring results were found for behavioral development. All scores were within normal ranges. Our results are in line with a previous study on children born to mothers with hematological malignancies, suggesting that these children are not at risk for neurodevelopmental problems at a median follow-up of 18.7 years (Aviles & Neri, Citation2001). However, in this study, in-depth testing was lacking.
Maternal outcomes of pregnant women with non-Hodgkin and Hodgkin lymphoma have been recently studied. These studies showed comparable maternal survival between pregnant and non-pregnant patients (Maggen et al., Citation2020, Citation2019). In our cohort, 10 mothers died of their disease (17.5%). The death of the mother is a major life event that potentially influences child development. Previous studies have shown an association between anxiety and stress during pregnancy with adverse birth outcomes (e.g., spontaneous abortion, preterm labor, growth restriction) and problems across several domains (cognitive, behavioral and emotional) in the child (Betchen et al., Citation2020; Van den Bergh et al., Citation2005). In subgroup analyses, receiving supportive care was associated with the death of the mother. Three children whose mothers died received support for social-emotional problems as reported by the fathers. The need for supportive care in these children may be explained by the stress associated with the loss of their mothers. Paternal roles become different, and fathers are often challenged to adjust to single parenthood while managing their own grief (Yopp & Rosenstein, Citation2013). Earlier studies have highlighted that widowed fathers often experience depressive feelings and feel incompetent across several domains (Boerner & Silverman, Citation2001; Nilsson et al., Citation2009). These families are at risk of high levels of distress and may explain the need for supportive care. Unfortunately, the needs of widowed fathers have been overlooked in literature and publications often only concern single parenthood in fathers because of separation or divorce. To the best of our knowledge, this is the first study that shows an association between maternal bereavement due to the cancer and the need for supportive care of the child. The complexity of managing hematological cancers during pregnancy highlights the need for a multidisciplinary approach in an experienced center where gynecologists, oncologists, pediatricians, neonatologists, nurses, and psychologists can easily contribute. Further research of psychosocial effects in families confronted with cancer during pregnancy is needed, including more in-depth testing using standardized assessments of stress, emotional functioning, and sensory processing to elucidate psychosocial risks in children born to mothers with a poor prognosis.
Our study has some limitations. The median follow-up period was 6.1 years and may have been too short to identify neurocognitive problems that become more apparent at later school-ages. In addition, our study group was small and postnatal environmental factors challenged the research on the long-term neurodevelopmental outcome of prenatal exposure to hematological cancers during pregnancy. Larger samples and follow-up until adult age are needed to investigate the impact on cognitive functions and psychosocial development after maternal hematological cancer is diagnosed during pregnancy.
In conclusion, our data did not detect major long-term neurodevelopmental problems in children prenatally exposed to hematological maternal malignancies. The reassuring data support the current policy to treat hematological cancer also during pregnancy. However, caution is indicated and surveillance of the emotional development of the child is needed, especially when the mother is deceased to cancer.
CNY-OA_20-124-File002.docx
Download MS Word (26.4 KB)Acknowledgments
Jeroen Blommaert, Kaat Philippe, Cettina Schellens, Lara Stroobants (UZ Leuven, Belgium), Monica Fumagalli (Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy), Christianne Lok, Vera Wolters (Netherlands Cancer Institute – Antoni van Leeuwenhoek, Amsterdam, The Netherlands).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed at https://doi.org/10.1080/09297049.2021.1902489.
Additional information
Funding
References
- Achenbach, T. M., & Rescorla, L. A. (2001). Manual for the ASEBA school-age forms & profiles. University of Vermont, Research Center for Children, Youth, & Families.
- Amant, F., Van Calsteren, K., Halaska, M. J., Gziri, M. M., Hui, W., Lagae, L., Willemsen, M. A., Kapusta, L., Van Calster, B., Wouters, H., Heyns, L., Han, S. N., Tomek, V., Mertens, L., & Ottevanger, P. B. (2012). Long-term cognitive and cardiac outcomes after prenatal exposure to chemotherapy in children aged 18 months or older: An observational study. The Lancet Oncology, 13(3), 256–264. https://doi.org/10.1016/S1470-2045(11)70363-1
- Amant, F., Vandenbroucke, T., Verheecke, M., Fumagalli, M., Halaska, M. J., Boere, I., Han, S., Gziri, M. M., Peccatori, F., Rob, L., & Lok, C. (2015). Pediatric outcome after maternal cancer diagnosed during pregnancy. New England Journal of Medicine, 373(19), 1824–1834. https://doi.org/10.1056/NEJMoa1508913
- Aviles, A., & Neri, N. (2001). Hematological malignancies and pregnancy: A final report of 84 children who received chemotherapy in utero. Clinical Lymphoma, 2(3), 173–177. https://doi.org/10.3816/CLM.2001.n.023
- Barker, D. J., Martyn, C. N., Osmond, C., Hales, C. N., & Fall, C. H. (1993). Growth in utero and serum cholesterol concentrations in adult life. BMJ (Clinical Research Ed), 307(6918), 1524–1527. https://doi.org/10.1136/bmj.307.6918.1524
- Bayley, N. B. (2005). Scales of infant and toddler development – third edition: Administration manual. Harcourt Assessment.
- Betchen, M., Grunberg, V. A., Gringlas, M., & Cardonick, E. (2020). Being a mother after a cancer diagnosis during pregnancy: Maternal psychosocial functioning and child cognitive development and behavior. Psycho-oncology, 29(7), 1148–1155. https://doi.org/10.1002/pon.5390
- Boerner, K., & Silverman, P. R.-J. O.-J. (2001). Gender specific coping patterns in widowed parents with dependent children. OMEGA - Journal of Death and Dying, 43(3), 201–216. https://doi.org/10.2190/GD5J-47U3-VR9P-Q63W
- Brenner, B., Avivi, I., & Lishner, M. (2012). Haematological cancers in pregnancy. Lancet, 379(9815), 580–587. https://doi.org/10.1016/S0140-6736(11)61348-2
- Cohen, M. J. (1997). Children’s memory scale. Les Editions du Centre de Psychologie Appliquée.
- De Haan, J., Verheecke, M., Van Calsteren, K., Van Calster, B., Shmakov, R. G., Mhallem Gziri, M., Halaska, M. J., Fruscio, R., Lok, C. A. R., Boere, I. A., Zola, P., Ottevanger, P. B., De Groot, C. J. M., Peccatori, F. A., Dahl Steffensen, K., Cardonick, E. H., Polushkina, E., Rob, L., Ceppi, L., Sukhikh, G. T., & Amant, F. (2018). Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: A 20-year international cohort study of 1170 patients. The Lancet Oncology, 19(3), 337–346. https://doi.org/10.1016/S1470-2045(18)30059-7
- De Sonneville, L. M. J. (2014). Handboek Amsterdamse neuropsychologische taken Amsterdam. Boom Testuitgevers.
- Forrester, G., & Geffen, G. (1991). Performance measures of 7–to 15-year-old children on the auditory verbal learning test. The Clinical Neuropsychologist, 5(4), 345–359. https://doi.org/10.1080/13854049108404102
- Garon, N. M., Piccinin, C., & Smith, I. M. (2016). Does the BRIEF-P predict specific executive function components in preschoolers? Applied Neuropsychology: Child, 5(2), 110–118. https://doi.org/10.1080/21622965.2014.1002923
- Korakiti, A. M., Zografos, E., Van Gerwen, M., Amant, F., Dimopoulos, M. A., & Zagouri, F. (2020). Long-term neurodevelopmental outcome of children after in utero exposure to chemotherapy. Cancers (Basel), 12(12), 3623. https://doi.org/10.3390/cancers12123623
- Lenaerts, L., Jatsenko, T., Amant, F., & Robert Vermeesch, J. (2019). Noninvasive prenatal testing and detection of occult maternal malignancies. Clinical Chemistry, 65(12), 1484–1486. https://doi.org/10.1373/clinchem.2019.306548
- Maggen, C., Dierickx, D., Cardonick, E., Mhallem Gziri, M., Cabrera‐Garcia, A., Shmakov, R. G., Avivi, I., Masturzo, B., Duvekot, J. J., Ottevanger, P. B., O’Laughlin, A., Polushkina, E., Van Calsteren, K., Woei‐A‐Jin, F. J. S. H., & Amant, F. (2020). Maternal and neonatal outcomes in 80 patients diagnosed with non-Hodgkin lymphoma during pregnancy: Results from the international network of cancer, infertility and pregnancy. British Journal of Haematology, 193(1). https://doi.org/10.1111/bjh.17103
- Maggen, C., Dierickx, D., Lugtenburg, P., Laenen, A., Cardonick, E., Smakov, R. G., Bellido, M., Cabrera-Garcia, A., Gziri, M. M., Halaska, M. J., Ottevanger, P. B., Van Calsteren, K., O’Laughlin, A., Polushkina, E., Van Dam, L., Avivi, I., Vandenberghe, P., Woei-A-Jin, F. J. S. H., & Amant, F. (2019). Obstetric and maternal outcomes in patients diagnosed with Hodgkin lymphoma during pregnancy: A multicentre, retrospective, cohort study. The Lancet Haematology, 6(11), e551–e61. https://doi.org/10.1016/S2352-3026(19)30195-4
- N. B. (1993). Bayley scales of infant development (2nd ed.). The psychological corporation.
- Nilsson, M. E., Maciejewski, P. K., Zhang, B., Wright, A. A., Trice, E. D., Muriel, A. C., Friedlander, R. J., Fasciano, K. M., Block, S. D., & Prigerson, H. G. (2009). Mental health, treatment preferences, advance care planning, location, and quality of death in advanced cancer patients with dependent children. Cancer, 115(2), 399–409. https://doi.org/10.1002/cncr.24002
- Odd, D. E., Emond, A., & Whitelaw, A. (2012). Long-term cognitive outcomes of infants born moderately and late preterm. Developmental Medicine and Child Neurology, 54(8), 704–709. https://doi.org/10.1111/j.1469-8749.2012.04315.x
- Parazzini, F., Franchi, M., Tavani, A., Negri, E., & Peccatori, F. A. (2017). Frequency of pregnancy related cancer: A population based linkage study in Lombardy, Italy. International Journal of Gynecological Cancer, 27(3), 613–619. https://doi.org/10.1097/IGC.0000000000000904
- Roseboom, T. (2018). De eerste 1000 dagen: het fundamentele belang van een goed begin vanuit biologisch, medisch en maatschappelijk perspectief. De Tijdstroom.
- Smidts, D., & Huizinga, M. (2010). BRIEF executieve functies gedragsvragenlijst: Handleiding. Hogrefe Uitgevers.
- Van den Bergh, B. R., Mulder, E. J., Mennes, M., & Glover, V. (2005). Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: Links and possible mechanisms. A Review. Neuroscience and Biobehavioral Reviews, 29(2), 237–258. https://doi.org/10.1016/j.neubiorev.2004.10.007
- Van Gerwen, M., Vandenbroucke, T., Verheecke, M., Van Calsteren, K., Halaska, M. J., Fumagalli, M., Fruscio, R., Gandhi, A., Veening, M., Lagae, L., Ottevanger, P. B., Voigt, J.-U., De Haan, J., Gziri, M. M., Maggen, C., Mertens, L., Naulaers, G., Claes, L., & Amant, F. (2020). Data describing child development at 6 years after maternal cancer diagnosis and treatment during pregnancy. Data Brief, 32, 106209. https://doi.org/10.1016/j.dib.2020.106209
- Vandenbroucke, T., Verheecke, M., Van Gerwen, M., Van Calsteren, K., Halaska, M. J., Fumagalli, M., Fruscio, R., Gandhi, A., Veening, M., Lagae, L., Ottevanger, P. B., Voigt, J.-U., De Haan, J., Gziri, M. M., Maggen, C., Mertens, L., Naulaers, G., Claes, L., Amant, F., Blommaert, J., & Drochýtek, V. (2020). Child development at 6 years after maternal cancer diagnosis and treatment during pregnancy. European Journal of Cancer, 138, 57–67. https://doi.org/10.1016/j.ejca.2020.07.004
- Wechsler, D. (1989). Wechesler preschool and primary scale of intelligence-revised. WPPSI-R: Psychological Corporation.
- Wechsler, D. (1997). WAIS-III: Psychological corporation San Antonio. Pearson.
- Wechsler, D. (2002). Wechsler preschool and primary scale of intelligence (3rd ed.). Psychological Corporation.
- Wechsler, D. (2003). Wechsler intelligence scale forchildren (4th ed.). Psychological Corporation.
- Wechsler, D.(2012). WPPSI-IV (Wechsler preschool and primary scale of intelligence).
- Wechsler, D. J. S. A. (1991). Manual for the Wechsler intelligence scale for children (3rd ed.). The Psychological Corporation.
- Wechsler, D. J. S. A. (2008). Wechsler adult intelligence scale–fourth edition (WAIS–IV) (Vol. 22, pp. 816–827). NCS Pearson.
- Wechsler, D. J. S. A. P. C. (2014). Wechsler intelligence scale for children–fifth edition (WISC-V). Pearson.
- Yopp, J. M., & Rosenstein, D. L. (2013). A support group for fathers whose partners died from cancer. Clinical Journal of Oncology Nursing, 17(2), 169–173. https://doi.org/10.1188/13.CJON.169-173