ABSTRACT
Although sex chromosomal trisomies (SCT) in children are highly prevalent and associated with an increased risk for neurodevelopmental difficulties including socio-emotional problems, little is known about underlying mechanisms that could drive this risk. Studying emotional reactivity and expressivity of young children with SCT in early childhood could identify deviations in early emotional development and potentially serve as risk markers to guide clinical care in developing interventions. Participants in the current study were 90 SCT children and 97 population-based controls, aged 1 to 7 years, who experienced a stress-inducing event in which physiological (heart rate) and observational data (expression of negative emotions) were collected. Results showed early disturbances in the emotion system of young children with SCT, in terms of blunted but prolonged emotional reactivity and a reduced emotional expressivity in response to stress. Further, the concordance between emotional reactivity (arousal response) and expressivity was significantly lower in SCT, compared to controls. Given the significant impact of emotions on adaptive day-to-day functioning, deviations in processing emotions could be an important underlying mechanism in explaining the heterogeneity and variability in developmental outcomes often described in individuals with SCT.
Sex chromosomal trisomies (SCT) are among the most common chromosomal duplications, with a high prevalence of 1–2 in 1000 births (Berglund et al., Citation2019; Bojesen et al., Citation2003; Groth et al., Citation2013; Morris et al., Citation2008). The chromosomal karyotypes include 47,XXY (Klinefelter syndrome) and 47,XYY (×YY syndrome) in males and 47,XXX (Trisomy X syndrome) in females. Studying genetic conditions as SCT from pregnancy on provides an unique opportunity to prospectively examine potentially at-risk development and identify early mechanisms that can contribute to outcomes (later) in life. This is especially relevant in the SCT population, since these individuals have an increased risk for various (neuro)developmental difficulties, including behavioral, learning, and socio-emotional problems (for a review see Urbanus, van Rijn & Swaab, Citation2020). To date, what drives these increased risks remains fairly unknown. To explain these neurodevelopmental difficulties, research has primarily focused on the area of information processing skills and has already identified difficulties in general intellectual functioning (albeit at the lower end of the typical range), social cognition, executive functions, and language (for a review on this topic, see van Rijn, Citation2019). Of equal importance is the perception and understanding of emotions, given that emotions are also crucial for our day-to-day functioning. Studying emotions in SCT is relevant: numerous studies have shown that individuals with SCT are vulnerable in their emotional development and often show difficulties in this area, including emotional outbursts (Visootsak & Graham, Citation2009), affective problems (Urbanus et al., Citation2020), and depressive symptoms (Tartaglia et al., Citation2010). Also, increased symptoms of psychiatric disorders associated with emotional difficulties such as autism spectrum disorders (ASD) are not uncommon in the SCT population (Ross et al., Citation2012; van Rijn et al., Citation2014). Since most of previous studies have examined older children and adults and mostly with a 47,XXY karyotype, information about the early emotional development in young children (before the age of 8 years) across all three karyotypes is limited. However, it is needed to identify early risk markers that help explain the increased risk for psychopathology and potentially guide clinical care in developing early and preventive interventions to support the overall development of these children. The current study aims to provide in this need for knowledge.
Important in the study of emotions, is that they are considered to be multifaceted and include whole-body processes, such as physiological and behavioral responses (Citation2013). Emotions involve person–situation interactions that compel attention and give rise to coordinated yet flexible responses that in turn modify the ongoing interaction (Citation2013). Thus, emotions serve a signaling function, in which they highlight events as relevant or irrelevant to an individual and help to identify which situations are attention-compelling (and which are not). To quantify the signaling function of emotions, emotional reactivity (also called affective arousal) can be assessed, which is the initial arousal response on a physiological level (Gross, Citation2013). After situations are signaled as relevant, self-regulatory processes activate the autonomic nervous system (Sapolsky, Citation2004), including the sympathetic nervous system (SNS) that stimulates increased respiratory rate and heart rate, preparing the body both physiologically and behaviorally to act (Porges & Furman, Citation2011). To illustrate: when in danger, an individual needs to attend their attention to the dangerous situation quickly (i.e., signal the event as relevant), which in turn will activate physiological changes, including heart rate acceleration, that enables a fight or flight response. The link with functional psychological outcomes in childhood day-to-day life is evident: sufficient emotional reactivity (in terms of SNS activity) in challenging situations is related to greater self-soothing, more attentional control, and greater capacity for social engagement (Blair & Peters, Citation2003; Calkins & Keane, Citation2004; Calkins et al., Citation2002). On the other hand, inadequate emotional reactivity has been linked to both childhood externalizing and internalizing behavior problems (Beauchaine, Citation2001; Boyce et al., Citation2001). In the SCT population, studies on emotions have primarily included behavioral measures and questionnaire data and only two other studies so far have examined direct psychophysiological indices of emotional reactivity and also yielded discordant results: the first that showed increased affective arousal in response to viewing emotion-evoking visual images (van Rijn et al., Citation2014) and the second that found a blunted affective arousal response to evoking social stimuli in young children with SCT (Urbanus et al., Citationunder review); thus highlighting the importance of further investigation of emotional processes in SCT.
Emotional reactivity, following emotion perception and appraisal, serves a key role in psychosocial functioning. By signaling the demands of the environment, emotional reactivity enables the coordination of behavioral responses that in turn facilitate adaptive behavior (Gross, Citation2013). As a behavioral response, the expression of emotions serves an important social and communicative function (Greenberg, Citation2004), in that the display of (facial) emotions can elicit behavior in others which in turn influences the ongoing interaction. For example, showing fear can elicit others to approach for help, whereas showing anger can signal others to avoid and withdraw (Marsh et al., Citation2005). In young children, the frequency and intensity of emotional expressivity has been linked to the quality of social relationships (Diaz et al., Citation2017; Eisenberg et al., Citation1993) and the child’s feelings of social competence (Waiden & Field, Citation1990). Individual differences in the expression of negative emotions were also found to be related to externalizing problem behavior in typical developing children (Eisenberg et al., Citation2001), highlighting that the amount and intensity of emotion expressivity can have differential effects on person–situation interactions. So far, studies on emotional expressivity in individuals with SCT typically examined the behavioral consequences of inappropriate emotional expression, such as emotional outbursts (van Rijn & Swaab, Citation2020; Visootsak & Graham, Citation2009), instead of examining the expression of emotions as the main focus of study. Even if studies examined emotional expressivity, it was usually done using self-reported data. The current study provides one of the first observational studies of emotional expressivity in children with SCT.
For adequate psychosocial functioning, a concordant system of matching emotional internal and external processes is key. When the overt display of emotions matches the internal arousal response (e.g., emotional concordance), it informs the environment on the internal state of the child which enables others to adequately respond to a child’s needs (Robinson et al., Citation1997). Discordance however can significantly hinder the engagement of the environment and confuse others about actual internal states (Mauss et al., Citation2011). In fact, caregivers and other social partners decide whether to engage with the child or to retreat from interaction following a child’s display of emotion and behavior (Denham, Citation1998). Thus, the expression of emotions and the concordance with the physiological arousal response is important in terms of adaptive social and communicative functioning. Concordance between these two emotional constructs has not yet been studied in individuals with SCT.
To the best of our knowledge, this study is the first to examine these components of emotional development in young individuals with SCT. Studying children at this young age (before the age of 7) can provide insight in early “at-risk” development. It is not surprising that a well-attuned emotion system and emotional management skills are key milestones for social and cognitive functioning, with foundations in the earliest years of life (Gross, Citation2013). We propose that differences in the emotional development, i.e., being over- or underaroused or having a discordant display of emotions, might already emerge during early childhood in children with SCT, potentially laying the groundwork for other developmental difficulties. In sum, the current study aims to investigate early emotional development of young children with an extra X or Y chromosome, in terms of emotional reactivity and emotional expression and its concordance, using a standardized behavioral assessment of a stressful event. Key to this study is that use of direct, objective, and sensitive measures of the emotion system in terms of physiological (heart rate) and observational data in a large sample of young and predominantly prenatally diagnosed children with an extra X or Y chromosome. As many earlier studies with SCT included postnatally diagnosed participants, this study includes a wider range of phenotypic characteristics. We examine these constructs of the emotion system during a stress-inducing event, as opposed to a resting state, because it is precisely those situations that are potentially “threatening” that elicit the emotional system to enable quick and adaptive responses.
Materials and methods
Participants
The current study is part of a larger international study (the TRIXY Early Childhood Study, centerd at Leiden University in the Netherlands, including research sites in the Netherlands and Colorado, in the United States of America [USA]). The TRIXY Early Childhood Study investigates the social, emotional, and behavioral development of young children with a trisomy of the X/Y chromosomes (TRIXY). For the current study, children aged 1 year to and including 7 years (at baseline) were included. Children with SCT were recruited from two sites: The Center of Expertise for Trisomy of the X and Y chromosomes (TRIXY) in the Netherlands that recruited children from the Dutch-speaking countries in Western Europe (n = 42) and the eXtraordinarY Kids Clinic in Developmental Pediatrics at the Children’s Hospital Colorado (CHCO) that recruited children from across the United States of America (USA, n = 48). Primary caregivers of children with SCT were contacted with the help of clinical genetics departments, pediatricians, and national advocacy or support groups for (parents of) individuals with SCT with recruitment flyers and postings on the internet and social media. Recruitment strategies for the SCT group resulted in three inclusion trajectories (see ): a) “information seeking parents,” b) ‘active prospective follow-up,’ and c) ‘clinically referred cases.’ Children in the control group were recruited from day care centers, public institutions, and elementary schools from the western part of the Netherlands. Inclusion criteria for all participants were that parents and/or children were Dutch- or English-speaking and children had no previous head injuries, severely impaired hearing or vision, and/or color-blindness.
Table 1. Demographic characteristics of the Sex Chromosome Trisomies (SCT) and control group.
In this study, 90 children with SCT and 97 age matched population-based controls participated (). The SCT group consisted of 30 girls with 47,XXX, 45 boys with 47,XXY, and 15 boys with 47,XYY. As for the timing of SCT diagnosis, 60 children (66.7%) had a prenatal diagnosis (i.e., because of [routine] prenatal screening, abnormal ultrasound findings, or advanced maternal age) and 30 children (33.3%) had a postnatal diagnosis (i.e., because of developmental delay, physical and/or growth problems, or medical concerns). Genetic testing results were reviewed to confirm sex chromosome trisomy in at least 80% of cells. Children from the control group were not subjected to genetic screening, due to ethical considerations of blood testing. They were considered to be a representative sample of the general population. In addition, given the prevalence of SCT is 1 in 1000, we reviewed the possible risk of having a child with undiagnosed SCT in our control group minimal and acceptable.
Background information of participants
Global intellectual functioning (GIF) was assessed with the Bayley Scales of Infant and Toddler Development (NSCT = 27, Ncontrol = 31, Bayley, Citation2006) in children aged 1 to 2 years, and the short-version of the Wechsler Preschool and Primary Scale of Intelligence third edition (NSCT = 61; Ncontrol = 64; WPPSI; Wechsler, Citation2002) in children aged 3 years or older. GIF scores for four children in the SCT group were missing. There was a significant difference in average full-scale intelligence scores between the SCT and control group, t(181) = −4.009, p < .001, d = .6. Although both groups on average scored within the average range, the SCT group scored lower (M = 96.63, SD = 18.14) than the control group (M = 106.27, SD = 14.32). Secondly, parental education level was assessed according to the Hollingshead criteria and ranged from category 1 (no formal education) to 7 (graduate professional training) (Hollingshead, Citation1975). When the child was raised by two parents (95%), educational level was averaged over both parents. Median parental education level was the same (6) in the SCT and control group (p = .637).
Ethics and procedure of the assessment
This study was approved by the Ethical Committee of the Leiden University Medical Center, Leiden, the Netherlands, and the Colorado Multiple Institutional Review Board (COMIRB) in the USA. Researchers from Leiden University were responsible for project and data‐management (i.e., training and supervision of researchers, processing and scoring of data). A written informed consent was signed by the primary caregivers of all participating children. Before the lab or home visit, participants were explicitly prepared with a visual information brochure and a copy set of the electrodes for the physiological assessment. Research took place in a quiet, stimuli-low room either at the university or in the family home, using written protocols detailing all procedures and verbal instructions to standardize assessments. Children were given time to familiarize before and after the electrodes were applied by playing an age-appropriate game, while seated in a car set to have a stable and framed position suited for physiological measurement.
Measures
Physiological arousal
Two electrodes were attached at the top center of the chest (10 centimeters below the suprasternal notch) and the bottom left of the ribs (10 centimeters above the bottom of the rib cage). Heart rate was recorded continuously during baseline and the unpredictable toy paradigm with AcqKnowledge (version 5.0.2, BIOPAC Systems Inc.). Recording was acquired through an Electrocardiogram amplifier (ECG100C) and a BIOPAC data acquisition system (MP150 Windows) with a sampling rate of 1.000 Hz. In AcqKnowledge a 0.5 Hz highpass filter and 50 Hz notch filter were applied to stabilize the ECG signal. Recorded physiological data were further processed by inspecting the detected R peaks in PhysioData Toolbox version 0.5.0 (Sjak-Shie, Citation2020). Motion artifacts were visually identified and excluded from the data. Heart rate data (beats per minute: BPM) were summarized in 30-second epochs in concordance with the behavioral data.
Baseline
To measure baseline, children watched a 3 minute video of a fish tank, which has been shown to be an adequate measure of resting state (Piferi et al., Citation2000). Heart rate (in BPM) over the course of the video was analyzed in epochs of 30 seconds each and the epoch in which children had the lowest heart rate was identified as representing resting state. This was done on group level, for the control group and the SCT group separately.
Emotional distress during stressful event
The standardized Unpredictable Mechanical Toy Task from the Laboratory Temperament Assessment Battery (Lab-TAB; Goldsmith et al., Citation1999) was chosen as a stressful event, to induce general emotional distress. It contains elements of nonsocial novelty and intrusiveness in which the toy (i.e., stressor) is, given the context, relatively inescapable. For the current study, a remote-controlled robot was chosen as the distressing stimulus, which has also been used in other studies with clinical groups examining emotional processing (e.g., in ASD: Zantinge et al. (Citation2018), in mood disorders: Savory et al. (Citation2020)). The procedure of the task was executed following the Lab-TAB manual. Caregivers were instructed to sit in the room out of direct sight, filling out questionnaires or reading magazines and to remain as uninvolved as possible while displaying a neutral face. An experimenter entered the room in a white laboratory coat and protection glasses placing a (novel to the child) robot 1.5 meters away from the child. The robot made three approaches of 30 seconds each, starting by walking toward the child, stopping 15 centimeters in front of the child moving its arms and emitting noise. Then, the robot walked backward, pausing for 5 seconds before moving forward again, repeating this sequence three times in total (i.e., stress phase). During the entire task, the experimenter did not make any eye-contact or communicate with the child. After stress phase, the experimenter left the room with the robot and the caregiver was instructed to sit with the child and watch an animated video of 2 minutes together (i.e., recovery phase). During recovery, caregivers were allowed to sooth and comfort their child if necessary, but the child stayed in the car seat for the remaining time of the video to allow for stability in the assessment of physiological recovery. The entire procedure was videotaped from two angles.
In case caregivers judged the experiment as too stressful for their child, they were allowed to stop the experiment and move forward to the recovery phase. Twenty-two percent of the parents (24 SCT, 18 controls) requested for an early termination. A total of 145 children completed the full experiment, of which 66 were children with SCT and 79 were controls. Non-completers were significantly younger (M = 2.92, SD = 1.52) than children who finished the experiment (M = 3.90, SD = 1.78), but there were no other significant group differences, e.g., in terms of research group (×2 (1) = 1.763, p = .184) or gender (×2 (1) = .160, p = .690).
To analyze the reactivity and recovery of the physiological system, four moments from the toy task were chosen for analysis: 1) initial stress (the first 30 seconds of the stress phase), 2) prolonged stress (the final 30 seconds of the stress phase), 3) initial recovery (the initial 30 seconds of the 120-second recovery phase), and 4) extended recovery (the 30-second period between 60 and 90 seconds of the 120-second recovery phase).
Observational coding of emotional expression
Videos of the unpredictable mechanical toy task were subsequently coded in 10-s epochs (with sound on) for emotional expression following the coding instructions from the Lab-TAB manual (Goldsmith et al., Citation1999) and the facial and bodily indicators of three basic emotions (fear, sadness, and anger) as described in the Facial Action Coding System (FACS; Ekman and Friesen (Citation1976)). The peak intensity of the emotions was coded within each 10-s epoch to catch the burst of facial and bodily expression during these intervals. Facial and bodily indicators of the three emotions were scored on a 4-point scale (0–3): neutral (0 – no sign of facial or bodily emotion), mild (1 - one observable facial or bodily sign), moderate (2 - two observable signs) and severe (3 – more signs). The scores were averaged across the available epochs per participant. A composite negative emotionality score was calculated derived by summing these averages. Inter-rater reliability (IRR) was assessed using a two-way mixed, absolute agreement intra-class correlation model (Hallgren, Citation2012). The IRR was substantial for emotional expression (intra-class correlation coefficient (ICC) = 0.81, p < .001). Four trained independent coders scored the recorded videos of which 25% were double coded. IRR was monitored continuously in regular consensus meetings. Discrepancies were discussed within the team to obtain a final consensus score. Distress vocalizations, as described in the Lab-TAB manual, were not included in the analyses since our study focused on the visual observable expression of emotion.
Data analysis
Due to missing data, the number of participants differed across analyses. Reasons for missing data included technological difficulties only, such as hardware or saving fail or a blocked camera view. For physiological data and the observational data taken together, data was missing from 12 children with SCT and 9 children with TD. Analyses were performed by excluding participants with missing values analysis-by-analysis. After inspection for outliers and normality checks regarding all data, no children were excluded.
First, baseline heart rate levels (BPM) between the controls and the SCT group were analyzed with an independent samples t-test, as well as within-group comparisons between the different karyotypes (ANOVA). Next, a GLM repeated measures analysis was performed with the between subject factor Group (SCT, controls) and the within-subjects factor Task (initial stress, prolonged stress, initial recovery, and extended recovery). Heart-rate data during the stressful event was corrected for baseline heart rate. To further analyze the heart rate pattern over time and potential group differences, paired sample t-tests were done for each research group separately. Subsequently, emotional expression was examined with a GLM repeated measures analysis with the between subject factor Group (SCT, controls) and the within-subjects factor Task (initial stress, prolonged stress). Also, to examine the concordance between expression and arousal response (Pearson) correlation analyses were performed between the arousal response from initial to prolonged stress phase and total emotional expression separately for both groups. When aforementioned analyses revealed significant group differences, of secondary interest to the study was whether these group effects were dependent of age. The moderating effect of age was assessed using PROCESS, a bootstrapping, nonparametric resampling procedure (Hayes, Citation2012). Bootstrapping analysis with 5000 resamples was done to test for a significant moderating effect using the SPSS macro developed by Hayes (Citation2012). Outcome variables and moderator variable (i.e., child’s age) were centered. In this analysis, the moderation effect is significant if the 95% bias corrected confidence interval for the moderator effect does not include zero. Finally, additional MANOVA’s were performed to examine the quality of the data and its representativeness based on a number of key background variables (karyotype, recruitment strategy, recruitment site). Level of significance was set at p = .05. For all significant effects, Cohen’s d addressed effect size (.2 = small effect; .5 = medium effect; .8 = strong effect, Cohen, Citation1977).
Results
Psychophysiological arousal during baseline
Baseline heart rate levels did not differ between children with SCT (M = 103.06, SD = 17.52) and controls (M = 100.94, SD = 13.90) (t(112)= .761, p = .448). Also, there were no significant differences between the three different karyotypes in the SCT group (F(2,76) = 2.702, p = .074): the mean baseline heart rate was 96.83 (SD = 16.50) for boys with 47,XYY, 108.97 (SD = 16.59) for boys with 47,XXY, and 101.84 (SD = 18.34) for girls with 47,XXX.
Psychophysiological arousal in response to stressful event
GLM repeated measure analysis revealed a significant main effect of Task (F(3,129) = 23.436, p < .001, eta =.353), a significant effect of Group (F(1,131) = 4.643, p < .05, eta =.034), and a significant Task * Group interaction effect (F(3,129) = 3.183, p < .05, eta =.069). In other words, physiological arousal changed in response to a stressful event, but this pattern differed between children with SCT and controls (see ). Within group comparison revealed a differential reactivity and recovery pattern between children with SCT and controls (see ). When faced with an unexpected stressor, children with SCT showed a weaker arousal response compared to controls (indicating a blunted response) and their recovery took longer compared to controls who showed an immediate initial recovery response.
Figure 1. Physiological reactivity and recovery patterns for Sex Chromosome Trisomies (SCT) group and control group during stressful event.
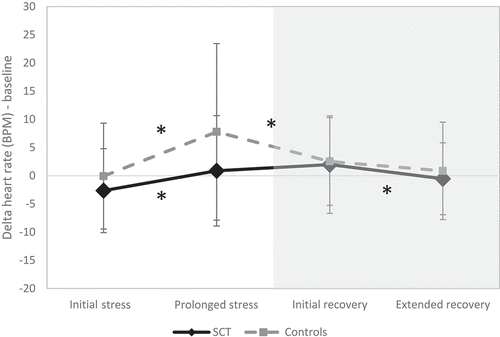
Table 2. Within group increases in arousal (heart rate) in response to stressful event.
Role of age in psychophysiological arousal
To examine whether the vulnerabilities in physiological reactivity and recovery were present across all ages in children with SCT, bias-corrected bootstrapping analyses (PROCESS) were conducted for the stress arousal response (from initial to prolonged stress) and the recovery arousal response (from prolonged stress to extended recovery). No significant moderation effect of child’s age was found for neither the stress response (b = −.07, SE =.92, t = −.07, p = .940, 95% confidence interval = > −1.88, 1.75) nor the recovery response (b = 1.14, SE = 1.16, t = .98, p = .328, 95% confidence interval = > −1.15, 3.43).
Emotional expression in response to a stressful event
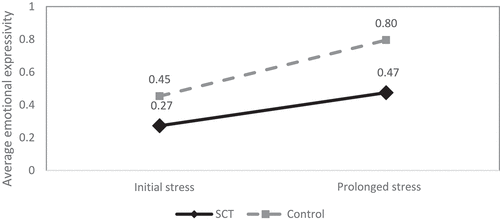
A GLM repeated measure analysis revealed a significant main effect of Task (F(1, 138) = 14.802, p < .001, eta =.097) and a significant effect of Group (F(1,138) = 4.591, p < .05, eta =.032). The interaction effect Task * Group was non-significant (F(1,138) = .929, p = .337). In controls, there was a significant increase in emotional expressivity from the initial to the prolonged stress phase of the task (also see ). This was also present for children with SCT, but significantly lower compared to the controls.
Figure 2. Average emotional expressivity during stress phases of the unpredictable toy task for Sex Chromosome Trisomies (SCT) group and control group.
Role of age in emotional expressivity
To examine whether the vulnerabilities in the expression of emotions were present across all ages in children with SCT, bias-corrected bootstrapping analyses (PROCESS) were conducted. No significant moderation effect of child’s age was found for emotional expressivity (b = −.04, SE =.19, t = −.21, p = .833, 95% confidence interval = −.42, .34).
Concordance between reactivity and expressivity
The results from the correlation analyses, performed in each research group separately, revealed significant relationships between the physiological arousal response and the total amount of emotional expressivity, during the stress phase. In controls, there was a significantly strong, positive correlation between arousal response and emotional expressivity (r = .728, p < .001). Although a significantly positive correlation was also found in the SCT group (r = .390, p < .01), this was significantly weaker in comparison to the control group, as shown by Fisher r-to-Z-transformation (z = > −2.783, p < .01). In other words, the concordance between arousal reactivity and emotional expressivity was significantly lower in the SCT group as compared to controls.
The role of karyotype, ascertainment bias and recruitment site
Karyotype
To examine whether the emotion vulnerabilities were present across all three karyotypes of SCT, a MANOVA was performed with karyotype as independent variable, and the three main outcome parameters (stress arousal response, recovery arousal response, and emotional expressivity) as dependent variables. There were no significant differences between the three karyotypes (Pillai’s trace =.155, F(6,100) = 1.403, p = .221): boys and girls with an extra X or Y chromosome showed similar patterns in the area of psychophysiological and emotional expressive outcomes.
Recruitment strategy
To examine whether recruitment strategy was relevant to the increased risk for emotion vulnerabilities, a MANOVA was performed with recruitment strategy within the SCT group (prospective follow-up, information seeking parents, clinically referred cases) as independent variable and the three main outcome parameters (stress arousal response, recovery response, and emotional expressivity) as dependent variables. There were no significant group differences (Pillai’s trace=.148, F(6,100) = 1.336, p = .248): how children were ascertained for enrollment in the study did not appear to affect the degree of psychophysiological and emotional expressive outcomes.
Research study site
To examine whether study site was related to emotion vulnerabilities, a MANOVA was performed with study site (the Netherlands, the United States of America) as the independent variable and the three main outcome parameters (stress arousal response, recovery response, and emotional expressivity) as dependent variables. There were no significant group differences (Pillai’s trace =.089, F(6,50) = 1.629, p = .194): children from the United States and The Netherlands showed similar vulnerabilities in the area of psychophysiological and emotional expressive outcomes.
Discussion
The present study examined emotional reactivity and expressivity in response to a stress evoking event in young children with an extra X or Y chromosome, compared to controls. Key to this study was the examination of both physiological components (e.g., affective arousal in heart rate) and observational behavioral components (facial and bodily expressivity) of the emotional system. By studying both parameters with sensitive and objective techniques, we aimed to understand the possible differential deficits and concordance in emotional processing in a young sample of genetically “at-risk” children. The key finding of the current study is that differential reactivity and recovery patterns were found for children with an extra sex chromosome (SCT) compared to controls. They not only showed a significantly lower arousal response (indicating a blunted response) but their recovery also took longer compared to controls who showed an immediate recovery response. In addition to emotional arousal, we found that children with SCT showed less emotional expressivity during both initial and prolonged stress. Furthermore, our study revealed less interplay between the physiological and behavioral components of the emotion response: children with SCT showed a significantly lower concordance between emotional arousal and expressivity compared to controls when faced with an unexpected (stressful) event. Taken together, these results provide the first evidence of significant vulnerabilities in the responsiveness and recovery of the emotional system of young children with SCT, which may be one of the key mechanisms underlying problems in social adaptive functioning.
Discordance between expression and arousal has not been described elsewhere in the literature on SCT, making our study the first to describe these results in this population. However, in other clinical groups also known for social-emotional difficulties, such children with autism spectrum disorders (ASD), discordance between arousal and expression has also been described (Zantinge et al., Citation2018). In hypothesizing about the nature of these differences in the affective system of children with SCT, it could be helpful to examine the underlying neurological networks in the brain that are thought to be involved in processing emotional information. For instance, numerous neural brain regions play some role in the emotion system, including subcortical areas such as the amygdala (Costafreda et al., Citation2008) as well as a set of cortical regions, including the anterior insula and dorsal anterior cingulate (Murphy et al., Citation2003). Interestingly, neuroimaging findings in SCT (specifically 47,XXY) show consistent neuro-anatomical and functional differences in these areas, including reduced gray-matter volume in both insula and temporal regions, including the amygdala and hippocampus (Hong & Reiss, Citation2014), suggesting that the physiological and behavioral vulnerabilities are likely anchored in (early) brain maturation. Further support for this early link between genes, brain, and behavior also comes from our finding that age was no significant contributor to physiological arousal or emotional expressivity. Average deviations in emotional processing were present across all ages in children with SCT, even as early as 12 months old. Our results match with those of other studies that also found early signs of an at-risk development in a subgroup of (although not all) children with SCT (Bouw et al., Citation2022; Kuiper et al., Citation2021; Urbanus et al., Citation2022; Zampini et al., Citation2021).
To the best of our knowledge, there are only three other studies to date that examined physiological arousal in individuals with SCT, for which the results vary. The first showed increased skin conductance levels in response to social information (emotion-evoking clips) compared to controls, in adults with 47,XXY (van Rijn et al., Citation2014). The second study (Bizzell et al., Citation2020) compared healthy controls to school-aged children with 47,XYY with and without an autism spectrum disorder, but found no significant groups differences in their arousal responses to sensory challenges (i.e., ambulance siren). The third study (Urbanus et al., Citationunder review), that included the same cohort of children as the current study, found a blunted physiological arousal response in response to social bids using video clips. Differences in these findings might relate to the nature of the stressor (e.g., social, nonsocial, neutral) and the context in which the arousal is measured (Stifter et al., Citation1989). The current study was designed to measure emotional reactivity without the possible interference of social interaction aspects, by introducing a stressor that did not require interaction with a second person. Comparing the results from the aforementioned studies to ours suggests that children with SCT might show a blunted arousal response in terms of their own emotions, a typical response to neutral stressors, and an increased arousal response responding to emotions of others. Replication of the current results as well as future studies that examine the influence of the nature of the stressors and the longitudinal pathway of arousal are needed to provide clarity. Nevertheless, our results on reduced emotional expressivity fit with how some young children with SCT are described on a behavioral level, including decreased assertiveness and more reserved, withdrawn, and shy behavior (Otter et al., Citation2010; Ratcliffe et al., Citation1990; Ross et al., Citation2012; van Rijn & Swaab, Citation2015). Similarly, our results match with other studies that found deviations in emotional expressivity, including difficulties in expressing negative emotions to others (van Rijn et al., Citation2008), regulating their emotions (van Rijn & Swaab, Citation2020), and identifying and verbalizing their emotions (Van Rijn et al., Citation2006).
The current results are of significant additive value to our current knowledge of mechanisms driving the risk for adaptive behavior problems of children with SCT. Traditionally, emotional and behavioral difficulties with SCT have been explained by deficits in information processing skills, including language difficulties and executive function difficulties (for a review see van Rijn, Citation2019). Based on our data, we offer another important underlying mechanism for explaining the clinical variety so often described in SCT: that of the emotion system. Understanding how vulnerabilities in the emotional system could impact adaptive functioning and behavior is key. Our results show that the signaling function of emotions, indicating that a situation is relevant and requires attention, works differently in children with SCT. These children appear less likely to signal challenging situations as potentially relevant whilst needing a longer recovery period from the same event. They show a reduced behavioral response to the situation, in terms of emotional expressiveness. The cohesion between these two components is less strong than in typical development, which makes communication of the “internal state” to the outside world more difficult. In other words, what happens on the inside (arousal) does not match with what happens on the outside (expression). In terms of day-to-day interaction, one can imagine that a disconcordance confuses a care-taker with respect to what a child needs in a specific situation, significantly hindering their adequate involvement in the child’s development and functioning. An optimal amount of emotional reactivity is needed for a child to be an active participant in day-to-day life: not too much, given that leads to continuous overwhelming experience of emotions, but also not too little, given that leads to no adequate behavioral activation (passivity). Having just the right amount of reactivity and expressivity helps individuals in their interaction with others by supporting communication of their inner state: it enables actively seeking out those situations that are good and avoiding those that are harmful (Gross, Citation2013).
When the signaling function of the emotional system does not work properly, a child cannot rely on its automatic function, which makes it difficult for the child to respond automatically and intuitively to changes in his or her environment (Gross, Citation2013). Organizing behavior so that it is adaptive to the demands of the situation at hand is thus impacted. This impaired emotional system could be an explanation on why children with SCT may experience difficulties in showing appropriate social and emotional behavior. Being human, most of our interactions and functioning take place within a highly complex and social world and well-regulated emotions help us to adaptively respond to changes in our social environment (van Rijn et al., Citation2012). Evidence for this hypothesis also comes from studies in typically developing children that show that inadequate emotional reactivity has been linked to behavioral problems (Beauchaine, Citation2001; Boyce et al., Citation2001). Discordance of arousal and expressivity is associated with later increased depressive symptoms and lower well-being (Mauss et al., Citation2011). Future studies should examine the link between emotion reactivity and responsivity and behavioral problems later in life in children with SCT, implementing a longitudinal design that goes beyond the scope of the current study. Nonetheless, our data provide the initial evidence that SCT can impact the pattern of reactivity and responsivity of the emotion system, putting these children at an early risk for socio-emotional developmental difficulties.
Strengths of our study include the use of sensitive and objective techniques, such as psychophysiology, in a young sample of predominantly prenatally diagnosed children with SCT. Studying children with a genetic disposition that can be diagnosed prenatally provides a unique opportunity to examine developmental genetic-behavioral-pathways, implementing a prospective approach that goes beyond describing problematic behavior. Instead it focuses on identifying early markers of “at risk” development that could guide early interventions to minimize future adverse outcomes. For clinical practice, our results are of significant value as well. Caregivers and professionals working with children with SCT should be aware of the differential aspects of the emotion system that could contribute to developmental vulnerabilities in early childhood. These children could benefit from orienting-supported interventions (such as guiding children’s attention to those situations that could be relevant) as well as help in expressing emotions in an adaptive way (such as mirroring or verbalizing emotional experiences). In addition, given children with SCT showed an (overall) prolonged reactivity to stress, they might need more time recovering to a “regulated” baseline state when faced with challenges: more than they might display in their behavior. Helping caregivers be aware of possible discordance in children with SCTs emotional expression may help them better support their interactions and responses to novelty.
Several factors limit the impact of the findings from the current study, for which related suggestions for future research are given. First, our study included no indices of arousal other than heart rate and observed expressive behavior in response to a stress-inducing stimulus. A range of different types of stimuli (including both positive and negative, social and nonsocial loading stimuli) should be explored further as well as other indices of the autonomic nervous system (ANS) including heart rate variability and information on the parasympathetic branch of the ANS (Benevides & Lane, Citation2015). Secondly, the current study did not include any cognitive measures or information on emotion regulation strategies. We did not examine how children dealt with the challenging situations in terms of emotion regulation strategies and how it relates to social, behavioral, and emotional functioning in day-to-day life. It would be interesting to examine the predictive value of emotion reactivity and its accompanying emotion regulation strategies over time, to provide insight in the longitudinal relationships of the emotion system and later developmental difficulties, as well as to guide intervention strategies. Whilst our study is of cross-sectional nature and our results showed that age was not a significant contributing factor to emotional reactivity nor responsivity, longitudinal studies are needed to support the developmental impact in individuals with SCT over time and its relation to neurodevelopmental disorders and psychopathology, including but not limited to ADHD and ASD. Further, these study results spark interest into how the genetic differences lead to differences in neurobiological responses, as well as how psychosocial and/or pharmacological interventions targeting emotional regulation may affect behavioral responses in children with SCT. This line of research could also include the study of the (specific) genetic influence of additional X and Y chromosomes on the social-emotional phenotype, examining differences between different karyotypes as well as comparisons with other (non-sex chromosomal) trisomies.
In conclusion, our findings show early disturbances in the emotion system of very young children with SCT, in terms of blunted and prolonged emotional reactivity and a reduced emotional expressivity in response to stress. We propose that the emotion system could be an important underlying mechanism in explaining the heterogeneity and variability in developmental outcomes so often described in individuals with SCT, given the significant impact emotions have on adaptive day-to-day functioning. The current findings are important for improvement of clinical care in individuals with SCT, given that increased awareness of the discordance between expression and arousal can be meaningful in psycho-education and intervention strategies of individuals with SCT.
Geolocation information
This study was conducted at two research sites: 1) TRIXY Center of Expertise at Leiden University, at Leiden in the Netherlands (GPS: 52.17042540046926, 4.477625989724528) and 2) the eXtraordinarY Kids Clinic in Developmental Pediatrics at the Children’s Hospital Colorado (GPS: 39.742333516072826, −104.83492759321679).
Acknowledgements
This study was funded by a grant from the Dutch Organization for Scientific Research (NWO funding # 016.165.397 to Sophie van Rijn). Work in Colorado was partially supported by infrastructure of NIH/NCATS Colorado CTSA Grant Number UL1 TR002535. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views. The authors thank the families that participated in our study, and the research assistants and students for their help with data collection and processing. The data that support the findings of this study are available from the corresponding author upon.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bayley, N. (2006). Bayley scales of infant and toddler development, third edition. San Antonio, TX: Harcourt Assessment, Inc.
- Beauchaine, T. (2001). Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology, 13(2), 183–214. https://doi.org/10.1017/S0954579401002012
- Benevides, T. W., & Lane, S. J. (2015). A review of cardiac autonomic measures: Considerations for examination of physiological response in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(2), 560–575. https://doi.org/10.1007/s10803-013-1971-z
- Berglund, A., Viuff, M. H., Skakkebæk, A., Chang, S., Stochholm, K., & Gravholt, C. H. (2019). Changes in the cohort composition of turner syndrome and severe non-diagnosis of Klinefelter, 47, XXX and 47, XYY syndrome: A nationwide cohort study. Orphanet Journal of Rare Diseases, 14(1), 1–9. https://doi.org/10.1186/s13023-018-0976-2
- Bizzell, E., Ross, J., Rosenthal, C., Dumont, R., & Schaaf, R. (2020). Sensory features as a marker of autism spectrum disorders. Journal of Autism & Developmental Disorders, 50(6), 2240–2246. https://doi.org/10.1007/s10803-019-03948-8
- Blair, C., & Peters, R. (2003). Physiological and neurocognitive correlates of adaptive behavior in preschool among children in head start. Developmental Neuropsychology, 24(1), 479–497. https://doi.org/10.1207/S15326942DN2401_04
- Bojesen, A., Juul, S., & Gravholt, C. H. (2003). Prenatal and postnatal prevalence of Klinefelter syndrome: A national registry study. The Journal of Clinical Endocrinology & Metabolism, 88(2), 622–626. https://doi.org/10.1210/jc.2002-021491
- Bouw, N., Swaab, H., Tartaglia, N., Cordeiro, L., & van Rijn, S. (2022). Early social behavior in young children with sex chromosome trisomies (XXX, XXY, XYY): Profiles of observed social interactions and social impairments associated with autism spectrum disorder (ASD). Journal of Autism and Developmental Disorders. Advance online publication. https://doi.org/10.1007/s10803-022-05553-8
- Boyce, W. T., Quas, J., Alkon, A., Smider, N. A., Essex, M. J., & Kupfer, D. J. (2001). Autonomic reactivity and psychopathology in middle childhood. The British Journal of Psychiatry, 179(2), 144–150. https://doi.org/10.1192/bjp.179.2.144
- Calkins, S. D., Dedmon, S. E., Gill, K. L., Lomax, L. E., & Johnson, L. M. (2002). Frustration in infancy: Implications for emotion regulation, physiological processes, and temperament. Infancy, 3(2), 175–197. https://doi.org/10.1207/S15327078IN0302_4
- Calkins, S. D., & Keane, S. P. (2004). Cardiac vagal regulation across the preschool period: Stability, continuity, and implications for childhood adjustment. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology, 45(3), 101–112. https://doi.org/10.1002/dev.20020
- Cohen, J. D. (1977). Statistical power analysis for the behavioural sciences. Academic.
- Costafreda, S. G., Brammer, M. J., David, A. S., & Fu, C. H. Y. (2008). Predictors of amygdala activation during the processing of emotional stimuli: A meta-analysis of 385 PET and fMRI studies. Brain Research Reviews, 58(1), 57–70. https://doi.org/10.1016/j.brainresrev.2007.10.012
- Denham, S. A. (1998). Emotional development in young children. Guilford Press.
- Diaz, A., Eisenberg, N., Valiente, C., Van Schyndel, S., Spinrad, T. L., Berger, R., Hernandez, M. M., Silva, K. M., & Southworth, J. (2017). Relations of positive and negative expressivity and effortful control to kindergarteners’ student–teacher relationship, academic engagement, and externalizing problems at school. Journal of Research in Personality, 67(1), 3–14. https://doi.org/10.1016/j.jrp.2015.11.002
- Eisenberg, N., Fabes, R. A., Bernzweig, J., Karbon, M., Poulin, R., & Hanish, L. (1993). The relations of emotionality and regulation to preschoolers’ social skills and sociometric status. Child Development, 64(5), 1418–1438. https://doi.org/10.2307/1131543
- Eisenberg, N., Cumberland, A., Spinrad, T. L., Fabes, R. A., Shepard, S. A., Reiser, M., Murphy, B. C., Losoya, S. H., & Guthrie, I. K. (2001). The relations of regulation and emotionality to children’s externalizing and internalizing problem behavior. Child Development, 72(4), 1112–1134. https://doi.org/10.1111/1467-8624.00337
- Ekman, P., & Friesen, W. V. (1976). Pictures of facial affect. Consulting Psychologists Press.
- Goldsmith, H. H., Reilly, J., & Lemery, K. S. (1999). The Laboratory temperament assessment battery: preschool version (technical manual). University of Wisconsin.
- Greenberg, L. S. (2004). Emotion–focused therapy. Clinical Psychology & Psychotherapy: An International Journal of Theory & Practice, 11(1), 3–16. https://doi.org/10.1002/cpp.388
- Gross, J. J. (Ed.). (2013). Handbook of emotion regulation (2nd ed., p. 669). Taylor & Francis Ltd.
- Groth, K. A., Skakkebæk, A., Høst, C., Gravholt, C. H., & Bojesen, A. (2013). Klinefelter syndrome—a clinical update. The Journal of Clinical Endocrinology & Metabolism, 98(1), 20–30. https://doi.org/10.1210/jc.2012-2382
- Hallgren, K. A. (2012). Computing inter-rater reliability for observational data: An overview and tutorial. Tutorials in Quantitative Methods for Psychology, 8(1), 23. https://doi.org/10.20982/tqmp.08.1.p023
- Hayes, A. F. (2012). PROCESS: a versatile computational tool for observed variable mediation, moderation, and conditional process modeling. University of Kansas, KS.
- Hollingshead, A. D. B. (1975). Four factor index of social status. Yale University.
- Hong, D. S., & Reiss, A. L. (2014). Cognitive and neurological aspects of sex chromosome aneuploidies. Lancet Neurology, 13(3), 306–318. https://doi.org/10.1016/S1474-4422(13)70302-8
- Kuiper, K., Swaab, H., Tartaglia, N., & van Rijn, S. (2021). Early developmental impact of sex chromosome trisomies on attention deficit‐hyperactivity disorder symptomology in young children. American Journal of Medical Genetics: Part A, 185(12), 3664–3674. https://doi.org/10.1002/ajmg.a.62418
- Marsh, A. A., Ambady, N., & Kleck, R. E. (2005). The effects of fear and anger facial expressions on approach-and avoidance-related behaviors. Emotion, 5(1), 119. https://doi.org/10.1037/1528-3542.5.1.119
- Mauss, I. B., Shallcross, A. J., Troy, A. S., John, O. P., Ferrer, E., Wilhelm, F. H., & Gross, J. J. Don’t hide your happiness! Positive emotion dissociation, social connectedness, and psychological functioning. (2011). Journal of Personality and Social Psychology, 100(4), 738–748. American Psychological Association. https://doi.org/10.1037/a0022410
- Morris, J. K., Alberman, E., Scott, C., & Jacobs, P. (2008). Is the prevalence of Klinefelter syndrome increasing? European Journal of Human Genetics, 16(2), 163–170. https://doi.org/10.1038/sj.ejhg.5201956
- Murphy, F. C., Nimmo-Smith, I. A. N., & Lawrence, A. D. (2003). Functional neuroanatomy of emotions: A meta-analysis. Cognitive, Affective, & Behavioral Neuroscience, 3(3), 207–233. https://doi.org/10.3758/CABN.3.3.207
- Otter, M., Schrander-Stumpel, C. T. R. M., & Curfs, L. M. G. (2010). Triple X syndrome: A review of the literature. European Journal of Human Genetics, 18(3), 265–271. https://doi.org/10.1038/ejhg.2009.109
- Piferi, R. L., Kline, K. A., Younger, J., & Lawler, K. A. (2000). An alternative approach for achieving cardiovascular baseline: Viewing an aquatic video. International Journal of Psychophysiology, 37(2), 207–217. https://doi.org/10.1016/S0167-8760(00)00102-1
- Porges, S. W., & Furman, S. A. (2011). The early development of the autonomic nervous system provides a neural platform for social behaviour: A polyvagal perspective. Infant and Child Development, 20(1), 106–118. https://doi.org/10.1002/icd.688
- Ratcliffe, S. G., Butler, G. E., & Jones, M. (1990). Edinburgh study of growth and development of children with sex chromosome abnormalities. IV. Birth Defects Original Article Series, 26(4), 1–44.
- Robinson, J. L., Emde, R. N., & Korfmacher, J. (1997). Integrating an emotional regulation perspective in a program of prenatal and early childhood home visitation. Journal of Community Psychology, 25(1), 59–75. https://doi.org/10.1002/(SICI)1520-6629(199701)25:1<59:AID-JCOP5>3.0.CO;2-Y
- Ross, J. L., Roeltgen, D. P., Kushner, H., Zinn, A. R., Reiss, A., Bardsley, M. Z., McCauley, E., & Tartaglia, N. (2012). Behavioral and social phenotypes in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. Pediatrics, 129(4), 769–778. https://doi.org/10.1542/peds.2011-0719
- Sapolsky, R. M. (2004). Why zebras dont get ulcers (3rd ed.). Holy McDougal.
- Savory, K., Garay, S. M., Sumption, L. A., Kelleher, J. S., Daughters, K., Janssen, A. B., Van Goozen, S., & John, R. M. (2020). Prenatal symptoms of anxiety and depression associated with sex differences in both maternal perceptions of one year old infant temperament and researcher observed infant characteristics. Journal of Affective Disorders, 264(1), 383–392. https://doi.org/10.1016/j.jad.2019.11.057
- Sjak-Shie, E. E. (2020). PhysioData Toolbox (Version 0.5.0). https://physiodatatoolbox.leidenuniv.nl
- Stifter, C. A., Fox, N. A., & Porges, S. W. (1989). Facial expressivity and vagal tone in 5-and 10-month-old infants. Infant Behavior & Development, 12(2), 127–137. https://doi.org/10.1016/0163-6383(89)90001-5
- Tartaglia, N., Cordeiro, L., Howell, S., Wilson, R., & Janusz, J. (2010). The spectrum of the behavioral phenotype in boys and adolescents 47, XXY (Klinefelter syndrome). Pediatric Endocrinology Reviews: PER, 8(01), 151. Retrieved July 15, 2022, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3740580/
- Tartaglia, N. R., Howell, S., Sutherland, A., Wilson, R., & Wilson, L. (2010). A review of trisomy X (47,XXX). Orphanet Journal of Rare Diseases, 5(1), 8. https://doi.org/10.1186/1750-1172-5-8
- Urbanus, E., Swaab, H., Tartaglia, N., Cordeiro, L., & van Rijn, S. (2020). The behavioral profile of children aged 1–5 years with sex chromosome trisomy (47,XXX, 47,XXY, 47,XYY). American Journal of Medical Genetics: Part C, Seminars in Medical Genetics, 184(2), 444–455. https://doi.org/10.1002/ajmg.c.31788
- Urbanus, E., Swaab, H., Tartaglia, N., Boada, R., & van Rijn, S. (2022). A cross-sectional study of early language abilities in children with sex chromosome trisomy (XXY, XXX, XYY) aged 1–6 years. Child Neuropsychology, 28(2), 171–196. https://doi.org/10.1080/09297049.2021.1960959
- Urbanus, E., Swaab, H., Tartaglia, N. R., & van Rijn, S. (under review). Physiological arousal in children with sex chromosomal trisomies: Responding to communicative bids Child Development.
- Van Rijn, S., Swaab, H., Aleman, A., & Kahn, R. S. (2006). X chromosomal effects on social cognitive processing and emotion regulation: A study with Klinefelter men (47, XXY). Schizophrenia Research, 84(2–3), 194–203. https://doi.org/10.1016/j.schres.2006.02.020
- van Rijn, S., Swaab, H., Aleman, A., & Kahn, R. S. (2008). Social behavior and autism traits in a sex chromosomal disorder: Klinefelter (47XXY) syndrome. Journal of Autism and Developmental Disorders, 38(9), 1634–1641. https://doi.org/10.1007/s10803-008-0542-1
- van Rijn, S., van’t Wout, M., & Spikman, J. (2012). Emotie en sociale cognitie [Emotion and social cognition. In R. Kessels, P. Eling, R. Ponds, J. Spikman, & M. van Zandvoort (Eds.), Klinische neuropsychologie [Clinical Neuropsychology] (pp. 267–290). Boom.
- van Rijn, S., Barendse, M., van Goozen, S., Swaab, H., & Guo, K. (2014). Social attention, affective arousal and empathy in men with Klinefelter syndrome (47,XXY): Evidence from eyetracking and skin conductance. PloS One, 9(1), e84721. https://doi.org/10.1371/journal.pone.0084721
- van Rijn, S., Stockmann, L., Borghgraef, M., Bruining, H., van Ravenswaaij-Arts, C., Govaerts, L., Hansson, K., & Swaab, H. (2014). The social behavioral phenotype in boys and girls with an extra X chromosome (Klinefelter syndrome and trisomy X): A comparison with autism spectrum disorder. Journal of Autism and Developmental Disorders, 44(2), 310–320. https://doi.org/10.1007/s10803-013-1860-5
- van Rijn, S., & Swaab, H. (2015). Executive dysfunction and the relation with behavioral problems in children with 47,XXY and 47,XXX. Genes, Brain, and Behavior, 14(2), 200–208. https://doi.org/10.1111/gbb.12203
- van Rijn, S. (2019). A review of neurocognitive functioning and risk for psychopathology in sex chromosome trisomy (47,XXY, 47,XXX, 47, XYY). Current Opinion in Psychiatry, 32(2), 79–84. https://doi.org/10.1097/YCO.0000000000000471
- van Rijn, S., & Swaab, H. (2020). Emotion regulation in adults with Klinefelter syndrome (47, XXY): Neurocognitive underpinnings and associations with mental health problems. Journal of Clinical Psychology, 76(1), 228–238. https://doi.org/10.1002/jclp.22871
- Visootsak, J., & Graham, J. M., Jr. (2009). Social function in multiple X and Y chromosome disorders: XXY, XYY, XXYY, XXXY. Developmental Disabilities Research Reviews, 15(4), 328–332. https://doi.org/10.1002/ddrr.76
- Waiden, T. A., & Field, T. M. (1990). Preschool children’s social competence and production and discrimination of affective expressions. The British Journal of Developmental Psychology, 8(1), 65–76. https://doi.org/10.1111/j.2044-835X.1990.tb00822.x
- Wechsler, D. (2002). WPPSI-III: Technical and interpretive manual. The Psychological Corporation.
- Zampini, L., Burla, T., Silibello, G., Capelli, E., Dall’Ara, F., Rigamonti, C., Ajmone, P. F., Monti, F., Zanchi, P., & Lalatta, F., Costantino, M. A., Vizziello, P. G. (2021). Preverbal skills in 8-month-old children with sex chromosome trisomies. First Language, 41(2), 200–217. https://doi.org/10.1177/0142723720962944
- Zantinge, G., van Rijn, S., Stockmann, L., & Swaab, H. (2018). Concordance between physiological arousal and emotion expression during fear in young children with autism spectrum disorders. Autism, 23(3), 629–638. https://doi.org/10.1177/1362361318766439
 Emotional reactivity and expressivity in young children with sex chromosome trisomies: evidence from psychophysiological and observational data
Emotional reactivity and expressivity in young children with sex chromosome trisomies: evidence from psychophysiological and observational data