ABSTRACT
To (i) determine whether accelerated long-term forgetting (ALF) can be found using standardized verbal memory test materials in children with genetic generalized epilepsy (GGE) and temporal lobe epilepsy (TLE), and (ii) to establish whether ALF is impacted by executive skills and repeat testing over long delays. One hundred and twenty-three children aged 8 to 16, (28 with GGE, 23 with TLE, and 72 typically developing; TD) completed a battery of standardized tests assessing executive functioning and memory for two stories. Stories were recalled immediately and after a 30-min delay. To examine whether repeat testing impacts long-term forgetting, one story was tested via free recall at 1-day and 2-weeks, and the other at 2-weeks only. Recognition was then tested for both stories at 2-weeks. Children with epilepsy recalled fewer story details, both immediately and after 30-min relative to TD children. Compared to TD children, the GGE group, but not the TLE group, showed ALF, having significantly poorer recall of the story tested only at the longest delay. Poor executive skills were significantly correlated with ALF for children with epilepsy. Standard story memory materials can detect ALF in children with epilepsy when administered over long delays. Our findings suggest that (i) ALF is related to poor executive skills in children with epilepsy, and (ii) repeated testing may ameliorate ALF in some children.
Epilepsy is a chronic medical condition associated with significant psychosocial, cognitive, and behavioral sequelae. When emerging in childhood, cognitive impairment exacerbates the level of disability beyond seizure symptomatology, exerting consequences for the child’s social, academic, and psychological development (Michaelis et al., Citation2018). While the adoption of effective, evidence-based restitutional memory strategies are of undisputed importance for children with epilepsy, there remains a paucity of them (Joplin et al., Citation2018; Kerr & Blackwell, Citation2015; Michaelis et al., Citation2018). Two profoundly underappreciated although well-established neurocognitive disturbances often seen in children with epilepsy are impairment in long-term memory formation (i.e., accelerated long-term forgetting; ALF; (Gascoigne et al., Citation2012, Citation2014; Grayson-Collins et al., Citation2019; Joplin et al., Citation2020, Citation2022), and executive dysfunction (Culhane-Shelburne et al., Citation2002; Hernandez et al., Citation2002; Hessen et al., Citation2018; Joplin et al., Citation2020, Citation2022; MacAllister et al., Citation2014; Sepeta et al., Citation2017). Recently, the International League Against Epilepsy (ILAE) Psychology Task Force was commissioned to report on the state of evidence of psychological treatment for improving health-related quality of life (Michaelis et al., Citation2018). However, the ILAE Psychology Task Force found few evidence-based recommendations for addressing neurocognitive disturbance, none of which targeted memory impairment nor executive functioning.
ALF is a memory disorder characterized by normal learning and recall of new information on standardized clinical testing over delays of up to 30 min, although a faster-than-normal rate of forgetting over the subsequent hours to weeks (Blake et al., Citation2000; De Renzi & Lucchelli, Citation1993). ALF has garnered the interest of memory researchers over the last two decades, as its presence and trajectory have been difficult to consistently place within prevailing models of memory (i.e., Multiple Trace Theory (MTT) and Classic Consolidation Theory; CCT). To date, ALF has most commonly been explained by disrupted long-term consolidation (consistent with CCT). ALF has typically been found in adults and children with temporal lobe epilepsy (TLE; Audrain & McAndrews, Citation2019; Gascoigne et al., Citation2014; Joplin et al., Citation2022; Lah et al., Citation2014; Mameniskiene et al., Citation2006; Narayanan et al., Citation2012) and typically attributed to subtle hippocampal abnormalities and/or seizure themselves. Most studies examining the relationship between hippocampal integrity and ALF have failed to find a correlation between ALF and hippocampal volume in patients with temporal lobe epilepsy (TLE).
ALF has also been found in children with Genetic Generalised epilepsy (GGE). As clinically obtained medical resonance imaging (MRI) scans of children with GGE are typically free of structural abnormalities, it was thought that in children with GGE, ALF was predominantly related to seizure activity (Gascoigne et al., Citation2012). Nevertheless, in a longitudinal study of children with GGE, ALF was found to persist even when children were free of seizures (Grayson-Collins et al., Citation2019). Hence, it is interesting to note that unlike clinically obtained structural MRIs, more advanced imaging, such as functional MRI, has revealed reduced connectivity in the default mode network of patients with GGE (Parsons et al., Citation2020). Parson and colleagues proposed that these reductions in connectivity may compromise network communication capacity, and adversely impact patient cognition. Moreover, it is possible that ALF is in part due to reductions in functional connectivity that disrupt a long-term memory consolidation process, which requires ongoing interaction between the hippocampus and neocortex. These reductions in connectivity may also be detrimental to other aspects of cognition that rely heavily on the functional integrity of widely distributed brain networks. Executive function, namely attention and working memory may be particularly sensitive to such disruptions. These cognitive functions also play a critical role in facilitating memory processes, especially for short-term memory processing (i.e., the phonological loop), the organization of learned materials during consolidation, and the timely retrieval of information (Baddeley, Citation1992). Thus, when executive skills are impaired, long-term memory recall may be profoundly impacted. It is well documented that executive dysfunction (such as deficits in executive attention) is associated with impaired long-term recall ability (Mayr et al., Citation2014; Skodzik et al., Citation2017). Mayr et al. (Citation2014) demonstrated the relationship between LTM and the control of attention through a spatial location paradigm. Through a series of theoretically driven studies exploring endogenous versus exogenous control over spatial attention, the authors purport that compromised attentional control may detrimentally impact memory traces encoded in LTM through interference.
Children with epilepsy have been found to have deficits in executive skills that are associated with poorer performance on standardized memory tests (Rzezak et al., Citation2012; Sepeta et al., Citation2017). For example, Sepeta et al. (Citation2017) discovered that children with focal epilepsy had greater difficulty with free recall (but not recognition) of verbal and visual information after a 20–30-min delay, compared to their typically developing (TD) peers. Additionally, lower scores on tests of executive skills (e.g., working memory and planning/organization) were linked to free recall of verbal and visual materials at standard delays. Joplin et al. (Citation2020) also observed impaired long-term memory for visual materials in children with GGE, which was related to working memory. To date, no study has investigated whether executive skills are related to long-term memory for verbal materials in GGE children, who are known to have deficits in executive functions, such as sustained attention, verbal/semantic fluency, and working memory (Chowdhury et al., Citation2014; Gelziniene et al., Citation2011; Loughman et al., Citation2014; Ratcliffe et al., Citation2020).
Whilst studies assessing verbal memory routinely use lists (Gascoigne et al., Citation2012, Citation2014; Joplin et al., Citation2020), single-presentation stories have been used less frequently. Story subtests place the content to be memorized within a contextualized and meaningful short narrative. This presents an important ecological difference from list-learning due to the critical need to remember extended, meaningful verbal information in everyday life (e.g., conversations, lectures, reading comprehension). Further, previous studies required participants to learn materials to criterion (Davidson et al., Citation2007; Gascoigne et al., Citation2012, Citation2014; Joplin et al., Citation2020, Citation2022). The current study presented stories as free recall tasks that (i) more realistically replicate memory demands in real life, and (ii) likely deploy different executive functions than list learning, which may be facilitated by organizational strategies such as “chunking” (i.e., the grouping together of semantically similar words). Memory for stories instead may demand executive resources such as attention and verbal short-term memory (i.e., the phonological loop).
Finally, it is of interest to note that while several studies employed list learning to criterion as the methodology, results have been divergent. Two studies found ALF in children with GGE (Gascoigne et al., Citation2012; Grayson-Collins et al., Citation2019) and another in children with TLE (Gascoigne et al., Citation2014). However, two further studies failed to identify ALF in children with GGE (Joplin et al., Citation2020) nor TLE (2022). While methodologically similar, the studies were not methodologically the same. Studies that found evidence of ALF on the list learning task-tested recall after one long delay (7 days). In contrast, the two studies that did not find evidence of ALF in children with epilepsy-tested recall at two long delays (1- and 14-days). It is possible that repeated testing over long in fact mitigated ALF. In the general population, there is convincing evidence that the practice of retrieval for newly acquired material can improve performance at testing over a delay, an effect which is sometimes even greater than further learning in healthy participants (see Roediger & Butler, Citation2011; Roediger & Karpicke, Citation2006). Furthermore, providing additional retrieval trials has been found to reduce forgetting in adults with ALF (Jansari et al., Citation2010; Ricci et al., Citation2019). Establishing whether additional retrieval opportunities eliminate or ameliorate ALF in children with epilepsy has important implications for targeted remediation for children whose development is critically dependent on knowledge acquisition.
The brevity of delays represents another issue of measurement, as research has established that some clinical populations (i.e., children with epilepsy) have shown differential patterns of memory deterioration over extended delays (i.e., delays of days or weeks) relative to TD children (i.e., as seen in Gascoigne et al., Citation2012, Citation2014). Typically, studies have used only one short and one long delay, often 30 min and one week, respectively (Davidson et al., Citation2007; Gascoigne et al., Citation2012, Citation2014). These studies also have historically investigated only one population of interest (i.e., GGE or TLE), precluding any comparison between these two clinically divergent populations.
The current study sought to address these gaps in the literature by employing repeat test intervals at one day and two weeks, comparing the performance of children with GGE and TLE on a free-recall story memory task (i.e., not learned to criterion for increased ecological validity) relative to their TD peers. Of particular interest was whether the type of epilepsy syndrome differentially impacted story recall performance relative to TD counterparts. Secondly, we sought to determine whether an additional rehearsal 1-day post learning would confer any benefit to memory retention for the three groups of interest. Based on previous research into ALF in children with epilepsy, we hypothesized that relative to the TD children, both groups of children with epilepsy would show ALF. Nevertheless, we expected that in children with TLE memory decay would be faster, and ALF thus would manifest earlier than in children with GGE. Consistent with research into spaced retrieval and active recall, we anticipated that having an additional opportunity to recall materials 1-day post-learning would facilitate performance at a 2-week delay. Lastly, consistent with research identifying a relationship between memory function for free-recall tasks after a 30-min delay and weaker executive functioning in children with epilepsy (Joplin et al., Citation2020, Citation2022; Sepeta et al., Citation2017), it was expected that having reduced executive attention and phonological loop repository would be associated with a steeper forgetting curve for Story Memory over long delays (i.e., ALF would be more prominent).
Method
The study was conducted in accordance with ethics approvals obtained by participating children’s hospitals. TD children were recruited from public schools via State Education Ethics and through passive snowballing via the peer networks of participating families.
Participants
One hundred and twenty-three children (28 with GGE, 23 with TLE, and 72 TD) participated in the study. Inclusion criteria were: (i) aged eight to 16 years; (ii) fluent in the English language; (iii) average or higher intellectual ability (Full-Scale IQ ≥ 79); and (iv) free of major sensory deficits/neurological disorders/major neurodevelopmental disorders (i.e., autism spectrum disorder). To be considered eligible to participate, children with epilepsy needed to have (i) a diagnosis of GGE or TLE (as determined by medical records and confirmed by treating neurologists), as well as (ii) evidence of normal development prior to the onset of epilepsy (as determined by medical records and/or initial interview with parents by one of the investigators, SJ). All participants with epilepsy were recruited through four tertiary-referral children’s hospitals. Patient records were inspected retrospectively by treating clinicians to identify children diagnosed with epilepsy who met the inclusion criteria. Parents of identified children were contacted for an intake interview to assess eligibility via the telephone. If they expressed interest and willingness to participate in the study, an interview was conducted to collect background information and verify that the child met the study inclusion criteria. Seventy-two TD children were recruited from the general public (i.e., state education and peer networks) via advertisements and word-of-mouth (snowball) recruitment. Inclusion and exclusion criteria were the same (sans epilepsy-specific criterion), as all TD children were required to be free of a history of seizures.
Materials
Intelligence
The two-subtest version of the Wechsler Abbreviated Scale of Intelligence, Second Edition (Vocabulary and Matrix Reasoning; WASI-II; Wechsler, Citation2011) was used to provide an estimate of the examinee’s FSIQ-2 (Full-Scale Intelligence Quotient, 2-subject version; M = 100, SD = 15).
Memory
Verbal memory (short- and long-term retention) was assessed using stories from the Story Memory subtest of the Wide Range of Assessment of Memory and Learning, second edition (WRAML-2; Sheslow & Adams, Citation2003). The subtest contained two short stories, which were read to participants and asked to recall as many details as possible immediately after the presentation and following a 20–30-min delay as per WRAML-2 manualised instructions. To avoid contamination at long delays, a recognition condition was not administered at baseline. Age-scaled scores (M = 10, SD = 3) were obtained to compare children’s performance to normative data.
To assess long-term memory formation and the impact of extra rehearsal on long-term recall, all participants were asked to recall (1) the first (A) story (i.e., the story most recalled) at 1 day and 2 weeks, and (2) the second (B) story (i.e., the story least recalled) at 2 weeks. In addition, a forced choice recognition (FCR) task was administered at 2 weeks (i.e., an experimental administration of recognition), following the recall of both stories. Raw scores (the number of story details recalled) obtained on immediate, 30-min, 1-day (where applicable), and 2-week delays were considered. The percentage of story details recalled on delays relative to the immediate recall was calculated and used in the current study.
Executive function
Two subtests (Verbal Fluency and Color-Word Interference) from the Delis-Kaplan Executive Function Scale (D-KEFS; Delis et al., Citation1994) were administered to assess verbal fluency, verbal generativity, and inhibition. Raw scores were transformed into age-scaled normative scores (M = 10, SD = 3). Additionally, four subtests (Digit Recall, Backward Digit Recall, Dot Matrix, and Odd-one-Out) from the Automated Working Memory Assessment (AWMA; Alloway, Citation2007) and one subtest (Creature Counting) from the Test of Everyday Attention for Children (TEA-Ch; Manly et al., Citation1999 were administered to assess working/short-term memory, and complex attention, respectively. Standard scores (M = 100, SD = 15) were derived from normative data for AWMA subtests. Timeliness and accuracy were factored into the normative scaled scores (M = 10, SD = 3) for Creature Counting.
Procedure
A standardized assessment of intelligence and memory was completed in a face-to-face individual testing session. Long-term recall and recognition of stories were assessed via a phone call. Participants were not forewarned of follow-up tasks to prevent rehearsing/route learning. Information relating to the developmental history of the child and relevant epilepsy variables (i.e., medication taken and seizure frequency at the time of assessment) was collected using a structured questionnaire administered to parents and cross-checked with medical records where possible.
Socio-economic status was gauged using the Socio-Economic Indexes for Areas (SEIFA; Australian Bureau of Statistics [ABS], Citation2011). The SEIFA is a product created by the ABS that ranks postal areas in Australia based on socio-economic advantages and disadvantages. The SEIFA produces an original value based on index rankings and quantiles (e.g., deciles). This measure was omitted for 3 participants from Canada.
The Global Assessment of Severity of Epilepsy (GASE) Scale was used to gauge overall epilepsy severity. The GASE is a single‐item, 7‐point global rating scale intended for neurologist‐report of epilepsy severity in children (Chan et al., Citation2015).
Statistical analyses
All statistical analyses were performed using SPSS statistical software version 19, with a standard alpha of 0.05. Data were screened for normality using visual inspection of histograms and review of a Skewness and Kurtosis. Kolmogorov–Smirnov tests were used to check the suitability of variables for parametric analyses. Analysis of Covariance (ANCOVA) was used to examine group differences on continuous normally distributed variables. Sex and age were included as covariates. Independent samples t-tests were used to compare the GGE and TLE groups on clinical variables. Paired samples t-tests were used to compare the magnitude of difference between (a) the story most recalled and (b) the story least recalled at two weeks within groups. For data that were not normally distributed, non-parametric statistics were used (i.e., The Kruskal–Wallis H test was used for SIEFA data, and the Mann – Whitney U-test for GASE scores). When results were statistically significant, pairwise comparisons using Fisher’s LSD were reported. Bivariate one-tailed Pearson Product-Moment correlations were used to examine the relationship between executive function scores on which statistically significant group differences were identified and AFL in the TLE and GGE groups. One-tailed hypothesis testing was considered appropriate as we retained specific predictions about the direction of the difference (i.e., that poorer executive skills would relate to poorer memory scores).
Repeated measurements of ANCOVA (Group × Delay), followed by post-hoc analyses, tested the main effects of group, delay, and interactions on Story Memory. When Mauchly’s test of sphericity was violated, the Greenhouse–Geisser correction was applied. Tukey’s HSD was used for post-hoc analyses that included the three groups when the main effect of Group or Delay was significant and when a significant interaction was identified. When Levene’s Test for Equality of Variances was significant, an equal variance was not assumed.
Results
The groups were comparable on SES (SEIFA, Table 5.1) although not on age (GGE < TLE, p = .042, although not TD, p = .659; TD ≯ TLE, p = .103), sex distribution (GGE > TLE; TD (X2(2) = 7.14, p = .008, TLE ≯ TD), and FSIQ-2 (TD > GGE, p < .001; TD ≯ TLE, p = .072; TLE ≯ GGE, p = .439). With respect to clinical characteristics, there were no significant differences between epilepsy groups on clinical variables, namely GASE scores, days since last seizure, seizure status at the time of assessment, and medication status ().
Table 1. Background and clinical variables.
Verbal memory (standardised and experimental administration)
A two-way (Group × Delay) ANCOVA, with age and sex as covariates, revealed a significant main effect of Group F(2, 118) = 23.79, p = < .001, ηp2 = 27.8%, although not Delay F(1, 118) = .085 p = .772, ηp2 = 1% and no interaction F (2, 118) = .16, p = .849, ηp2 = 3%. Post hoc analyses indicate that TD children recalled more story details than did those with GGE (p < .001) and TLE (p < .001). Children with TLE and GGE performed similarly on the delayed recall task (p = .969).
Long-term memory formation
Two separate two-way (Group × Delay) ANCOVAs were conducted (one for each story) to test between-group differences in the percentage of Story Memory detail recalled at short and long delays (see for descriptive statistics); see for long-term experimental recognition scores). ALF was not found to be related to age of seizure of first seizure, nor seizure frequency ().
Table 2. Scaled scores were obtained on (i) a standardized administration of the story memory subtest of the wide range assessment of memory and learning – second edition; (ii) recognition scores obtained on experimental tests of verbal memory after two weeks.
A two-way repeated measures ANCOVA for the story most recalled () indicated a main effect of Delay (F(1.70, 142.60) = 5.54, p = .007, ηp2= .062), and Group (F(2, 84) = 3.32, p = .041, ηp2 = .073) when adjusting for age, sex, and immediate recall scores. There was no significant interaction (F(3.40, 142.60) = 1.33 p = .267, ηp2 = .031). Multiple comparisons using Tukey’s HSD indicated that TD children recalled significantly more story details overall than did those with GGE (p = .019) and those with TLE (p = .004). There was no significant difference in recall of story details overall between children with epilepsy (p = .872).
Figure 1. Mean percentage recall (relative to the immediate recall; covaried for age) of story details obtained by the TLE, GGE and TD groups of (a) the story most recalled at 30-min, 1-day, and 2-weeks and (b) the story least recalled at 30-min and 2-weeks. Error bars represent standard error.
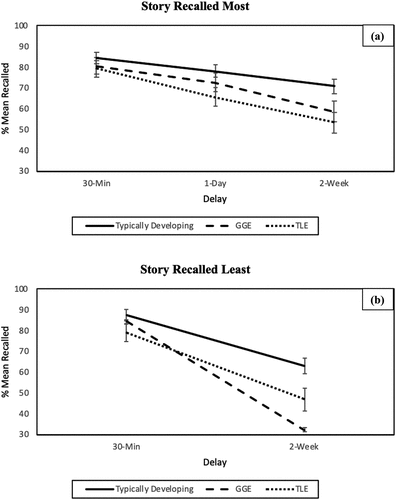
A two-way repeated measures ANCOVA (controlling for age, sex, and immediate recall scores) of the story recalled least () indicated a significant main effect of Group (F(2, 77) = 6.13, p = .003, ηp2 = .139), no main effect of Delay (F(1, 77) = 3.12, p = .081, ηp2 = .039) and a significant Delay by Group interaction (F(2, 77) = 8.25, p = .001, ηp2 = .178). Pairwise comparisons indicated that TD children recalled significantly more story details overall than did those with GGE (p = .001) and those with TLE (p = .018). There was no significant difference in the overall recall of story details between children with epilepsy (p = .338). The interaction was explored using independent samples t-tests, which indicated that TD children recalled significantly more story details at 30 min and 2-week delays relative to children with GGE (p = .02 and p < .001, respectively) and children with TLE (p = .016 and p = .002, respectively). Children with TLE and GGE did not differ in recall of the story, least recalled at either 30 min (p = .919) or 2 weeks (p = .098) delays.
Magnitude of a decline from a short (30-min) to a very long (2 weeks) delay
To compare the magnitudes of a decline (% loss) in recall of the story most recalled and the story least recalled from the short (30-min) to the extended (2-week) delay, we calculated a “difference score” (recall at 30-min – recall at 2-weeks) for each story in each group. Negative values indicate a drop in performance.
ANCOVAs were used to investigate the magnitude of memory drop for both stories. A statistically significant difference was detected between the TLE, GGE, and TD children for the story most recalled (F(2, 69) = .5.50, p = .006), see . Pairwise comparisons indicated that TD children experienced significantly less memory drop (as measured by the Magnitude of Difference score) than did the GGE group (p .037) and TLE group (p .003). The difference between the GGE and TLE group was not statistically significant (p .401); see .
Figure 2. A drop in recall of (a) the story most recalled and (b) the story least recalled from a 30-minute delay to a two-week delay. Higher scores (i.e., closer to zero) indicate better performance. Error bars represent standard error.
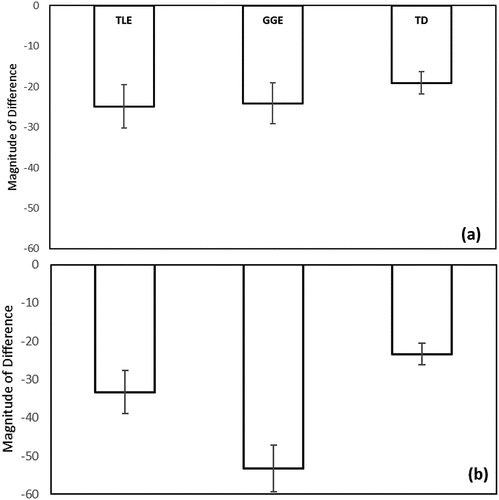
The analysis for the story least recalled revealed a significant difference between TLE, GGE, and TD children, (F(2, 69) = 11.20, p = < .001). Pairwise comparisons indicated that TD children showed less loss in recall than the GGE (p < .001) and TLE groups (p = .033), see . The difference between the GGE and TLE group was borderline significant (p = .056).
Two separate one-way ANOVAs were conducted to assess between-group differences in recognition, one for each story. The ANOVAs revealed significant main effects of Group for the story most recalled (F(2, 117) = 21.73, p < .001, ηp2 = .271) and the story least recalled (F(2, 107) = 43.23, p < .001, ηp2 = .447). Multiple comparisons using Tukey’s HSD indicated that TD children correctly recognized significantly more details from both stories relative to children with epilepsy (GGE: p = <.001; TLE: p = <.001 and GGE: <.001; TLE: p = .023, respectively). Recognition scores of children with GGE and TLE were comparable for the story most recalled (p = .126) although not the story least recalled, where children with TLE recognized more details compared to those with GGE (p = <.001).
Paired samples t-tests were used to examine the magnitude of drop within each group. The TD (t(36) = 2.98, p = .005) and GGE (t(18) = 4.12, p < .001) group (although not TLE; t(17) = 1.87, p = .079) and had a significant difference in performance on the story most recalled when compared to the story least recalled at two weeks.
Executive functions
provides scores obtained by children with TLE, GGE, and TD children on tests of executive function. ANVOCA was used to explore group differences, controlling for age and sex (). Children with TLE performed worse than TD children on the Letter Fluency and Category Fluency subtests of the DKEFS. Children with GGE performed significantly more poorly than TD children and those with TLE on Letter Fluency, Category Fluency, Inhibition, Inhibition/Switching, but not on Category Switching. Finally, the children with GGE performed significantly worse than those in the TD group on the subtests from the AWMA (Digit Recall, Backwards Digit Recall, Odd-one-out), and Creature Counting from the TEA-Ch (on timeliness, although not accuracy).
Table 3. Scaled scores obtained on tests of executive functions and verbal memory: between-group comparison using ANCOVA controlling for age and sex.
Executive function subtests were correlated with long-term memory scores to explore the relationship between executive skills and ALF. In TD children, there were no significant correlations between scores on measures of executive functions and recall of either story at two weeks. In children with TLE, the story most recalled was correlated with the Category Fluency subtest of the DKEFS (r = .42, p = .025). The story least recalled was correlated with the Letter Fluency (r = .46, p = .018) and Category Fluency (r = .41, p = .035) in children with TLE. In children with GGE, there were no significant correlations between executive function and the story most recalled at 2 weeks. However, the story least recalled correlated with Creature Counting – timing score (r = .61, p = .005), Digit Recall (r = .50, p = .027), Digits Backwards (r = .57, p = .007), and Letter Fluency (r = .45, p = .027).
Discussion
The current study aimed to investigate (i) the presence of ALF for story memory in children with GGE and TLE, (ii) the impact of repeat testing over long delays on memory formation, and (iii) and to what extent ALF relates to executive function. It was hypothesized that both children with TLE and GGE would evidence accelerated rates of forgetting relative to TD children. We further hypothesized that TLE and GGE groups would manifest differential patterns of decline across the two-week period. Moreover, we anticipated that having an additional opportunity to recall materials 24-h post learning would have an enhancive effect on performance at a 2-week delay, thus offsetting ALF. Finally, it was expected that reduced performance on tasks of executive functioning would be associated with worse recall ability over long delays (i.e., ALF).
ALF for story memory in children with epilepsy
This study found that children with GGE experienced an accelerated and marked rate of memory deterioration relative to both TD children and those with TLE on a free recall story memory task. While children with GGE had greater difficulty with this task at a two-week delay than children with TLE and those that were TD, they conversely appeared to experience the most benefit from repeat testing. That is, an active, spaced recall 24 h post-learning enhanced recall for children with GGE. The finding that children with GGE fare worse on the long-term memory task than those with TLE is surprising, as children with GGE (who – as opposed to children with TLE – have intact hippocampal structures) are not traditionally expected to have clinical memory problems. Children with GGE were shown to perform on par with children with TLE on short delays. Thus, the finding that they may experience a more rapid and impactful rate of forgetting than children with TLE has implications for both (i) the theoretical underpinning of our understanding of memory consolidation and (ii) the clinical management of patients with GGE. These findings are largely consistent with Classic Consolidation Theory (CCT) (Alvarez & Squire, Citation1994; Squire et al., Citation1984), which suggests that a slowly changing neocortical network comes to function as a permanent repository of long-term memory over days, weeks, months, or years after acquisition (Alvarez & Squire, Citation1994). Accordingly, individuals with GGE who experience seizures and epileptiform discharges may be at risk of functional disruption during the consolidation phase, leading to insidious and clinically undetected long-term memory failure. It is possible that children with GGE have a wider network disruption. In addition, in children with GGE epilepsy is genetically related. More recently, researchers have looked at underlying genetic processes, such as seizure-related consolidation. These theories suggest that epileptogenesis may, in fact, act to “hijack” and disturb biological memory processes (Das & Luczak, Citation2022).
Retrieval and space-based practice
It was found that children with GGE – who experienced the most pronounced ALF in the current study – also experienced the most benefit from an additional opportunity to recall materials. When compared, GGE children performed on par with TD children and children with TLE at a two-week delay when given an opportunity to recall materials one-day post learning. This finding is consistent with research examining the effect of both retrieval-based practice (i.e., learning through testing) and spaced practice (i.e., learning distributed across multiple sessions) on enhancing memory retention (Greene, Citation2008; McCabe et al., Citation2010; Roediger & Karpicke, Citation2006). The effect on memory appears enduring and impactful, irrespective of the spacing schedule (Karpicke & Bauernschmidt, Citation2011). Retrieval and space-based practice certainly have been applied in both clinical and educational settings with demonstrated efficacy (Gordon, Citation2020).
There is a myriad of evidence demonstrating that additional rehearsals facilitate long-term memory formation in healthy participants (Bahrick, Citation1979; Dunlosky et al., Citation2013; Roediger & Karpicke, Citation2006) as well as in neurodiverse populations (Brooks et al., Citation2006; Creighton et al., Citation2013; Gordon, Citation2020). This research shows that a simple and practical strategy, such as recalling content one-day post learning, may protect against subsequent memory deterioration. Such strategies have also been shown to benefit adult patients demonstrating ALF (Jansari et al., Citation2010; Ricci et al., Citation2019). Whether retrieval and space-based practice confer enduring benefit over longer delays will be important for future research to examine in more depth, alongside the optimal timeframe and frequency these strategies should be implemented.
The role of executive functioning in memory formation and consolidation
Finding a relationship between executive control and ALF was expected, and consistent with the reconceptualization of epilepsy as a network disorder. Even in focal epilepsies, seizure activity can migrate to the widely distributed cortical or subcortical sites (Wilson & Baxendale, Citation2014). As predicted, executive skills were more impaired in children with epilepsy. We found that children with GGE experienced more executive difficulties than children with TLE. Both children with GGE and TLE had executive deficits in verbal generativity, which was predictive of ALF for the story, which did not offer an additional rehearsal (i.e., the story least recalled). This finding suggests that children with epilepsy may have compromised phonological loop and central executive functioning relative to their TD peers. Thus, executive components of memory may, in fact, be more critical than appreciated for functional memory formation and retrieval. It is difficult to explain the difference in findings more comprehensively as research investigating the role of executive skills in long-term memory recall remains in it’s infancy.
When impaired, long-term memory recall may be heavily impacted, as seen in the current study in children with GGE. These results were consistent with the findings of Sepeta et al. (Citation2017), Joplin et al. (Citation2020), and Joplin et al. (Citation2022). While strategy and organization are likely less critical in the recall of stories (relative to structured lists, as used in other studies of ALF), participants with a compromised verbal short-term memory repository (i.e., phonological loop) may fail to encode content when their short-term memory becomes saturated. Given the ecological relevance and importance of one’s ability to remember everyday information (i.e., for which they are intrinsically afforded only a single exposure), this finding carries significant clinical and educational implications. If children with GGE experience an impoverished ability to recall stories, this impairment may be generalizable to difficulties with autobiographical memory, everyday memory, somatic memory, and source memory.
The findings have been reported and interpreted with consideration of some methodological limitations. Some exploratory analyses were likely underpowered for meaningful investigation of within-group differences, such as a comparison of performance on the two stories within each clinical group. Further, many of the correlational analyses were somewhat liberal and exploratory, and thus we cannot exclude the likely inflation of pairwise-error probability. Our findings may not be generalizable to all children with GGE, as the condition encompasses a wide range of epilepsy syndromes. Furthermore, the current study was conducted using children from specialized epilepsy services at four tertiary-referral children’s hospitals, rather than from the general community. Moreover, did not collect data on the number of seizures that occurred between delays. Nevertheless, seizures may be subclinical and the number of observed seizures may not be representative of the epileptiform activity. Hence, we could not exclude the possibility that subclinical epileptiform activity potentially contributed to ALF.
The findings reported herein suggest that children with GGE are more prone to LTM failure than both their typically developing peers and those with TLE. This leads to the assumption that coadjutant factors are preventing the identification of memory failure in children with GGE, as (i) they are not expected to have memory failure by virtue of their syndrome type (which typically is considered to be more benign), and (ii) should LTM failure be suspected, the trajectory of LTM decay in children with GGE is insidious in its onset, and thus evades detection in a standardized clinical setting.
The adoption of effective, evidence-based restitutional memory strategies is of critical importance for children with epilepsy. These provide a better opportunity for optimizing academic achievement, learning, and typical social development. Strategies that are simple and practical to implement in the child’s clinical, home, and school setting are likely to be adopted more readily and therefore produce the best outcomes. The evidence supporting retrieval and space-based practice for memory enhancement is robust, and this research offers preliminary support for the possible benefit for some children with epilepsy. It was also found that failure was associated with greater difficulties of executive dysfunction (i.e., short-term/working memory and verbal generativity). This suggests that ALF, therefore, may in part be secondary to deficits in the central executive and phonological loop. This warrants further investigation, and future studies may explore the benefit of both retrieval and space-based practice and executive skill training both independently and adjunctly as clinical interventions targeting ALF in children with epilepsy. Clinically, it is essential to include tests of executive dysfunction in neuropsychological assessments of children with epilepsy. Failing to detect and rehabilitate memory and executive problems early in life is likely to have a long-lasting and far-reaching impact on a child’s development.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alloway, T. P. (2007). Automated working: Memory assessment: Manual. Pearson.
- Alvarez, P., & Squire, L. R. (1994). Memory consolidation and the medial temporal lobe: A simple network model. Proceedings of the National Academy of Sciences of the United States of America, 91(15), 7041–7045. https://doi.org/10.1073/pnas.91.15.7041
- Audrain, S., & McAndrews, M. P. (2019). Cognitive and functional correlates of accelerated long-term forgetting in temporal lobe epilepsy. Cortex, 110, 101–114. https://doi.org/10.1016/j.cortex.2018.03.022
- Baddeley, A. (1992). Working memory. Science, 255(5044), 556–559. https://doi.org/10.1126/science.1736359
- Bahrick, H. P. (1979). Maintenance of knowledge: Questions about memory we forgot to ask. Journal of Experimental Psychology: General, 108(3), 296–308. https://doi.org/10.1037/0096-3445.108.3.296
- Blake RV, Wroe SJ, Breen EK, McCarthy RA. (2000). Accelerated forgetting in patients with epilepsy: evidence for an impairment in memory consolidation. Brain, 123(Pt 3), 472–483.
- Brooks, B. L., Weaver, L. E., & Scialfa, C. T. (2006). Does impaired executive functioning differentially impact verbal memory measures in older adults with suspected dementia? Clinical Neuropsychology, 20(2), 230–242. https://doi.org/10.1080/13854040590947461
- Chan, C. J., Zou, G., Wiebe, S., & Speechley, K. N. (2015). Global assessment of the severity of epilepsy (GASE) scale in children: Validity, reliability, responsiveness. Epilepsia, 56(12), 1950–1956. https://doi.org/10.1111/epi.13216
- Chowdhury, F. A., Elwes, R. D., Koutroumanidis, M., Morris, R. G., Nashef, L., & Richardson, M. P. (2014). Impaired cognitive function in idiopathic generalized epilepsy and unaffected family members: An epilepsy endophenotype. Epilepsia, 55(6), 835–840.
- Creighton, A. S., van der Ploeg, E. S., & O’Connor, D. W. (2013). A literature review of spaced-retrieval interventions: A direct memory intervention for people with dementia. International Psychogeriatrics, 25(11), 1743–1763. https://doi.org/10.1017/S1041610213001233
- Culhane-Shelburne, K., Chapieski, L., Hiscock, M., & Glaze, D. (2002). Executive functions in children with frontal and temporal lobe epilepsy. Journal of the International Neuropsychological Society, 8(5), 623–632. https://doi.org/10.1017/S1355617702801308
- Das, R., & Luczak, A. (2022). Epileptic seizures and link to memory processes. AIMS Neuroscience, 9(1), 114–127. https://doi.org/10.3934/Neuroscience.2022007
- Davidson, M., Dorris, L., O’Regan, M., & Zuberi, S. M. (2007). Memory consolidation and accelerated forgetting in children with idiopathic generalized epilepsy. Epilepsy & Behavior, 11(3), 394–400. https://doi.org/10.1016/j.yebeh.2007.05.004
- Delis, D., Kramer, J., Kaplan, E., & Ober, B. (1994). California verbal learning test - Children's edition. The Psychological Corporation.
- De Renzi, E., & Lucchelli, F. (1993). Dense retrograde amnesia, intact learning capability and abnormal forgetting rate: A consolidation deficit? Cortex, 29(3), 449–466. https://doi.org/10.1016/S0010-9452(13)80253-5
- Dunlosky, J., Rawson, K. A., Marsh, E. J., Nathan, M. J., & Willingham, D. T. (2013). Improving students’ learning with effective learning techniques: Promising directions from cognitive and educational psychology. Psychological Science in the Public Interest, 14(1), 4–58. https://doi.org/10.1177/1529100612453266
- Gascoigne, M. B., Barton, B., Webster, R., Gill, D., Antony, J., & Lah, S. S. (2012). Accelerated long-term forgetting in children with idiopathic generalized epilepsy. Epilepsia, 53(12), 2135–2140. https://doi.org/10.1111/j.1528-1167.2012.03719.x
- Gascoigne, M. B., Smith, M. L., Barton, B., Webster, R., Gill, D., & Lah, S. (2014). Accelerated long-term forgetting in children with temporal lobe epilepsy. Neuropsychologia, 59, 93–102. https://doi.org/10.1016/j.neuropsychologia.2014.04.012
- Gelžinienė, G., Jurkevičienė, G., Marmiene, V., & Adomaitiene, V. (2011). Specific features of memory and executive functions in patients with juvenile myoclonic epilepsy. Epilepsy & Behavior, 20(3), 512–517.
- Gordon, K. R. (2020). The advantages of retrieval-based and spaced practice: Implications for word learning in clinical and educational contexts. Language, Speech, and Hearing Services in School, 51(4), 955–965. https://doi.org/10.1044/2020_LSHSS-19i-00001
- Grayson-Collins, J., Gascoigne, M. B., Barton, B., Webster, R., Gill, D., & Lah, S. (2019). Longitudinal study of accelerated long-term forgetting in children with genetic generalized epilepsy: Evidence of ongoing deficits. Cortex, 110, 5–15. https://doi.org/10.1016/j.cortex.2017.08.028
- Greene, R. L. (2008). 2.06 - Repetition and spacing effects. In J. H. Byrne (Ed.), Learning and memory: A comprehensive reference (pp. 65–78). Academic Press.
- Hernandez, M. T., Sauerwein, H. C., Jambaqué, I., De Guise, E., Lussier, F., Lortie, A., Dulac, O., & Lassonde, M. (2002). Deficits in executive functions and motor coordination in children with frontal lobe epilepsy. Neuropsychologia, 40(4), 384–400. https://doi.org/10.1016/S0028-3932(01)00130-0
- Hessen, E., Alfstad, K., Torgersen, H., & Lossius, M. I. (2018). Tested and reported executive problems in children and youth epilepsy. Brain and Behavior, 8(5), e00971. https://doi.org/10.1002/brb3.971
- Jansari, A. S., Davis, K., McGibbon, T., Firminger, S., & Kapur, N. (2010). When “long-term memory” no longer means “forever”: Analysis of accelerated long-term forgetting in a patient with temporal lobe epilepsy. Neuropsychologia, 48(6), 1707–1715. https://doi.org/10.1016/j.neuropsychologia.2010.02.018
- Joplin, S., Gascoigne, M., Barton, B., Webster, R., Gill, D., Lawson, J. A., Mandalis, A., Sabaz, M., McLean, S., Gonzalez, L., Smith, M.-L., & Lah, S. (2022). Accelerated long-term forgetting in children with temporal lobe epilepsy: A timescale investigation of material specificity and executive skills. Epilepsy & Behavior, 129, 108623. https://doi.org/10.1016/j.yebeh.2022.108623
- Joplin, S., Gascoigne, M., Webster, R., Gill, D., Lawson, J., Barton, B., Mandalis, A., Sabaz, M., & Lah, S. (2020). Accelerated long-term forgetting in children with genetic generalised epilepsy: The temporal trajectory and contribution of executive skills. Epilepsy & Behavior, 113C, 107444. https://doi.org/10.1016/j.yebeh.2020.107471
- Joplin, S., Stewart, E., Gascoigne, M., & Lah, S. (2018). Memory rehabilitation in patients with epilepsy: A systematic review. Neuropsychology Review, 28(1), 88–110. https://doi.org/10.1007/s11065-018-9367-7
- Karpicke, J. D., & Bauernschmidt, A. (2011). Spaced retrieval: Absolute spacing enhances learning regardless of relative spacing. Journal of Experimental Psychology: Learning, Memory, and Cognition, 37(5), 1250–1257. https://doi.org/10.1037/a0023436
- Kerr, E. N., & Blackwell, M. C. (2015). Near-transfer effects following working memory intervention (Cogmed) in children with symptomatic epilepsy: An open randomized clinical trial. Epilepsia, 56(11), 1784–1792. https://doi.org/10.1111/epi.13195
- Lah, S., Mohamed, A., Thayer, Z., Miller, L., & Diamond, K. (2014). Accelerated long-term forgetting of verbal information in unilateral temporal lobe epilepsy: Is it related to structural hippocampal abnormalities and/or incomplete learning? Journal of Clinical and Experimental Neuropsychology, 36(2), 158–169. https://doi.org/10.1080/13803395.2013.874405
- Loughman, A., Bowden, S. C., & D'Souza, W. (2014). Cognitive functioning in idiopathic generalised epilepsies: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews, 43, 20–34.
- MacAllister, W. S., Vasserman, M., Rosenthal, J., & Sherman, E. (2014). Attention and executive functions in children with epilepsy: What, why, and what to do. Applied Neuropsychology: Child, 3(3), 215–225. https://doi.org/10.1080/21622965.2013.839605
- Mameniskiene, R., Jatuzis, D., Kaubrys, G., & Budrys, V. (2006). The decay of memory between delayed and long-term recall in patients with temporal lobe epilepsy. Epilepsy & Behavior: E&B, 8(1), 278–288. https://doi.org/10.1016/j.yebeh.2005.11.003
- Manly, T., Robertson, I. H., Anderson, V., & Nimmo-Smith, I. (1999). TEA-Ch: The Test of everyday attention for children. Thames Valley Test Company.
- Mayr, U., Kuhns, D., & Hubbard, J. (2014). Long-term memory and the control of attentional control. Cognitive Psychology, 72, 1–26. https://doi.org/10.1016/j.cogpsych.2014.02.001
- McCabe, D. P., Roediger, H. L., McDaniel, M. A., Balota, D. A., & Hambrick, D. Z. (2010). The relationship between working memory capacity and executive functioning: Evidence for a common executive attention construct. Neuropsychology, 24(2), 222–243. https://doi.org/10.1037/a0017619
- Michaelis, R., Tang, V., Goldstein, L. H., Reuber, M., LaFrance, W. C., Jr., Lundgren, T., Modi, A. C., & Wagner, J. L. (2018). Psychological treatments for adults and children with epilepsy: Evidence-based recommendations by the international league against epilepsy psychology task force. Epilepsia, 59(7), 1282–1302. https://doi.org/10.1111/epi.14444
- Narayanan, J., Duncan, R., Greene, J., Leach, J. P., Razvi, S., McLean, J., & Evans, J. J. (2012). Accelerated long-term forgetting in temporal lobe epilepsy: Verbal, nonverbal and autobiographical memory. Epilepsy & Behavior: E&B, 25(4), 622–630. https://doi.org/10.1016/j.yebeh.2012.06.038
- Parsons, N., Bowden, S. C., Vogrin, S., & D’Souza, W. J. (2020). Default mode network dysfunction in idiopathic generalised epilepsy. Epilepsy Research, 159, 106254. https://doi.org/10.1016/j.eplepsyres.2019.106254
- Ratcliffe, C., Wandschneider, B., Baxendale, S., Thompson, P., Koepp, M. J., & Caciagli, L. (2020). Cognitive Function in Genetic Generalized Epilepsies: Insights from Neuropsychology and Neuroimaging. Frontiers in neurology, 11, 144. https://doi.org/10.3389/fneur.2020.00144
- Ricci, M., Wong, T., Nikpour, A., & Miller, L. A. (2019). Testing the effectiveness of cognitive interventions in alleviating accelerated long term forgetting (ALF). Cortex, 110, 37–46. https://doi.org/10.1016/j.cortex.2017.10.007
- Roediger, H. L. 3rd, & Butler, A. C. (2011). The critical role of retrieval practice in long-term retention. Trends in Cognitive Sciences, 15, 20–27.
- Roediger, H. L., & Karpicke, J. D. (2006). Test-enhanced learning: Taking memory tests improves long-term retention. Psychological Science, 17(3), 249–255. https://doi.org/10.1111/j.1467-9280.2006.01693.x
- Rzezak, P., Guimarães, C. A., Fuentes, D., Guerreiro, M. M., & Valente, K. D. (2012). Memory in children with temporal lobe epilepsy is at least partially explained by executive dysfunction. Epilepsy & Behavior, 25(4), 577–584.
- Sepeta, L. N., Casaletto, K. B., Terwilliger, V., Facella-Ervolini, J., Sady, M., Mayo, J., Gaillard, W. D., & Berl, M. M. (2017). The role of executive functioning in memory performance in pediatric focal epilepsy. Epilepsia, 58(2), 300–310. https://doi.org/10.1111/epi.13637
- Sheslow, D., & Adams, W. (2003). Wide range assessment of memory and learning (WRAML). NCS Pearson.
- Skodzik, T., Holling, H., & Pedersen, A. (2017). Long-term memory performance in adult ADHD. Journal of Attention Disorders, 21(4), 267–283. https://doi.org/10.1177/1087054713510561
- Socio-Economic Indexes for Areas. (2011). Australian Bureau of Statistics. https://www.abs.gov.au/ausstats/[email protected]/DetailsPage/2033.0.55.0012011
- Squire, L., Cohen, N., & Nadel, L. (1984). The medial temporal region and memory consolidation: A new hypothesis. In H. Weingartner & E. Parker (Eds.), Memory consolidation: psychobiology of cognition (pp. 185–210). Lawrence Elbaum.
- Wechsler, D. (2011). Wechsler abbreviated scale of Intelligence, Second Edition (WASI-II). Harcourt Assessment.
- Wilson, S. J., & Baxendale, S. (2014). The new approach to classification: Rethinking cognition and behavior in epilepsy. Epilepsy & Behavior, 41, 307–310. https://doi.org/10.1016/j.yebeh.2014.09.011
Appendix
Table A1. Scores obtained on experimental tests of memory.
Table A2. Person product movement correlations by group between seizure variables and long-term verbal recall of both stories (most recalled and least recalled).
Table A3. Post Hoc comparison of executive function tests.
