?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Considering the deficiency of time elapsed for phase-stepping interferometric techniques and the need of developing non-contact and on-line measurement with high accuracy, a single-shot phase-shifting triple-interferometer (PSTI) is developed for analysis of characteristics of transparent structures and optical path difference (OPD) measurements. In the proposed PSTI, coupled three interferometers which generate four interference patterns, and a polarizer array is used as phase shifters to produce four spatially separated interferograms with π/2-phase shifts, which are recorded in a single capture by a camera. The configuration of the PSTI allows dynamic measurements (4D measurements) and does not require vibration isolation. We have applied the developed system to examine the size and OPD of cells, and the slope of thin films
Introduction
The development of phase-shifting interferometry began with the techniques implemented by Carre and Crane in the decade of the 60s (Citation1, 2). This technique in interferometry was later applied in surface reconstruction, measurement of wavefront aberrations, microscopy, fluid mechanics and heat transfer by researchers, such as Bruning, Wyant, Moore, Hardy and Creath, among others (Citation3–6). In this technique, the interferograms are recorded with a CCD camera, measuring the intensity variation of sequential phase shifts, through which it can determine the phase of the object under test for it is necessary to calibrate the system parameters to generate interferograms with relative phase shifts with the same intensity and fringe modulation (Citation7–9). Conventionally, phase-shifting interferometry is very sensitive to vibrations because image acquisition in various phase-shifting sequences could easily introduce measurement errors from environmental influences (Citation10, 11). These techniques allow static measurements of the optical phase; however, it is also necessary to develop techniques that allow performing dynamic measurements of the optical phase. With the knowledge of the optical phase, characteristic parameters of the sample under study can be calculated, allowing non-contact analysis of such structures, because there is a great variety of phase objects, such as acetates, glass, transparent fluids, microorganisms, cells and tissue among others, and additionally the interferometric techniques are allowing full-field images of interest in industry, the biomedical sector and metrology.
A variety of techniques are currently being developed to obtain several phase shifts in a single capture of the camera, allowing dynamic measurements of objects under study. Some of these techniques use parallel interferograms (Citation12), holographic ones (Citation13, 14) or pixelated phase masks attached to a CCD camera (Citation15, 16), among others, to generate from 2–to 4 to 9 interferograms simultaneously (Citation17–22). Nevertheless, some of the components utilized in these arrangements are still expensive and their implementation requires cameras calibration and development of algorithms for vibration suppression. In this research, we introduce a novel simultaneous phase-shifting interferometer which employs three coupled interferometers: a polarizing cyclic path interferometer (PCPI) that generates the interferogram, and two Michelson systems that replicate patterns to generate four simultaneous phase-shifted interferograms. The system developed is free of diffractive or mechanical elements, which is free of errors generated by piezoelectric translators, and since to the system that generates the primary interferogram is a PCPI; the PSTI is immune to environmental vibrations. The phase reconstruction is performed by using a phase-shifting algorithm which uses a four-step phase-shifting method with phase differences of π/2 (Citation23).
For this study, a microscope system has been added at the entrance of the PSTI, with a microscope objective (MO) with magnification of 60X, for the purpose of being able to analyze biological samples, such as red blood cells and the legs of a pseudoscorpion (Citation24–26) which are analyzed using the interferometer in non-shearing mode (Citation27–30). The microscope objective has a numerical aperture of 0.85, the working distance is 0.3 mm (wavelength range 400–700 nm), the effective focal length is 2.9 and the clear aperture is 4.5 mm. The objective is optically corrected for a conjugate distance of 160 mm (the lenses are antireflection coated for the visible spectrum).
The synthetic samples, such as particles and thin films deposited on a microscopic slide, were analyzed calculating its slope in the direction of the shear introduced by the PSTI. The experimental results show that the method is capable of performing 4D interferometric measurements and of minimizing influences from environmental disturbances.
Polarizing phase-shifting interferometry
The optical arrangement is shown in Figure . We used a diode pumped solid-state (DPSS) Laser with 400 mW in power (λ = 532 nm), and operating temperature from 10 to 35 °C. The laser beam is filtered, and the collimating lens, L0, generates a planar wavefront, which impinging on the microscope module, where the sample will be positioned. A polarizer filter, P0, is placed at an angle of 45° (see Figure ), so the light coming out after the polarizing beam splitter, PBS, will have a perpendicular and a parallel component of the same intensity. Once the light has gone through the PCPI and the quarter wave plate (Q), it will have crossed circular polarization states. At the output of the PCPI, the beams A and B are obtained with a lateral shift of x0. If these two beams superimpose, due to their mutual orthogonal polarization states, at this stage S-1 no interferogram can be detected. However, an interference pattern is observed when placing an auxiliary linear polarizer (see Stage-1 of the Video 1).
Figure 1. Polarization phase-shifting triple-interferometer. Li: Lens. MO: Microscope objective. BS: Beam splitter. PBS: Polarizing Beam splitter. Pi: Lineal polarizer filters. Mi: Mirrors. Beam cross section a = 2 mm. x0 = 2.5 mm. L1 = 100 mm (Video 1).
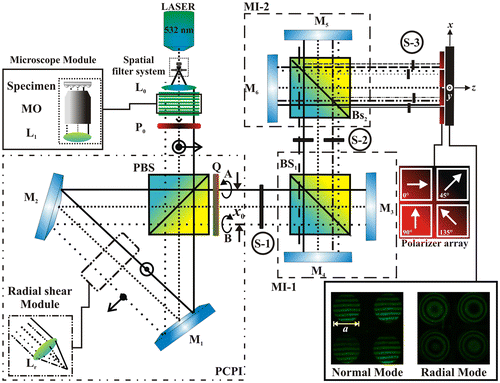
Coupled systems for generated four interferograms
The interferogram with crossed circular polarization (S-1) go into the first Michelson interferometer (MI-1), which multiplexes the pattern, generating two interferograms in S-2, (see Stage-2 of the Video 1).
The second MI-2 duplicates each of the two patterns emerging from MI-1, to generate a total of four interferograms in S-3. Phase shifts can be introduced into each one of the fringe patterns when a linear polarizer is placed at different angles on each pattern. (see Stage-3 of the Video 1) (Citation18–20). The function of coupled Michelson interferometers, is to replicate the incident interferogram, similar to the effect generated by a diffraction grating, but without disadvantages of the modulation of the fringes. The proposed system can operate in normal mode and radial mode, and generate radial shear interferograms that can be useful for analyzing samples with different symmetries.
In summary, the replicas are obtained with the two coupled Michelson configurations, which are also used to position the four interferograms in the image plane. The phase shift is done by the mask of polarizers placed at the output of the system in the image plane (Video 1)
Phase calculation method
A common algorithm used to calculate the phase is the four-step method (Citation23). We know that although only three interferograms are required to solve for the three variables of the phase-shifting method, experimentally, more than three interferograms are often digitized for ease of computation, noise suppression and reduction in sensitivity to phase stepper error. In the four-step technique, the nominal phase step is π/2, and the phase takes values of 0, π/2, π and 3π/2. Considering that four interferograms are acquired in a single shot and are recorded in the same conditions. Using these values, the intensities at each point in images 1–4 are:(1)
(1)
and the phase at each point is:(2)
(2)
As expected, due to the averaging over more images in the four-step technique, it has a lower error than the three position technique. The evaluated phase is wrapped between −π and π due to the arctangent function. In order to remove the background phase Δϕ(x, y)ref, the phase retrieval procedure should include a reference phase in step where the background is measured beforehand (Citation9, 10); in other words, the background phase is calculated without the target object. Thus, the final phase will be:(3)
(3)
where Δϕ(x, y)ref is the phase without the test object and Δϕ(x, y)obj is the phase calculated with the test objects.
Phase unwrapping
Let us consider Δϕw as the wrapped phase obtained from the n-step phase-shifting algorithm. Since the images are obtained from a simultaneous phase-shifting interferometer, we implement the iterative unwrapping method proposed by Kerr et al. (Citation31, 32) to obtain the unwrapped phase Δϕu. In order to improve the results, we added a mask that filters the excess of information corresponding to the background and carrier signal. The mask is created from the mean intensity Im of the patterns(4)
(4)
where n is the number of steps made by our system, Ii is the intensity of each i-th pattern. This image allows us to detect the region of interest (ROI) where the sample is located. For each ROI, a 2D Gaussian function is applied in order to avoid harmonics and unwrapping errors. The applied filter is:(5)
(5)
where ϕf is the filtered phase, m is the mask function and H is the 2D Gaussian function, this filter removes sharp edges and details.
Results and discussion
To show the feasibility of the proposed set-up and to verify the applications, biological and synthetic samples have been tested in the normal and radial mode of operation of the interferometer. The four interferograms were acquired by a 3.0-megapixel CMOS sensor (colour camera) of 2048 × 1536 pixels (pixel size, 3.2 × 3.2 μm). The microscope objective used had a magnification M = 60X and a numerical aperture NA = 0.85. The CMOS camera was adjusted to capture the images of four interference patterns simultaneously. We can capture several images over the same detector field, since we have low frequency interferograms with respect to the inverse of the pixel spacing, the influence of errors in the capture seems to be rather small if noticeable (for this case, the spatial resolution of each interferogram is 580 × 380). To process the phase, it is first necessary to align the interferograms on the screen, this is achieved by moving the mirrors M3–M6, to adjust the separation and position in the x–y axes, subsequently, the four interferograms are separated digitally, using the method described in Ref. (Citation20), which consists of using an iris diaphragm to locate a common zone of the interference replicas used as reference points; this procedure locates each centroid of the points captured by the camera that belong to the shifted interference patterns used. After locating each reference point, a geometric mask is selected in the algorithm, taking into account the first centroid, to obtain the common interference pattern region. Through this procedure, our algorithm is capable of selecting the four interference pattern locations, and using digital image processing we obtain the wrapped phase data.
In this case, the phase difference provides the partial derivative of the out-of-plane displacement: the slope in the shear’s direction (Citation18). If we want to obtain the phase, we have to integrate the slope in both directions; nevertheless, in this stage of the work we present the measurement of the slope since it gives us the information of the variations of the object (Citation18).
Analysis of the temporary stability of the system
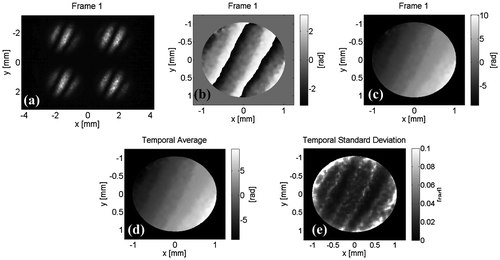
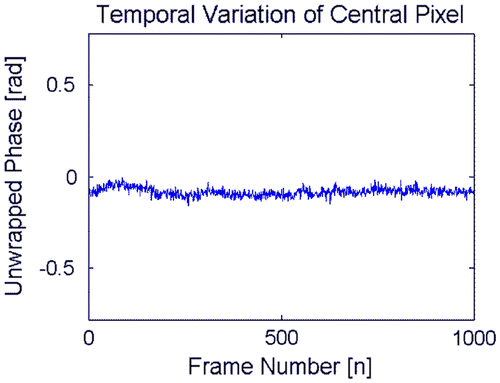
We analyzed the stability of the system by capturing 1000 frames (10 fps), and show its variation in each pixel (see Figures and ). Figure (a) shows the four interferograms (for one representative frame), the wrapped phase (Figure (b)) and its corresponding unwrapped phase in Figure (c). Figure (d) explaining the average and Figure (e) the standard deviation of the variation by each pixel from all the 1000 frames. The Figure show the temporal variation of the unwrapped phase selecting the central pixel of Figure , the average obtained correspond to −.084 rad with a standard deviation of 0.022 rad; this result showing that the set-up is insensitive to external vibrations and hence a high-quality vibration isolation table is not necessary to conduct the experiment.
Biological structures
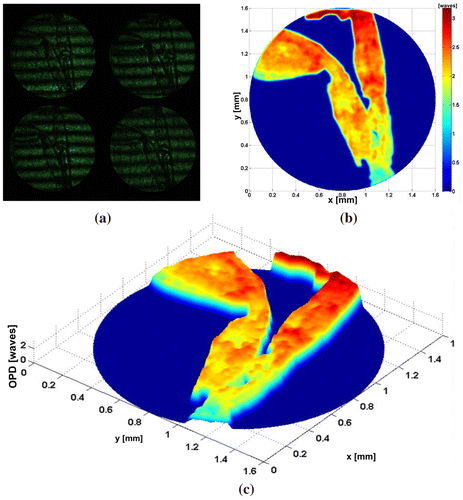
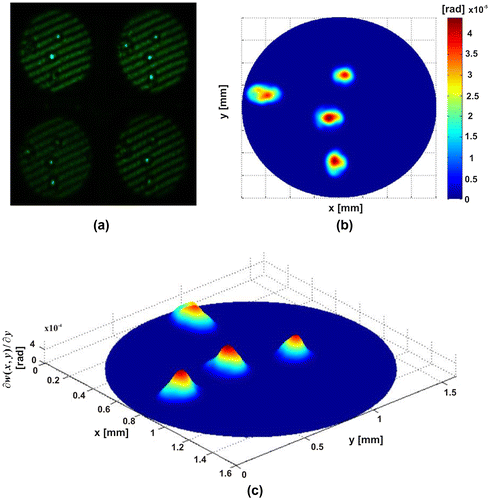
Figure shows experimental results for one of the pseudoscorpion legs (pseudoscorpionida order). This was fixed on a slide (Citation33), which was a few millimetres long. These tiny arachnids have long chelicerae, as scorpions, but the segmented abdomen is short and they lack a terminal sting. The interesting part to study corresponds to the legs of the ventral region, since this is one way of partially identifying the order of the species (Citation34). Figure (a) shows the four interferograms obtained in one shot. Figures (b) and (c) show the Optical Path Difference (OPD) of the sample. Figure shows the (OPD) induced by a group of Red Blood Cells (RBC), which were deposited by ‘smear’ on a microscope slide. Their morphology is obtained as a very important parameter in the biomedical field to diagnose diseases.
Figure 5. Human red blood cells (a) π/2-shifted interferograms captured in a single shot. (b) OPD of sample of RBC. (c) OPD of single RBC. (d) Transversal section of RBC.
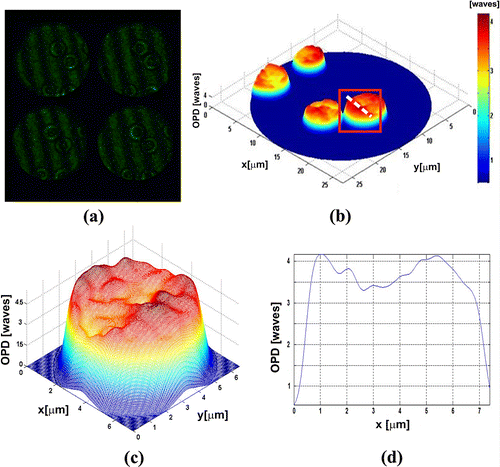
Figure (a) shows the four interferograms captured in one shot of the camera with the proposed system. Figure (b) presents the OPD for all RBC under study. Figure (c) shows the OPD obtained for the cell enclosed in a rectangle shown in Figure (b). Figure (d) shows the cross section of the RBC; it can be noticed that the OPD, and it allows us to calculate the mean thickness, which is calculated as OPD/Δn = 2.8 μm, where Δn mean value of the RBC refraction index, was, assumed 1.395 for the RBC (Citation35–37). In order to show the advantages of the proposed system, we present the experimental results obtained with dynamic biological structures, see Figure . The Video 2 has shown a group of RBC which moves by gravity. Also, a white blood cell is observed crossing the field of the camera. This result shows that optical systems can differentiate the cells beside of their characteristics, in both cases we utilized a CSI operating in the non-shearing mode, The non-shearing mode is refereeing on the input beams generated with a displacement x0 larger than beam size a (). This by moving mirrors M1 and M2, enabling one to change the spacing x0 between the beam centres (Citation18–21), and then generate the interference patterns by moving the mirrors M3–M6 (Citation29, 30). Experimental results referring to Figures , and were obtained by a non-shearing mode and Figures and in a shearing mode implementation.
Synthetic structures
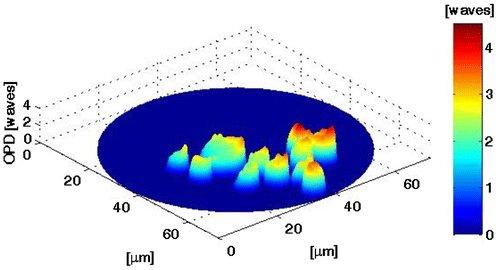
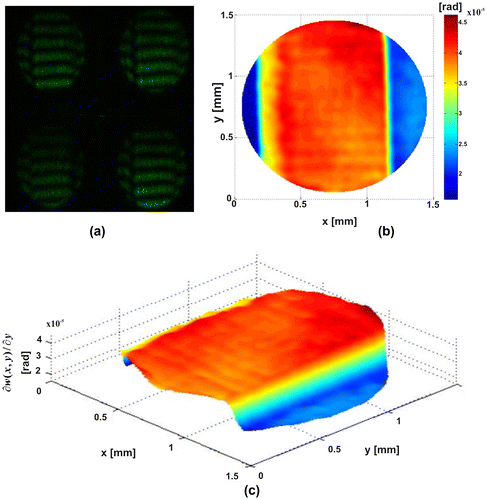
In the results shown in Figures –, it shows that the phase difference provides the partial derivative of the out-plane displacement for this case, the slope in the shear direction. Figure shows the results obtained for plastic microparticles deposited on a microscopic slide. Figure (a) presents the simultaneous interferograms generated by PSTI. Figures (b) and (c) show the slope, the average diameter is 0.2 mm. In Figure , we show a thin film generated with a layer of immersion oil, which was deposited by gravity onto a microscopic slide. Figure (a) presents the simultaneous interferograms generated by PSTI. Figures (b) and (c) show the slope. In both cases, the phase object introduces a change in the reference surface, these variations are observed in the slope associated with the changes of the phase.
Radial Mode
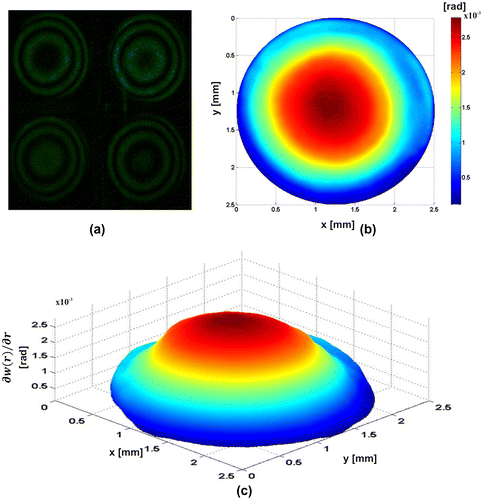
The system can be modified to become a radial shear interferometer when a lens is placed on the trajectory of PCPI. The result corresponds to obtaining two images with a slightly different magnification which will be focused at the camera plane.
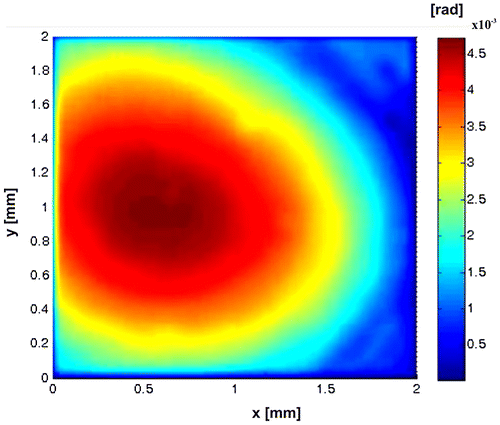
Figure shows the characteristic patterns of these symmetries. Figure (a) shows the four radial-shearograms obtained in one shot; the fringes obtained are contours of r[∂r(x,y)/∂x]. Figures (b) and (c) show the radial slope. In this configuration, the system can be used to test lenses and spherical surfaces (Citation38–47). In Figure , representative frame of the temporal variation of the slope calculated for this case is displayed, in the Video 3, can observe the phase variations induced by the evaporation of alcohol.
Final remarks
Conventional phase-shifting interferometry is very sensitive to vibrations because image acquisition in several phase-shifting sequences could easily introduce measurement errors from environmental influences. We introduce a new simultaneous phase-shifting interferometer which employs a polarizing phase-shifting triple interferometer to generate four simultaneous phase-shifted interferograms in one capture of the CMOS camera. Phase reconstruction is performed using a developed phase-shifting algorithm which uses a four-step phase-shifting method with phase differences of π/2.
The proposed system could be applied for the study of biological tissue, in vivo analysis of the morphology of RBC, calculation of deformation in microstructures, finally, the dynamic events allow measuring variations and properties of organic and synthetic structures. The advancements in simultaneous phase-shifting interferometry have led to development to dynamic methods (4D measurements), which can operate in the presence of environmental disturbances but preserves measurement accuracy.
Supplemental data
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09500340.2017.1300697
Disclosure statement
No potential conflict of interest was reported by the author.
Funding
This research was supported by the Initiative for the creation of the First Optics and Photonics Engineering undergraduate program in Mexico.
Supplementary_material.zip
Download Zip (2.9 MB)Acknowledgements
The author thanks Dra Amalia Martínez García and Dr Gustavo Rodríguez Zurita for their helpful suggestions. The author would like to thank the anonymous reviewers for their valuable comments and suggestions to improve the quality of the paper.
References
- Carré, P. Installation et utilisation du comparateur photoelectric et interferential du bureau international des poids et mesures [Installation et utilisation du comparateur photoelectric et interferential du bureau international des poids et mesuresInstallation and use of the photoelectric and interferential comparator of the International Bureau of Weights and Measures]. Metrologia. 1966, 2, 13–23.10.1088/0026-1394/2/1/005
- Crane, R. Interference Phase Measurement. Appl. Opt. 1969, 8, 538–542.
- Bruning, J.H.; Herriott, D.R.; Gallagher, J.E.; Rosenfeld, D.P.; White, A.D.; Brangaccio, D.J. Digital Wavefront Measuring Interferometer for Testing Optical Surfaces and Lenses. Appl. Opt. 1974, 13, 2693–2703.10.1364/AO.13.002693
- Wyant, J.C. Use of an AC Heterodyne Lateral Shear Interferometer with Real-time Wavefront Correction Systems. Appl. Opt. 1975, 14, 2622–2626.10.1364/AO.14.002622
- Moore, D.T. Gradient Index Optics Design and Tolerancing. Ph. D. Thesis, University of Rochester, 1973.
- Hardy, J.W.; Lefebvre, J.E.; Koliopoulos, C.L. Real-Time Atmospheric Compensation. J. Opt. Soc. Am. 1977, 67, 360–369.10.1364/JOSA.67.000360
- Creath, K. Phase Measurement Interferometry Techniques. In Progress in Optics: Wolf, E., Ed.; Elsevier Science Publisher, Amsterdam, 1988; pp 349–393.
- Schwider, J. Advanced Evaluation Techniques in Interferometry. In Progress in Optics: Wolf, E., Ed.; North-Holland, Amsterdam, 1990; pp 271–359.
- Greivenkamp, J.E.; Bruning, J.H. Phase Shifting Interferometry. In Optical Shop Testing: Malacara, D., Ed.; Wiley, Hoboken, NJ, 2007; pp 547–666.
- de Groot, P.J. Vibration in Phase-Shifting Interferometry. J. Opt. Soc. Am. A 1995, 12, 354–365.10.1364/JOSAA.12.000354
- Millerd, J.E.; Brock, N.J. Methods and Apparatus for Splitting Imaging and Measuring Wavefronts in Interferometry. US Patent 6,304,330 and 6,522,808, 2001.
- Min, J.; Yao, B.; Gao, P.; Guo, R.; Zheng, J.; Ye, T. Parallel Phase-shifting Interferometry Based on Michelson-like Architecture. Appl. Opt. 2010, 49, 6612–6616.10.1364/AO.49.006612
- Barrientos-García, B.; Moore, A.J.; Pérez-López, C.; Wang, L.; Tschudi, T. Transient Deformation Measurement with Electronic Speckle Pattern Interferometry by Use of a Holographic Optical Element for Spatial Phase Stepping. Appl. Opt. 1999, 38, 5944–5947.
- Barrientos-García, B.; Moore, A.J.; Pérez-López, C.; Wang, L.; Tschudi, T. Spatial Phase-stepped Interferometry Using a Holographic Optical Element. Opt. Eng. 1999, 38, 2069–2074.
- Sasada, M.; Fujii, A.; Awatsuji, Y.; Kubota, T. Parallel Quasi-phase-shifting Digital Holography Implemented by Simple Optical Set up and Effective Use of Image-sensor Pixels. In Technical Digest of the 2004 ICO International Conference: Optics and Photonics in Technology Frontier, International Commission for Optics, 357–358, 2004.
- Millerd, J.; Brock, N.; Hayes, J.; North-Morris, M.; Novak, M.; Wyant, J. Pixelated Phase-mask Dynamic Interferometer. Proc. Interferometry XI 2004, 5531, 304–314.
- Awatsuji, Y.; Sasada, M.; Kubota, T. Parallel Quasi-phase-shifting Digital Holography. Appl. Phys. Lett. 2004, 85, 1069–1071.10.1063/1.1777796
- Flores Muñoz, V.H.; Toto-Arellano, N.I.; López, B.; Martínez García, A.; Rodríguez-Zurita, G. Measurement of Red Blood Cell Characteristic Using Parallel Phase Shifting Interferometry. Optik. 2015, 126, 5307–5309.10.1016/j.ijleo.2015.09.019
- Toto-Arellano, N.-I.; Martínez-García, A.; Rodríguez-Zurita, G.; Rayas-Álvarez, J.A.; Montes-Perez, A. Slope Measurement of a Phase Object Using a Polarizing Phase-Shifting High-Frequency Ronchi Grating Interferometer. Appl. Opt. 2010, 49, 6402–6408.10.1364/AO.49.006402
- Rodriguez-Zurita, G.; Toto-Arellano, N.-I.; Meneses-Fabian, C.; Vázquez-Castillo, J.F. One-Shot Phase-shifting Interferometry: Five, Seven, and Nine Interferograms. Opt. Lett. 2008, 33, 2788–2790.10.1364/OL.33.002788
- Toto-Arellano, N.I.; Serrano-García, D.I.; García, A.; Zurita, G.; Montes-Pérez, A. 4D Profile of Phase Objects through the Use of a Simultaneous Phase Shifting Quasi-common Path Interferometer. J. Opt. 2011, 13, 115502.10.1088/2040-8978/13/11/115502
- Kiire, T.; Nakadate, S.; Shibuya, M. Simultaneous Formation of Four Fringes by Using a Polarization Quadrature Phase-shifting Interferometer with Wave Plates and a Diffraction Grating. Appl. Opt. 2008, 47, 4787–4792.10.1364/AO.47.004787
- Malacara, D.; Servín, M.; Malacara, Z. Phase Detection Algorithms. In Interferogram Analysis for Optical Testing, 2nd ed. Malacara, D., Ed.; Taylor & Francis Group, Boca Raton, FL, 2005; pp 259–355.
- Popescu, G.; Ikeda, T.; Dasari, R.R.; Feld, M.S. Diffraction Phase Microscopy for Quantifying Cell Structure and Dynamics. Opt. Lett. 2006, 31, 775–777.
- Popescu, G.; Badizadegan, K.; Dasari, R.R.; Feld, M.S. Observation of Dynamic Subdomains in Red Blood Cells. J. Biomed. Opt. 2006, 11, 059802.10.1117/1.2362857
- Popescu, G.; Ikeda, T.; Goda, K.; Best-Popescu, C.A.; Laposata, M.; Manley, S.; Dasari, R.R.; Badizadegan, K.; Feld, M.S. Optical Measurement of Cell Membrane Tension. Phys. Rev. Lett. 2006, 97, 218101.10.1103/PhysRevLett.97.218101
- Sarkar, S.; Bhattacharya, K. Polarization Phase Shifting Cyclic Interferometer for Surface Profilometry of Non-birefringent Phase Samples. J. Mod. Opt. 2013, 60, 185–189.10.1080/09500340.2013.765051
- Serrano-García, D.; Martínez-García, A.; Toto-Arellano, N.I.; Otani, Y. Dynamic Temperature Field Measurements Using a Polarization Phase-Shifting Technique. Opt. Eng. 2013, 53 (11), 112202.
- Toto-Arellano, N.-I.; Serrano-García, D.-I.; Martínez-García, A. Parallel Two-step Phase Shifting Interferometry Using a Double Cyclic Shear Interferometer. Opt. Express. 2013, 21, 31983–31989.10.1364/OE.21.031983
- Toto-Arellano, N.-I.; Flores-Muñoz, V.H.; Lopez-Ortiz, B. Dynamic Phase Imaging of Microscopic Measurements Using Parallel Interferograms Generated from a Cyclic Shear Interferometer. Opt. Express. 2014, 22, 20185–20192.10.1364/OE.22.020185
- Kerr, D.; Kaufmann, G.H.; Galizzi, G.E. Unwrapping of Interferometric Phase-fringe Maps by the Discrete Cosine Transform. Appl. Opt. 1996, 35 (5), 810–816.10.1364/AO.35.000810
- Ghiglia, D.C.; Romero, L.A. Robust Two-dimensional Weighted and Unweighted Phase Unwrapping that Uses Fast Transforms and Iterative Methods. J. Opt. Soc. Am. A 1994, 11 (1), 107–117.10.1364/JOSAA.11.000107
- Márquez-Luna, J. Técnicas de colecta y preservación de insectos [Insect collection and conservation techniques]. Bol. Soc. Entomol. Aragonesa. 2005, 37, 385–408.
- Hoffmann, A. Relación bibliográfica preliminar de las arañas de México [Preliminary bibliography report of spiders from Mexico]. Inst. Biol. UNAM. Publ. Esp. 1976, 54, 117–120.
- Shock, I.; Barbul, A.; Girshovitz, P.; Nevo, U.; Korenstein, R.; Shakeda, N.T. Optical Phase Nanoscopy in Red Blood Cells Using Low-coherence Spectroscopy. J. Biomed. Opt. 2012, 17, 101509.10.1117/1.JBO.17.10.101509
- Pham, H.; Ding, H.; Sobh, N.; Do, M.; Patel, S.; Popescu, G. Off-axis Quantitative Phase Imaging Processing Using CUDA: Toward Real-time Applications. Biomed. Opt. Express. 2011, 2, 1781–1793.10.1364/BOE.2.001781
- Mico, V.; Zalevsky, Z.; García, J. Common-path Phase-Shifting Digital Holographic Microscopy: A Way to Quantitative Phase Imaging and Superresolution. Opt. Commun. 2008, 281, 4273–4281.10.1016/j.optcom.2008.04.079
- Hariharan, P.; Sen, D. Radial Shearing Interferometer. J. Sci. Instrum. 1961, 38 (11), 428–432.10.1088/0950-7671/38/11/305
- Liu, D.; Yang, Y.; Shen, Y. System Optimization of Radial Shearing Interferometer for Aspheric Testing. Proc. SPIE 2007, 6834, 68340U.10.1117/12.760132
- Wang, M.; Zhang, B.; Nie, S. Radial Shearing Interferometer for Aspheric Surface Testing. Proc. SPIE 2002, 4927, 673–676.10.1117/12.471690
- Kohno, T.; Matsumoto, D.; Yazawa, T. Radial Shearing Interferometer for in-Process Measurement of Diamond Turning. Proc. SPIE 1997, 3173, 280–285.10.1117/12.294519
- Zhang, B.; Ma, L.; Wang, M.; He, A. Aspheric Lens Testing by Means of Compact Radial Shearing Interferometer with Two Zone Plates. Laser Technol. 2007, 31, 37–46. (in Chinese).
- Kowalik, W.; Garncarz, B.; Kasprzak, H. Corneal Topography Measurement by Means of Radial Shearing Interference: Part I – Theoretical Consideration. Optik. 2002, 113 (1), 39–45.10.1078/0030-4026-00113
- Kowalik, W.; Garncarz, B.; Kasprzak, H. Corneal Topography Measurement by Means of Radial Shearing Interference: Part II – Experiment Results. Optik (Stuttg.) 2002, 113 (1), 46–50.
- Kowalik, W.; Garncarz, B.; Kasprzak, H. Corneal Topography Measurement by Means of Radial Shearing Interference: Part III – Measurement Errors. Optik. 2003, 114 (5), 199–206.10.1078/0030-4026-00247
- Li, D.; Wang, P.; Li, X.; Yang, H.; Chen, H. Algorithm for near-field Reconstruction Based on Radial-Shearing Interferometry. Opt. Lett. 2005, 30, 492–494.10.1364/OL.30.000492
- Yang, Y.; Lu, Y.; Chen, Y. A Radial Shearing Interference System of Testing Laser Pulse Wavefront Distortion and the Original Wavefront Reconstructing. Proc. SPIE 2005, 5638, 200–204.10.1117/12.570656