ABSTRACT
Getting students to understand the particulate nature of matter is a major challenge for chemistry education. In upper secondary school students commonly struggle to distinguish between intra- and intermolecular bonding and analyse chemical bonding in terms of electronegativity. In this study, we explore how creative drama may be used in chemistry education to facilitate students’ explorations of electronegativity and chemical bonding in Swedish upper secondary chemistry education. The study was conducted as a design-based intervention in three cycles in two schools. The interventions (which lasted for 30–60 minutes) were single-lesson-interventions consisting of drama activities and discussions in whole-class and student groups. A qualitative content analysis produced themes that captured the ways in which the students explored electronegativity and chemical bonding and how creative drama enabled collective student agency. The findings indicate that creative drama enabled the students to link the electronegativity and polarity of molecules to formations of molecular grid structures using their own bodies to represent how molecules were organised in different states of matter. The results also indicate that creative drama in chemistry education may enhance student agency in their explorations of electronegativity and the linking of electronegativity to intramolecular and intermolecular bonding.
Introduction
A major challenge for chemistry education is to develop students’ understanding of the particulate nature of matter (Harrison & Treagust, Citation2002; Taber & Coll, Citation2002), which is a prerequisite for a thorough understanding of chemical bonding (Othman et al., Citation2008). In upper secondary school, challenges regarding the particulate nature of matter are commonly expressed in terms of students’ struggle to distinguish between intra- and intermolecular bonding (Othman et al., Citation2008) and to analyse chemical bonding in terms of electronegativity (Burrows & Reid Mooring, Citation2015; Harrison & Treagust, Citation1996; Nahum et al., Citation2008; Peterson & Treagust, Citation1989; Taber & Coll, Citation2002). In this study, drama is used in chemistry education to facilitate students’ explorations of the particulate nature of matter, chemical bonding and electronegativity. Some studies suggest that drama can be a useful support to science learning (Dorion, Citation2009; Ødegaard, Citation2003; Yoon, Citation2006), but studies in science education are limited and the potential for using drama to facilitate students’ theoretical reasoning in chemistry needs further scrutiny and design development.
In this study a form of drama for educational purposes (Arieli, Citation2007; Braund, Citation2013) called creative drama is used. Creative drama involves a teacher guiding students in the dramatisation of a story or concept being taught. It is a form of improvised play intended to provide students with opportunities to explore chemical bonding concepts by using their own bodies. The rationale for focussing on creative drama in this study is its potential for facilitating processes that make chemical concepts personally meaningful to the students (Arieli, Citation2007). Here we conceptualise the making concepts personally meaningful in terms of agency and as ‘the ability [for students] to construct and transform independently one's own life activity.’ (Davydov et al., Citation2003, p. 63). Developing student agency refers to the enabling of students to define her-/himself in the world of life and to become engage in different kinds of activity and is a driving force of individual engagement (cf. Goulart & Roth, Citation2010). Agency is something that students achieve (do) in a situation rather than something they own (have) (Biesta & Tedder, Citation2007; Caiman & Lundegård, Citation2014). This study focusses on the potential of supporting student agency in exploring theoretical chemical relationships by means of creative drama.
Background
Chemical bonding and electronegativity
Chemical bonding is a key concept in chemistry education (Nahum et al., Citation2010). Multiple studies describe how students struggle to grasp the bonding concept (Bergqvist & Chang Rundgren, Citation2017; Nahum et al., Citation2010; Nicoll, Citation2001; Taber & Coll, Citation2002). Studies have also shown that students who do not master concepts related to chemical bonding in secondary school will likely struggle to understand higher-level chemical bonding (Burrows & Reid Mooring, Citation2015; Nahum et al., Citation2010; Nicoll, Citation2001).
In order to develop an understanding of chemical bonding, students have to interpret a variety of symbolic representations and abstract concepts (Taber & Coll, Citation2002). For example, students have to understand how covalent bonding and ionic bonding differ from intermolecular bonding, understand what is meant by electronegativity, the polarity of the bond and how the polarity of the bond is determined (Henderleiter et al., Citation2001).
Nicoll (Citation2001, p. 708) emphasises that ‘bonding, electronegativity, and molecular structure are the cognitive keys that students need to be able to unlock the door to understanding the microscopic world of chemistry’. Nicoll (Citation2001) further argues that these concepts are foundational to a more advanced conceptual understanding and that they are often revisited in successive chemistry courses that students take. Hence, electronegativity and polarity are key concepts in understanding chemical bonding (Nahum et al., Citation2010).
Foundational concepts of chemical bonding
In this study, we focus on chemical concepts that are foundational and, in our experience, difficult for Swedish first year upper secondary students to master during the introductory course of chemistry (mandatory for students studying the natural science subjects): the particulate nature of matter, distinguishing between intra- and intermolecular bonding, hydrogen bonding, electronegativity and polarity. The Swedish curriculum for Chemistry in upper secondary school emphasises that matter and chemical bonding are part of the core content of Chemistry: ‘Models and theories of the structure and classification of matter’ and ‘chemical bonding and its impact on e.g. the occurrence, properties and application areas of organic and inorganic substances’ (Swedish National Agency of Education, Upper Secondary Education Curriculum, Citation2011).
Particulate nature of matter
Othman et al. (Citation2008) showed in a research overview that difficulties understanding the particulate nature of matter are related to a lack of understanding of the relationship between the particulate nature of matter and the macroscopic properties of the subjects. The relationship between macroscopic properties and the submicro and symbolic representations have been described in Johnstone's triangle (Johnstone, Citation1982). The macro level is the observable or perceptible phenomenon and describes what we can see, hear or measure. The submicro level describes arrangements of particle units such as atoms, molecules and ions and electrons, and the symbolic level is the language of chemical formulas and symbols which describe the substances and particles (Johnstone, Citation1982). Several studies have revealed that students struggle to switch between the different levels of representation (De Jong et al., Citation2013; Gilbert & Treagust, Citation2009; Harrison & Treagust, Citation2002; Seifeddine Ehdwall & Wickman, Citation2018). Students often use one form of representation, usually macro, for describing what they can measure, hear or see, and do not move between modes of representation when they are asked to describe and explain a chemical phenomenon (Kozma & Russel, Citation1997).
To distinguish between intra- and intermolecular bonding
Difficulties distinguishing between intra- and intermolecular bonding are expressed on the symbolic level. For example, students will often represent the water molecule as a diatomic molecule of hydrogen and an atom of oxygen, or the water molecule is drawn as three separate atoms (De Jong et al., Citation2013). Othman et al. (Citation2008) have also shown in a study of diagnostic tests on students aged 15–16 that many of the students had difficulty distinguishing between intra- and intermolecular bonding. This was expressed as the inability to write about intermolecular bonds as broken when substances melted or boiled, and in perceiving covalent bonds as weak (cf. Henderleiter et al., Citation2001; Peterson & Treagust, Citation1989; Schmidt et al., Citation2009).
Hydrogen bonding
Research suggests that students’ alternative models for hydrogen bonds limit hydrogen bonds to the presence of oxygen and hydrogen atoms. For example, Schmidt et al. (Citation2009) found, when investigating German upper secondary school students’ models for intermolecular forces, that students would talk about boiling as involving the breaking of covalent bonds. Henderleiter et al. (Citation2001) have also shown, in a study of university students (who had passed their second course of a two-semester sequence of organic chemistry in a US university), that the students still expressed perceptions of hydrogen bonding which did not match the scientific discourse – for example, that hydrogen bonds could be induced and that boiling would break covalent bonds instead of hydrogen bonds.
Electronegativity and polarity
Students’ difficulties distinguishing between intra- and intermolecular forces have been interpreted as a lack of understanding of the concept of electronegativity – the atom's ability to attract electrons from other atoms (Harrison & Treagust, Citation1996; Nahum et al., Citation2008; Taber & Coll, Citation2002). Nicoll (Citation2001) showed in an interview study that first-year chemistry students struggled to explain concepts of electronegativity and polarity. The students associated electronegativity primarily with ionic compounds – the ability to gain or lose electrons instead of the process of attracting electrons – and some students appeared to know about the concept of polarity, but did not associate it with electronegativity. The results showed that some students were able to explain the concepts of electronegativity and polarity in line with the scientific discourse but could not put them together in context (cf. Burrows & Reid Mooring, Citation2015). In sum, research shows that students have difficulty grasping the concept of electronegativity and applying electronegativity in context to understand the difference between intramolecular and intermolecular bonding. Students tend to disregard the role of electronegativity and conflate the concepts of polarity, covalent bonding, electronegativity and intermolecular forces (Peterson & Treagust, Citation1989).
Using creative drama to explore chemical bonding
In this study, creative drama is used to facilitate making molecular bonding at the submicro level visible to students, and as a means for students to describe macro level chemical observations with submicro level models of chemical bonding. A review of research on the teaching of chemical bonding emphasise the role of visual models in to supporting student learning of chemical bonding (Nahum et al., Citation2010). Whereas previous research has pointed to the potential of using two- and three-dimensional visualisations to facilitate students’ theoretical reasoning concerning chemical bonding (for example Venkataraman, Citation2009; Wu et al., Citation2001; Zhang & Linn, Citation2011), this study focusses specifically on the potentials of using drama where students use their own bodies as semiotic resources. An assumption is that the bodily experiences afforded in a drama activity may have consequences for student learning and the possibilities to support student agency (Andrée, Citation2012; Goulart & Roth, Citation2010; King, Citation2012) in exploring chemical bonding.
Multiple studies have suggested that creative drama may be an effective tool for enhancing students’ conceptual understanding of scientific concepts (Alrutz, Citation2004; Arieli, Citation2007; Dorion, Citation2009; Hendrix et al., Citation2012; Ødegaard, Citation2003; Osama, Citation2016; Yoon, Citation2006). In the previous research of drama in science education, the scientific concepts targeted have been related to electricity, mixtures and solutions (Arieli, Citation2007), sound and solar energy (Hendrix et al., Citation2012) and molecules in different states of matter (Arieli, Citation2007; Osama, Citation2016). However, research has primarily been based on a pre- and post-test methodology. Few attempts have been made to investigate how drama actually works in classroom practice (cf. Braund, Citation2015). Hence, research that zooms in on student participation in drama activities in the science classroom is needed to explore where the potential for conceptual learning lies.
Facilitating collaborative learning through creative drama, allowing a more experienced student to mediate learning for a less experienced student, can thus promote science learning (Hendrix et al., Citation2012; Osama, Citation2016). Pre- and poststudies of creative drama interventions have suggested that creative drama may enhance understanding of scientific concepts for low- as well as average- and high-achieving students (Aubusson et al., Citation1997; Osama, Citation2016). In creative drama students may reconstruct their knowledge of chemistry by moving between a model or description from the textbook and a three-dimensional living model with their own bodies. This enables the students to reconstruct and develop their understanding of specific scientific concepts (Ødegaard, Citation2015). In relation to chemical bonding, Danckwardt-Lillieström et al., Citation2018 showed that when the students (in creative drama) created meaning in the bodily mode together with the verbal and the written modes, other types of semiotic work were afforded than are usually offered in chemistry education. The ways of meaning-making can be expanded by widening the types of semiotic resources used in teaching (e.g. bodily resources such as voice, gestures, facial expressions; digital resources such as audio, video and simulations; text resources such as different kinds of paper, scissors, tape, crayons, etc.) compared to what is commonly used in chemistry teaching when teaching electronegativity and chemical bonding.
A recognised challenge of working with creative drama is the generation and promotion of anthropomorphic language. For example, when students are told to ‘be atoms’. Research has warned that simplified explanatory models can obstruct future chemistry learning (Bergqvist & Chang Rundgren, Citation2017; Nahum et al., Citation2008; Taber & Coll, Citation2002). However, Manneh et al. (Citation2019) showed, in a study of chemistry students during problem-solving classes in an introductory course at university level, that students’ use of anthropomorphic expressions was beneficial for their learning since it enabled them to engage with technical expressions (cf. Watts & Bentley, Citation1994). Simplifying the chemical language may thus function as scaffolding in students’ work to acquire chemical concepts and processes, since these can act as anchors for the development of scientific explanations within a scientific discourse (Taber, Citation2002; Taber & Coll, Citation2002). Given the risks of oversimplification, it is important that chemistry teachers are aware of such limitations and support students in discerning the limitations of simplified models (Bergqvist & Chang Rundgren, Citation2017).
A significant feature of creative drama is that the teacher can engage in a dialogue with the students to promote learning and guide them to the turning point – moving from everyday language to a scientific language (Mortimer & Scott, Citation2006). Thus, student conceptions of chemical bonding can be interrogated through dialogue and discussion, the limitations of simplified models can be discussed, and the students may be provided with opportunities to scrutinise, evaluate and adjust their learning about electronegativity and chemical bonding.
The aim of this study is to explore how creative drama in upper secondary chemistry education may support students’ conceptual development of electronegativity, chemical bonding and the particulate nature of matter. Thus, we seek to investigate how students establish relationships between concepts and how such conceptual relations are challenged and investigated by the students in the drama activity, as well as in what ways the drama activity may afford student meaning-making in relation to chemical bonding.
Theoretical perspective
In recognising science learning as a complex social activity, we draw on the notion of agency. This study focusses on student agency as the driving force of students’ engagement (Goulart & Roth, Citation2010). Inspired by the work of Roth and his colleagues (Goulart & Roth, Citation2010; Roth, Citation2007) on dialectics in science education and King’s (Citation2012) work on interactions in a context-based chemistry classroom, we adopt a dialectical sociocultural lens where agency is conceptualised in the two dialectical relationships of agency/structure and agency/passivity. Agency and structure are theorised as a dialectic relationship where structures in themselves include social arrangements, relationships and practices that can exert power and constrain people's lives (Osterkamp, Citation1999). In the chemistry classroom, the material structures and resources (in the form of laboratory facilities, whiteboards, particular student's desks and so on), combined with social structures thus mediate what a teacher or student can do (King, Citation2012). The practice shapes the individual, but the individual also shapes the practice (Hewson, Citation2010; Sewell, Citation1992). Altering conditions in the form of structural and material resources may offer opportunities for agentic student engagement in chemistry learning (Andrée & Lager-Nyqvist, Citation2013). In relation to creative drama in chemistry education, questions of agency concern how the material and social resources provided opportunity and responsibility for the students to transform their understanding of chemistry.
Agency and passivity presuppose one another (Roth, Citation2007). A student may be receptive to learning, which can be recognised by bodily and facial expressions. Roth (Citation2007) emphasises that a student can be receptive to learning in two ways: unconsciously and consciously. In the former, agency is not invoked, but in the latter agency is invoked. Passivity can be described as unconscious student receptivity where ‘a student may choose consciously to participate in a group discussion on the current chemistry topic she is learning. The student is choosing to be receptive to learning rather than learning passively since passivity is an unconscious act’ (King, Citation2012, pp. 78–79). Hence, in order to enable learning, the students need to be agential, observed through their verbal and non-verbal actions as well as passive, observed through their willingness to learn and openness to learning (Hwang & Roth, Citation2009). The introduction of creative drama in chemistry teaching involves the dialectics of agency/structure as well as agency/passivity.
Research questions
The research questions addressed are as follows:
In what ways may creative drama afford students’ conceptual exploration of electronegativity and chemical bonding?
In what ways may creative drama support collective student agency in exploring electronegativity and chemical bonding?
Method
The study was conducted in the form of design-based research (DBR) with a focus on creating knowledge that will be useful to the planning of teaching in the complex environment of the chemical classroom (The Design-Based Research Collective, Citation2003). Design-based research builds on intervention through a cyclic process of redesign and analysis where a design of a teaching activity is tested, evaluated and tested again (Cobb & Gravemeijer, Citation2006). Teachers and researchers work together and try to create meaningful changes in the classroom and learning (The Design-Based Research Collective, Citation2003). By examining different aspects of the learning process and how the classroom can be designed to support the learning process the design-based study may contribute with an understanding of what can be described as a learning ecology (Cobb et al., Citation2003). Design-based research is thus an important methodology for understanding how, when and why innovations in education works in practice (The Design-Based Research Collective, Citation2003).
Designing an intervention
The interventions were designed with two tentative design principles as guiding heuristics. Both principles were based on prior teaching experiences and previous research on the use of drama in science education and research on chemical bonding specifically (see Background). First, we intended to create opportunities for the students to work symbolically at both the submicro and the macro levels (Johnstone, Citation1982) and enable reflections on the different organisational levels by offering the students different semiotic modes. In designing creative drama, we also intended to emphasise the transitions between the symbolic, macro and microscopic world so that students would develop models of bonding on these three levels (Nicoll, Citation2001). Second, we wanted to create opportunities for the students to interact with each other and to make their bodily formations of molecules visible in the classroom. By making students’ bodily formations of molecules visible in the classroom, we intended to create richer opportunities for student meaning-making via peer learning and opportunities for the teacher to gain insight into the conceptual reasoning in the student groups.
An intervention in the form of a research lesson including creative drama activities was designed with the design principles as the starting point. The research lesson which was implemented in three cycles included the following tasks:
Small-group task. Form a hydrogen fluoride molecule with your own bodies where you show which of the atoms is most electronegative. Provide an oral account of the formations.
Whole-class activity. Form the liquid phase of hydrogen fluoride with a large group of students and alternate between the different phases of matter.
Small-group task. Form a water molecule with your bodies where you show which of the atoms is most electronegative. Provide an oral account of the formations.
Whole-class activity. Form the liquid phase of water with a large group of students and alternate between the different phases of matter.
In order to support the students’ scientific reasoning and semiotic work, the students were encouraged to use any semiotic resources available in the classroom (e.g. coloured paper, textbooks, computers, digital tablets, cellphones, scissors, clothes pegs and tape) in combination with the bodily mode.
The tasks were intended to enable the students to move between the macro, submicro and symbolic levels in order to afford student meaning-making on the particulate nature of matter, electronegativity, chemical bonding and how to connect these concepts to each other. The learning objectives for the lesson were to enable students to distinguish between stronger intramolecular bonds and weaker intermolecular bonds and to enable students to reason about molecular submicro level interaction in relation to macrolevel phenomena such as substances’ different phases of matter. The whole-class tasks were designed to provide students with opportunities to explore interactions between several molecules. At the end of the whole-class tasks (task 2 and 4), the students were provided to talk with their peers and teacher about why they had positioned oneself to one another the way they did.
A rationale for the choice of molecules was to facilitate the students’ first bodily formation by working with the hydrogen fluoride molecule which consists of only two atoms and is straight, unlike the more complex water molecule where the angle becomes important for the molecule's polarity. The students were familiar with both molecules since both are frequently used in class and in the textbooks. In addition, models of hydrogen fluoride molecules are commonly used in the research literature to investigate pupils’ understanding of electronegativity (Burrows & Reid Mooring, Citation2015; Peterson & Treagust, Citation1989).
The students are expected to link electronegativity (the ability of an atom to attract electrons from other atoms to itself, cf. Danielsson Thorell & Johansson, Citation2016) to the appearance of the polarity of the bond. Although covalent bonding, polar covalent bonding and ionic bonding are to be understood as a continuum, most textbooks (Bergqvist et al., Citation2013) and educators (Taber et al., Citation2012) present them as three distinct types of bonding.
Implementing the intervention in three cycles
A total of four research lessons were conducted in three cycles in two municipal upper secondary schools. The participating schools were located in local municipalities in vincity of the university and had expressed interest in exploring drama in upper-secondary chemistry education. One of the schools (School A) were the school where the first author worked as a teacher at the time. After informal probing in nearby municipalities, another school (School B) was selected for participation where a teacher who had been nominated for participation also expressed interest in using drama. Both participating teachers had longstanding experience in teaching upper-secondary school chemistry and had also experimented on their own with drama as a teaching method. The schools have somewhat different catchment areas. School A was situated in a suburban area where more than half of the students had a migrant background (which in this case refers to a person born abroad, or a person born in Sweden with two foreign-born parents). School B was situated in the inner city where about one fith of the students had migrant background (Swedish National Agency of Education, Citation2020).
provides an overview and summary of the collected data. The participating students in all three cycles were aged 16–17 years, in their first year of the natural science programme. The data were collected in the three cycles. The first and second cycles were conducted at School A with the first author acted as a teacher during the data collection. In Cycle 3 (including two research lessons), the study was conducted in collaboration with the second teacher at School B (see ).
Table 1. The table provides an overview and summary of the collected data during the three cycles of intervention in the study.
In all interventions, the teacher introduced the drama activity with the help of two students who were asked to visualise a hydrogen fluoride molecule. Several students suggested that it was hydrogen bonding or ionic bonding between the atoms, but in all cycles the respective teacher clarified in dialogue with the students that it was covalent bonding between the atoms and that the bonding in the drama activity was represented by the interconnected arms in the student-constructed molecule. The activity enabled a discussion in the groups and in the classroom about the difference between hydrogen bonding, ionic bonding and polar covalent bonding. In all three cycles the teacher completed the introduction by clarifying the first task. For example, the teacher in Cycle 2 said:
So you should select two peers who are a hydrogen fluoride molecule and then you should mark the molecule in some way which atom is most electronegative. Now you have to think and discuss for a few minutes and then I want you to present your bodily molecules for each other.
In Cycle 1, twenty-five students participated. Prior to the drama activity they participated in a teaching sequence on chemical bonding, which included intramolecular bonding (ionic bonding, polar and non-polar covalent bonding), the concept of electronegativity, intermolecular bonding (dipole–dipole bonding, intermolecular hydrogen bonding and Van der Waals bonding) and metal bonding. The research lesson lasted about 30 minutes and was video- and audio-recorded with one stationary video camera located at the back of the classroom and two audio recorders.
In Cycle 2, twenty-six students participated. Prior to the research lesson they participated in a teaching sequence on chemical bonding, wich included intramolecular bonds (as described in Cycle 1), the concept of electronegativity and were in the middle of a teaching sequence regarding intermolecular bonds. Certain concepts (e.g. hydrogen bonding) that the students raised in the research lesson had not yet been covered in lectures. The research lesson lasted 60 minutes and was video- and audio-recorded with five cameras and four audio recorders.
In Cycle 3, students from two classes participated (26 students in the first and 28 students in the second). Prior to the drama activity they participated in a teaching sequence on chemical bonding as described in Cycle 1. Unlike the students in Cycle 1 and 2, the students in Cycle 3 did not use any chemistry textbook, but they had a web-based resource. The research lessons lasted for 60 minutes and were audio- and video-recorded with one moving camera and three stationary cameras, with three audio recorders in the first group and four in the second.
Formal written consent was provided by the participating students in accordance with the ethical guidelines provided by the national science council (Swedish Research Council, Citation2017).
Revisions between the cycles
The main purpose of the intervention in Cycle 1 was to open up to develop the students’ understanding of hydrogen bonding. The students’ interaction and semiotic work in the creative drama revealed a lack of ability to connect electronegativity to molecular polarity and intermolecular bonding. This became very visible as some students did not know how to position themselves against the other bodily molecules at the phase transitions of the substances. Hence, we decided to expand the purpose of the drama activity to focus more on exploration of electronegativity and chemical bonding. Further we observed in the transcripts that the students were not given time to develop their conceptual exploration and reasoning about chemical bonding. Therfore we wanted to create more space for the students’ conceptual exploration both within and between student groups. Thus, we decided to extend the creative drama from 30 minutes in Cycle 1 to 60 minutes in Cycle 2. Our results from Cycle 1 displayed that the students presented their molecules in dialogue with the teacher who was walking around the classroom. To support the student interaction between groups the students were asked to present and explain their molecular formations to the whole class. To additionally support student agency in discerning and evaluating the bodily formations the students were instructed by the teacher to document selected student molecules with their digital tablets. These photos were later used as a basis for continued group discussions and a written individual assignment.
Cycle 3 was conducted in School B with Teacher B who had not previously worked with creative drama. The implementation was prepared after detailed planning by the first author and Teacher B. Our results from Cycle 2 displayed that even though the students had more time for the tasks (1–4), the teacher acted as a director with the students participating in a teacher-directed visualisation of chemical bonding and electronegativity. Based on our findings we decided that Teacher B would use the same task instructions that had been used in the intervention in Cycle 2, although with an increased emphasis on making the students explain to their peers why they had visualised the molecules or positioned themselves in the way they had done in the drama activity. Teacher B would also encourage students to use various form of semiotic resources and explain the rationales for how they created their bodily molecules. In addition, teacher B would pay attention to supporting the students in performing collective semiotic work on intermolecular bonding by offering the students to change phase several times (tasks 2 and 4). Hence during the research lessons, there were some differences in the implementation compared to Cycle 2. Teacher B invited the students to explain the concepts, and worked more like a mediator compared to teacher A (the first author) in Cycle 1 and Cycle 2. The students in Cycle 3 were given more space for their exploration of chemical bonding in the classroom compared to the students in previous cycles.
Data processing and analysis
We used qualitative content analysis as described by Graneheim and Lundman (Citation2004) and Erlingsson and Brysiewicz (Citation2017) to investigate the ways in which creative drama facilitates students’ exploration of electronegativity and chemical bonding and how creative drama may enhance collective student agency in exploring electronegativity and chemical bonding.
The analytical process is illustrated in . To investigate how the students explored electronegativity and chemical bonding in creative drama and the emergence of collective student agency we discerned meaning units (1) – words, sentences or paragraphs containing aspects related to each other through their content or context. In the next step, these meaning units were condensed into codes (2) – names that most exactly described a particular meaning unit. Then we grouped those codes that related to each other through their content or context into categories (3) and, finally, as an expression of the latent content of the data material, we grouped categories into themes (4).
Table 2. Examples of how the qualitative thematic content analysis were applied in the study.
In the study, each category functioned as an umbrella for various content that was addressed in students’ semiotic work in the creative drama and that was related in some way. For example, the codes ‘fighting for the electrons’ and ‘The most electronegative atom has the strongest pull on electrons’ were both associated with students’ work on electronegativity and how to display electronegativity within the bodily mode using anthropomorphic expressions, and were therefore sorted under the category ‘Display electronegativity within bodily mode together with anthropomorphic expressions’ (see ). When discerning themes in students’ semiotic work we revised the transcripts and the audio-visual data material as a whole, searching for patterns. For example, the theme Displaying electronegativity and chemical bonding with anthropomorphic expressions illustrates how the students explored chemical concepts through creative drama (see ).
In all three cycles, we discerned the themes Displaying electronegativity and chemical bonding with anthropomorphic expressions and Amplifying bodily configurations with other semiotic resources. For the latter we discerned the category Artefact creation in all cycles but some of the codes were different. For example in Cycle 1 the most common code was Drawing chemical formula-paper on the body, in Cycle 2 Artefacts in form of electrons and in Cycle 3 Artefacts in form of protons. In Cycle 2 and Cycle 3 we discerned the theme Introducing movements to display electronegativity and the theme Resolving epistemic dissonance sparked by bodily prompting. In all cycles the theme Participation in directed visualisation and by directing of visualisation unfold but the cycles differ in categories, where Cycle 1 and Cycle 2 mainly were categorizised in Participation in directed visualisation and Cycle 3 by Directing of visualisation.
Validity and reliability
Throughout the study we have used different strategies to ensure valildity and reliability. Validity is contingent upon the mode of research (Newton & Burgess, Citation2008). This article reports on a design based educational research seeking to advance knowledge on how creative drama in upper secondary chemistry education may support students’ conceptual development in chemistry teaching. Thus, the mode of this research can be characterised as knowledge-generating. The primary forms of validity for knowledge-generating research are outcome and process validity (Newton & Burgess, Citation2008). Pivotal for ensuring both outcome and process validity is the critical and reflectice dialogue between the authors in the analysis of the results (cf. Coe, Citation2013). Throughout the study, in addition to the first author, the second and third author participated in implementation, analysis and documentation of the study. The authors collaborated closely in a critical and reflective dialogue during the process of analysis and the analysis has further been verified in seminars with other researchers. Also, the involvement of Teacher B in Cycle 3 contributes to strengthening the validity with respect to dialogic validity (cf. Newton & Burgess, Citation2008). Dialogic validity is akin to peer-review processes of scientific research but related to the scrutiny of research of other practitionners. In this study, Cycle 3 enabled us to explore the creative drama in a different classroom setting in collaboration with a teacher (Teacher B) who had no prior experience of creative drama. To further assure validity in the form of external validity or transferability we have sought to provide ‘thick descriptions’ of the research lessons (Geertz, Citation1973).
Reliability within qualitative research has to do with wether the results of a qualitative study are dependable and might help us understand a different situation (Golafshani, Citation2003). The intervention in Cycle 3 contributes to the reliability of the study since the results of this study do not only reflect the teaching practices of a teacher who might be considered an expert in using creative drama. The Cycle 1 and Cycle 2 were conducted by teacher A who had previous experience of using creative drama in chemistry teaching, whereas Cycle 3 was conducted by Teacher B who had no prior experience of creative drama. Hence, the teachers who participated in the study had different experiences of working with creative drama in chemistry teaching but were both able to work productively with their students on the tasks included in the research lesson.
Results
The results, which are based on collected data from both group and whole class interactions, are presented in two sub-sections in the form of themes regarding (I) ways of exploring and demonstrating electronegativity and chemical bonding, and (II) the emergence of collective student agency in exploring and demonstrating electronegativity and chemical bonding. The themes are presented in the two sub-sections with empirical examples of how the different themes were expressed in the research lessons.
Part I: ways of exploring and demonstrating electronegativity and chemical bonding
In multiple groups, semiotic work was performed by first using the textbook or notes to draw a structural formula for the molecule. In addition, the students performed semiotic work by transducing verbal instructions directly into the creation of artefacts, which were used to amplify their bodily visualisations of electronegativity and chemical bonding. The students were able to use the bodily mode together with a range of semiotic resources in the form of artefacts such as paper electrons, paper protons and clothes pegs, to solve the task. During the visualisation of hydrogen fluoride molecules, the students struggled to determine whether the covalent bond consisted of one or two electrons. For example, when two students in a group prepared their presentation of the bond in the hydrogen fluoride molecule one of the students said: ‘No, it's not like this, he only has, it's just one (electron) between us’ and the other student answers ‘Yes’, whereby a third group member clarifies that there should be two electrons in the electron pair bond and that they need to hold on to two cloth pegs. In addition, when the students visualised the bond between the atoms, questions arose as to which atom is most electronegative. Hence, the visualisation of electrons enabled the students to explore the intramolecular covalent bond and electronegativity. In addition, the artefact creation in several groups combined with bodily mode facilitated an understanding of the relationship between electronegativity, the polarity of the molecule, the angle of the water molecule and the formation of hydrogen bonding.
Below we describe the themes depicting the different ways of exploring and demonstrating electronegativity and chemical bonding from our analysis of the creative drama activity:
Displaying electronegativity and chemical bonding with anthropomorphic expressions.
Students introduce movements to display electronegativity
Amplifying bodily configurations with other semiotic resources.
Displaying electronegativity and chemical bonding with anthropomorphic expressions
This theme describes how the students used anthropomorphic expressions as a means to create meaning about electronegativity and chemical bonding throughout the creative drama. Human characteristics were thus attributed to the atoms in the form of emotions, sexes, attitudes, physical strength and age.
Several groups visualised electronegativity with happy/positive or negative/sad facial expressions. illustrates how one of the student groups emphasised that ‘(f)luorine (was) the most electronegative atom … ’ while visualising the hydrogen fluoride molecule shown in below.
The most electronegative atom, fluorine, was visualised by the student with a sad (negative) expression while the electropositive hydrogen atom was visualised by the student with a happy (positive) expression. The students thus made use of everyday connotations of positive/negative as happy/sad. To add symbolic meaning, the students put paper signs of coloured paper on themselves to label the atoms they represented. The students visualised the intramolecular covalent bond by linking arms.
Other examples of anthropomorphism in the creative drama included naming the hydrogen atom ‘weakly hydrogen’ because it did not ‘pull’ the electrons. In this group hydrogen was described hydrogen as ‘an old man because he was first’, while oxygen was assigned a property such as ‘strong’ because the atom had the highest electronegativity and ability to ‘most strongly pull’ the electrons. Other student groups attributed human characteristics in the form of different sexes to the atoms or positive and negative attitudes. One student said ‘(y)ou can think of it in different ways. Come up with stories to understand’. The students thus used anthropomorphisms to make narrative meaning of the chemical concepts in the creative drama.
Students introduce movements to display electronegativity
This theme describes how the students introduced movements in several groups when they demonstrated electronegativity with their bodies to the whole class. Conceptually, the students displayed the force between the electrons; in the drama activity, however, this attraction was displayed with the use of moving student bodies. Movement was also pivotal to the collective dramatisation of liquid water.
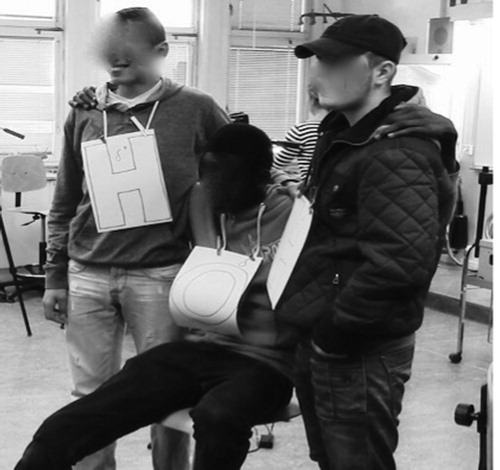
One example came from a group presenting their bodily formation of the water molecule. The group consisted of Anders (seated on the left in the picture, who has drawn the formula of the water molecule structure on paper), Eric (who represented oxygen, who is in the middle of the picture), and Martin and Pela (representing hydrogen atoms, placed on either side of Eric). In the example, Anders began using notes to draw a structural formula of the water molecule on paper to figure out how to visualise electronegativity.
Excerpt 1: Chemical bonding as a continuum
1. Anders: And oxygen has higher electronegativity because it has less distance to the electrons so that it can pull them more. Shows his textual representation drawn on a paper to the audience (see a)
2. Eric grasps his friends’ arms (see b).
3. Anders rolls the paper and looks at the student molecule (See c).
4. Eric: I, I’m pulling the other two, yes, oxygen has higher electronegativity
so, I’m pulling these ones * but never becoming an ion.
*Pulls his friends’ arms closer* (see c).
Amplifying bodily configurations with other semiotic resources
This theme described how all groups reinforced bodily expressions by using additional symbolic representations. The following examples illustrate how the students created and used different artefacts to reinforce bodily configurations. When the group of students worked with their water molecule, they created blue cardboard boxes to represent electron pairs (see ). Eric, the oxygen atom, stands on a chair and holds the blue cardboard boxes together with his peers Ingo and Lars representing hydrogen atoms.
Excerpt 2: Why does the water molecule have an angle?
When the group presented their water molecule to the whole class they said:
5. Eric: I have high electronegativity, so I have the electrons closer to me and become a what is called the negative pole.
6. Lars: ‘Okay, then I’ll ask why * is there an angle on the water molecule?
* points with his hand to Eric, looks at the teacher, smiles and looks at Eric. *
7. Eric: Because there are some electrons * that press, which press down **
* looks at Lars * * * looks towards classmates **
8. Lars: Up * here.
* Pointing and looking at Eric's head, then Ingo *.
9. *Ingo smiles*.
10. Eric: Yes, up here.
11. Eric lifts up his hands and places the cardboard boxes on his head, looks upwards and smiles, then looks towards the class.
12. Everyone laughs.
Several student groups produced artefacts in the form of paper protons to visualise electronegativity (see ). A group of students in the excerpt below were working on the task of visualising a hydrogen fluoride molecule and demonstrating which of the atoms was most electronegative. They started the task by looking at the periodic table on a computer screen and came to the conclusion that fluoride was the most electronegative atom. Then they discussed how they would represent electronegativity and started discussing how they could somehow represent electrons by using coloured paper:
Excerpt 3: Electronegativity depends on the number of protons
13. Lisa: Well, the electrons should be like that * or whatever – actually they are not round. *Pointing to a paper with the chemical sign for fluorine*.
14. Peter: No.
15. Lisa: They are kind of here and there.
16. Christine: Yes, that's how it is.
17. Lisa: But when you draw them, they are.
18. Christine: When you draw them, they are so.
19. Peter: Should I do, should I do minus signs? *
*Points with a pen to the paper he intends to write on*.
20. Britta: Mmm.
21. Lisa: Then we can have plus and minus
(…)
22. Lisa: But it is good to do and show because electronegativity depends on how many protons are in the nucleus.
23. Peter: No, it depends on how far it is between the nucleus and (valence electrons).
24. Lisa: It also depends on how many protons there are in the nucleus.
25. Peter. Yes, ok.
26. Lisa: Because it gets more attraction.
27. Peter. Yeah, that's right, exactly.
(…)
28. Lisa: Okay, but then we should show, in some way, how many protons there are.*
*grabs a red paper*.
When the student group worked with the task of designing a water molecule and showing which atom was the most electronegative while explaining ‘how they thought about it’ they created artefacts that represented the position of the poles on the molecule.
The students created artefacts by using a long piece of tape that they attached to a paper with a plus sign, which the students who performed hydrogen atoms held between them (see ). In their performance the students emphasised that the water molecule was angled and that it had a ‘minus side’ and a ‘plus side’ and that the number of protons affected the electronegativity of the atoms. The oxygen atom, having eight protons, ‘could more effectively pull in the shared valence electrons’ and was thus the most electronegative atom. The visualisation thus enabled the students to explore electronegativity in relation to intramolecular bonding.
Part II: collective student agency in exploring and demonstrating electronegativity and chemical bonding
In the whole-class activities, the creative drama activity provided opportunities for the students to engage in semiotic work, visualising intermolecular chemical bonding and phase transitions. The two themes discerned were as follows:
Resolving epistemic dissonance sparked by bodily prompting.
Participation in directed visualisation and by directing of visualisation.
Resolving epistemic dissonance sparked by bodily prompting
In all groups, the bodily formations of the molecules of their peers prompted semiotic work in other groups. Specifically, when students showed the angle of a water molecule by placing the student representing oxygen upwards or downwards, questions arose in the groups. Hence, the students did not only visualise that the water molecule had an angle, but almost all groups reflected on why there was an angle. The bodily formations of molecules in some groups thus prompted epistemic work in other groups.
Oxygen upwards or downwards?
In one group, from Cycle 2, the students negotiated whether the oxygen would be placed ‘upwards’ or ‘downwards’ in the water molecule. One student in the group suggested how they could perform the molecule placing oxygen upwards. Another student in the group objected that this was wrong, backing the claim with reference to the discursive chemical model of ‘Mickey Mouse’ (where the ‘face’ represents the oxygen atom and the two ‘ears’ represent the hydrogen atoms, which means that the oxygen is placed ‘down’ in the molecule). The objecting student was supported by his peers and the group visualised the molecule with one student representing the oxygen atom sitting on a chair, with the two students representing hydrogen atoms standing on either side (se ).
Later, when other groups posed to take pictures and presented their water molecules to the class, the group was confronted with formations where the oxygen atom was placed upwards (see ). This sparked a renegotiation of the place of oxygen. Later in the lesson, the group changed their visualisation of the water molecule and placed oxygen ‘upwards’. The example illustrates how the drama activity enabled students to grasp one of the limitations of the well-known Mickey Mouse model. The epistemic dissonance that arose among the students created opportunities for the students to revise the ‘up and down’ view of the water molecule.
Polar or non-polar molecule?
Another example concerns how the bodily configurations of one group of students (Laura, Kristin, Anna and Sten) facilitated an exploration of electronegativity and chemical bonding (). Laura and Kristin have placed themselves on chairs while Anna is standing on the floor next to the bench. Laura (on the left chair in the picture) says that ‘it is a polar covalent bond between the oxygen and hydrogen atoms’ (represented by artefacts in the form of yellow papers that are electrons and clothes pegs held in the students’ hands) while Anna pulls her hands towards her body. Sten, ready to take a photo (sitting to the right in the picture), objects that ‘(i)t is not polar’. Laura then explains. ‘Anna pulls them in here most strongly, so that they are closer to her than me’ while Anna shows with her bodily mode how the electrons in the form of the yellow paper come closer to her.
Sten then claims that there will be two negative poles, while Anna disagrees and explains, using her own and her classmate's bodily mode, that there will be two positive poles instead; ‘and then it will be like all electrons down there’. She points to her feet and continues, ‘we have protons and then we become the positive pole’, while pointing to Kristin and herself. Sten then notices that the molecule has one negative pole and two positive poles and exclaims, ‘I don't understand how it is polar. There is only one negative pole, but the positive pole is just like in two places’. After the first group presented their water molecule and emphasised that the water molecule had a positive and negative pole, Sten then asked a girl in another group: ‘do not all, do not all molecules become polar?’, and she responded, ‘not if they have the same atomic number’. The discussion was interrupted because the group itself had to report on the ‘stage’ in the classroom.
During the final task when the students were asked to form liquid water, Sten sat on the bench and looked at his friends on the floor. He asked the girls in the group next to him: ‘Aren’t all molecules polar?’ Sten claimed that the water molecule was symmetrical, but Britta responded, ‘no, he showed that – that's why everyone is in V-formation’ while pointing and nodding towards the student group who were getting ready to perform. Then Sten asks:
Excerpt 4: Sten ‘need to know’
29. Sten: Which substances are polar? *
*Looks toward Lisa and Britta. *
30. Lisa: So … eee.
31. Sten: If oxygen was non-polar and water was polar and carbon dioxide was non-polar …
32. Lisa: If they have free electron pairs, carbon dioxide has no free electrons – have double bond between a carbon atom and oxygen – so then there are no free electrons left and then …
33. Sten: Water also has …
34. Lisa: Water has free electrons left because the hydrogen only needs two so then oxygen has two free electron pairs and they are formed like this … * *Showing together with Britta, hands in the air, the shape of a water molecule and where the electron pairs should be placed. * ()
35. Sten: That's how you know.
36. Lisa: mmm yes exactly.
37. Sten: Yes, I understand.
Participation in directed visualisation and by directing of visualisation
In some parts, the creative drama activity was more or less directed. In some instances, the teacher acted as a director with the students participating in a teacher-directed visualisation of chemical bonding and electronegativity. In other instances, students acted as directors, displaying a strong sense of agency.
Visualisation directed by the teacher
In the whole-class activity, the teacher instructed the students to visualise liquid hydrogen fluoride. The pairs of student molecules moved to form a ‘giant molecule’, connecting their arms with other pairs of students in the shape of a ring. The teacher instructed the students how to place themselves and placed the students’ bodies where she wanted them ().
In the drama activity, paired arms had become an expression to show covalent bonding and the teacher then wanted to show the students how to visualise that hydrogen fluoride was still in a diatomic form when hydrogen fluoride changed phase. Hence, the teacher broke up the ring structure by re-dividing the students into diatomic molecules. The teacher then continued to clarify in verbal mode, on the basis of the students’ bodily mode, how dipole–dipole bonding occurs. The teacher then proceeds to link the dipole–dipole bond to the term hydrogen bond which has not been covered theoretically in previous lessons. For the students, the remaining part of the activity became a lecture where the teacher used the student bodies to talk about hydrogen bonding. When the students were asked to change from a liquid to a gas phase, they were urged by the teacher to run around, two by two and be far apart. The students then participated in the teacher-directed play where the teacher used the student bodies as semiotic resources while verbally explaining why she had placed the bodies as she had.
Students direct the visualisation with peers
The following episode illustrates how students also participated in the direction of the visualisation with their peers.
Excerpt 5: Karl and Elisa like each other – it will be a good ‘match’
The students have formed liquid hydrogen fluoride on the teacher's advice. They walk slowly around the classroom, get closer to each other, and the teacher asks a question.
38. Teacher: Slow down a little – now we try to check. Is there anyone who can help from outside and check if we have any connections between these molecules and what they could possibly be called?
39. Sture: (Student from the ‘audience’) Hydrogen bonds.
40. Teacher: Yes, show such a thing * – where do you find a hydrogen bond?
*Looking at Sture*.
41. Sture: * Between two couples huh? **
*Walks towards the student molecules* (See a).
**Looking at the teacher*.
42. Teacher: Yes, and where and somewhere was ok between two couples … it sounds good …
43. Sture: … because they contain F which is one of NÖFF * NOFF ** … and then to hydrogen.
*Watching the teacher*.
** Pointing between student molecules * (See a).
44. Teacher: Yes, and where somewhere exactly do you think they are or is there someone who … *Rickard is close …
*Looking at Rickard *.
45. Rickard: Karl (to the right in a) and Elisa (to the left in a) like each other.
*Looks at Karl and Elisa*.
46. Teacher: Way? Rickard: Because eee Elisa is negative and Karl positive …
*Elisa and Karl look at each other and smile; then look at Rickard*.
47. Richard: … and then it will be a good match and then Fanny (between Elisa and Karl in b) and Ingmar (to the left in b) also think …
48. *Fanny shows by moving towards Ingmar*
49. Richard: … because Ingmar is positive and Fanny is negative …
Discussion
The analysis of the interventions shows that the creative drama activity facilitated exploration of causes of electronegativity. In some student groups movement was introduced by the students to demonstrate forces of electronegativity acting on the atoms. The movement enabled an exploration of how electronegativity gives rise to the polarity in molecules and how electronegativity and chemical forces are related in a continuum. The drama activity made it possible for the students to link electronegativity to the polarity of the separate molecules (that they had already created in the individual groups) with the polarity in the whole-class interaction and how the intermolecular hydrogen bond may be formed. The creative drama thus allowed the students to work symbolically at the micro level and enable reflections on the two organisational levels. When the collective semiotic work linked electronegativity with the polarity of the molecules, opportunities for students to develop their understanding of intermolecular bonding and phase changes at macro level emerged. Hence, creative drama opened up for the students to distinguish between intra and intermolecular bonding which according to Henderleiter et al. (Citation2001) is the foundation for understanding how intermolecular bonding arise.
Affordances and constraints of ‘coming up with stories’ on chemical bonding
The creative drama activities enabled the students to pay attention to and discuss the limitations of commonly used models in chemistry teaching such as the Mickey Mouse model and ‘NOFF’. The students’ interpretation of models was made visible in action, which enabled the teacher to give the students tools for developing their conceptual understanding. In line with Waldrip et al. (Citation2010), creative drama may offer students opportunities to construct their own representations which may lead to conceptual gains and high level of student's engagement with learning and reasoning regarding electronegativity and chemical bonding. Although the creative drama helped focus the students’ attention on the limitations of some models, the drama activity was based on the use of other models. For example, visualising covalent bonds with connected arms, ran the risk that students would think that electron pairs in molecules hold together because it allows the atoms to receive eight electrons in their outer shell (the octet rule) and that the shared electron pair constituted the bond. Here, chemistry teachers need to be sensitive to the limitations of models, and the risks of oversimplification. According to Danckwardt-Lillieström et al. (Citation2018, p. 264) ‘the bodily models may serve as an anchor for more scientific descriptions and explanations and provide a foundation for students to master the scientific discourse’. The students in this study experienced chemistry teaching with a focus on Bohr's atomic model and bonding divided into separate units. Our results show that students were given an opportunity to understand chemical bonding as a continuum which may be a consequence of the focus on linking electronegativity to chemical bonding in the drama activity. Thus, the results contribute to underscoring the importance of providing an experiential basis for engaging with chemical bonding and that drama may provide such an experiential basis.
Previous research has problematised the use of different anthropomorphic expressions in chemistry education (Bergqvist & Chang Rundgren, Citation2017; Nahum et al., Citation2008; Taber & Coll, Citation2002). In the creative drama, anthropomorphic expressions functioned as a means to create meaning about electronegativity and chemical bonding. Hence, the students used in creative drama what Lemke (Citation1990) calls ‘humanised’ language compared to a ‘normal’ natural science language. As one student pointed out, it may be beneficial to ‘come up with stories to understand’ which demonstrates that the students are aware that the stories function as a tool for learning. Drawing on Manneh et al. (Citation2019), the anthropomorphic expressions may help students to better understand electronegativity and chemical bonding, which, with the help of the teacher, can then be developed to align with scientific discourse. However, potentially ambiguous or incorrect conceptions might arise from the bodily visualisation. Thus, it is highly important that a teacher organising drama activities are aware of limitations of simplified models and act to provide students with opportunities to react on incorrect or limited models (cf. Danckwardt-Lillieström et al., Citation2018).
Student agency
A distinctive feature of the creative drama was that a need arose for students ‘to know why’ in all research lessons. Drawing on the dialectical lens of agency/structure, the drama activity enabled both the material structure and the social structure to enhance the students’ agency (King, Citation2012). For example, in the first case students created their own artefacts while using their own bodies to visualise electronegativity and chemical bonding. In the latter, the structure of small group work may have provided the students with opportunities to exercise agency as they worked with creative drama. When the students were asked to demonstrate the intermolecular bond between the molecules collectively, they discussed, negotiated and acted as learning resources for each other. The students who demonstrated an understanding of how water molecules bond in the liquid phase acted with agency, which was observed in their verbal and non-verbal actions as they organised and operated as learning resources. The students who did not know how to position themselves in relation to the other students, demonstrated through bodily movements their willingness and openness to learning. This is in line with previous research emphasising that creative drama may enhance understanding of scientific concepts for low- as well as average- and high- achievement students (Aubusson et al., Citation1997; Osama, Citation2016).
The visualisation of the students’ bodily formations created opportunities for students to pay attention to differences in representations of chemical bonding, which got consequences for the students’ collective agency. The emergent epistemic tensions/contradictions that become recognised by the students in the creative drama constituted opportunities for collective work concerning the conceptual relations where students acted as learning resources for one another. For example, verbal and non-verbal actions of Sten displayed a conscious willingness and openness to learning from his peers. This may be understood as an instance of agency in line with what Hwang and Roth (Citation2009) emphasised as pivotal to enable learning.
During the third Cycle research lessons, the students were invited to engage in collective semiotic work. The teacher provided the social structure that facilitated a joint exploration of how electronegativity is linked to intermolecular bonding. Drawing on the dialectical lens of agency/structure, in the first case the social structure did not favour students’ collective agency, whereas in the second the teacher provided the students with more space for conceptual exploration. Hence, when the students directed the drama they enacted both personal and collective agency.
In conclusion, our findings indicate that using creative drama as a teaching strategy in chemistry education may create opportunities for agentic students’ engagement in chemistry. In line with previous research, we have shown that creative drama may enable students to deepen their understanding of scientific concepts (Arieli, Citation2007; Ødegaard, Citation2015; Osama, Citation2016). Further our findings show that there is potential for creative drama to facilitate collective student agency in quite abstract theoretical explorations of chemical concepts and conceptual relations such as electronegativity, intramolecular and intermolecular bonding. However, continued research is needed to further explore how the structural and material resources of creative drama afford agency in chemistry learning. Of particular theoretical interest for further research are questions concerning the entanglement of epistemic dissonance and agency and questions concerning under what conditions epistemic dissonance might afford agency, and vice versa, in relation to science teaching pedagogies drawing on drama, imagination and play (cf. Andrée & Lager-Nyqvist, Citation2013).
Generalisation of the results
Drawing on Larsson (Citation2009), one line of generalisation is generalisation through contextual similarity and recognition of patterns. The multimodal analysis in this study is based on a rich empirical material that has enabled a so-called thick description (Geertz, Citation1973), which creates a basis for the reader (for example teachers) to draw conclusions about the context similarity and recognising the patterns of our results in their own classrooms. Contextual similarities may occur both within the same area (electronegativity and chemical bonding), across areas in Chemistry, across other science subjects, or even across other school subjects. The research reported includes description and interpretation of processes that have emerged from situations with human activity. We do not claim that the themes discerned in this study will always occur in context-like situations but that the communicative patterns discerned may be recognised in new cases. This includes, for example, how students may use semiotic resources when interacting in creative drama activities and the potential of epistemic dissonance to create opportunities for conceptual learning. Further research is needed to investigate how creative drama may be refined to further support student learning of chemical bonding and in other areas of Chemistry as well as other school subjects and educational levels.
Design principles for creative drama in chemistry education
Based on the results presented in this article and previous research (Danckwardt-Lillieström et al., Citation2018) we suggest the following principles for designing creative drama intended to support students’ exploration of electronegativity and chemical bonding:
Design Principle 1: Create opportunities for students to work symbolically at both the sub-micro and macro levels and enable reflections at different organizational levels by offering students different semiotic resources as they materialize scientific concepts and processes with their bodies. Our findings indicate that in order for students to be given opportunity to conceptually link electronegativity with the polarity of the molecules and intermolecular bonding, the teaching should enable reflections at the two organizational levels (micro and macro) and opportunities in collective semiotic work to investigate phase changes at the macro level. When teaching chemical bonding through creative drama it seems beneficial for the students’ conceptual learning to provide opportunities for students to explore conceptual relationships by means of a range of semiotic modes, including bodily mode. Hence, students are provided an experiential basis for engaging with chemical bonding and differences in the visualisations may afford the emergence of productive epistemic dissonance.
Design Principle 2: Create opportunities for students to interact with each other and the teacher in an environment where students’ collective agency is promoted. The results show that visualisation of the students’ bodily formations created opportunities for students to pay attention to differences in representations of chemical bonding and afforded collective agency. In this study, the emergent epistemic tensions/contradictions that were recognized by the students in the creative drama constituted opportunities for collective work concerning the conceptual relations where students acted as learning resources for one another. Thus, drama activity may create opportunities for students to work at a collective level with the conceptual relations of chemical bonding. A prerequisite is that teachers provide sufficient space and time for students to interact with each other and to discuss how chemical concepts could be performed differently in the drama activity.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alrutz, M. (2004). Granting science a dramatic license: Exploring a 4th grade science classroom and the possibilities for integrating drama. Teaching Artist Journal, 2(1), 31–39. https://doi.org/10.1207/S1541180XTAJ0201_6
- Andrée, M. (2012). Altering conditions for student participation and motive development in school science: Learning from Helena’s mistake. Cultural Studies of Science Education, 7(2), 425–438. https://doi.org/10.1007/s11422-011-9314-x
- Andrée, M., & Lager-Nyqvist, L. (2013). Spontaneous play and imagination in everyday science classroom practice. Research in Science Education, 43(5), 1735–1750. https://doi.org/10.1007/s11165-012-9333-y
- Arieli, B. (2007). The integration of creative drama into science teaching [unpublished doctoral thesis]. Kansas state university, College of education.
- Aubusson, P., Fogwill, S., Barr, R., & Perkovic, L. (1997). What happens when students do simulation-role-play in science? Research in Science Education, 27(4), 565–579. https://doi.org/10.1007/BF02461481
- Bergqvist, A., & Chang Rundgren, S.-N. (2017). The influence of textbooks on teachers’ knowledge of chemical bonding representations relative to students’ difficulties understanding. Research in Science & Technological Education, 35(2), 215–237. https://doi.org/10.1080/02635143.2017.1295934
- Bergqvist, A., Drechler, M., De Jong, O., & Chang Rundgren, S. N. (2013). Representations of chemical bonding models in school textbooks-help or hindrance for understanding? Chemistry Education Research and Practice, 14(4), 589–606. https://doi.org/10.1039/C3RP20159G
- Biesta, G., & Tedder, M. (2007). Agency and learning in the lifecourse: Towards an ecological perspective. Studies in the Education of Adults, 39(2), 132–149. https://doi.org/10.1080/02660830.2007.11661545
- Braund, M. (2013). Critical episodes in student teachers’ science lesson using drama in grades 6 and 7. Mathematics, Science and Technology Education, 17(1-2), 4–13. https://doi-org.ezp.sub.su.se/10.1080/10288457.2013.826966
- Braund, M. (2015). Drama and learning science: An empty space? British Educational Research Journal, 41(1), 102–121. https://doi.org/10.1002/berj.3130
- Burrows, N. L., & Reid Mooring, S. (2015). Using concept mapping to uncover students’ knowledge structures of chemical bonding concepts. Chemistry Education Research and Practice, 16(1), 53–66. https://doi.org/10.1039/C4RP00180J
- Caiman, C., & Lundegård, I. (2014). Pre-school children’s agency in learning for sustainable development. Environmental Education Research, 20(4), 437–459. https://doi.org/10.1080/13504622.2013.812722
- Cobb, P., Confrey, J., diSessa, A., Lehrer, R., & Schauble, L. (2003). Design experiments in educational research. Educational Researcher, 32(9), 9–13. https://doi.org/10.3102/0013189X032001009
- Cobb, P., & Gravemeijer, K. (2006). Design research from a learning design perspective. In J. Akker, K. Gravemeijer, S. McKenney, & N. Nieveen (Eds.), Educational design research (pp. 45–85). Routledge.
- Coe, R. J. (2013). Conducting your research. In J. Arthur, M. Waring, R. Coe, & L. Hedges (Eds.), Research methods and methodologies in education (pp. 41–52). SAGE Publications Ltd.
- Danckwardt-Lillieström, K., Andrée, M., & Enghag, M. (2018). Creative drama in chemistry education: A social semiotic approach. Nordic Studies in Science Education, 14(3), 250–266. https://doi.org/10.5617/nordina.5869
- Danielsson Thorell, H., & Johansson, E. (2016). Reaktion 1 Kemi 1 Gymnasiet & Vux. Natur & Kultur.
- Davydov, V. V., Slobodchikov, V. I., & Tsukerman, G. A. (2003). The elementary school student as an agent of learning activity. Journal of Russian and East European Psychology, 41(5), 63–76. https://doi.org/10.2753/RPO1061-0405410563
- De Jong, O., Blonder, R., & Oversby, J. (2013). How to balance chemistry education between observing phenomena and thinking in models. In I. Eilks & A. Hofstein (Eds.), Teaching chemistry: A studybook (pp. 97–126). Sense Publishers.
- The Design-Based Research Collective. (2003). Design-based research: An emerging paradigm for educational inquiry. Educational Researcher, 32(1), 5–8. https://doi.org/10.3102/0013189X032001005
- Dorion, K. R. (2009). Science through drama: A multiple case exploration of the characteristics of drama activities used in secondary science lessons. International Journal of Science Education, 31(16), 2247–2270. https://doi.org/10.1080/09500690802712699
- Erlingsson, C., & Brysiewicz, P. (2017). A hands-on guide to doing content analysis. African Journal of Emergency Medicine, 7(3), 93–99. https://doi.org/10.1016/j.afjem.2017.08.001
- Geertz, C. (1973). Thick description: Toward an interpretive theory of culture. In C. Geertz (Ed.), The interpretation of cultures (pp. 310–323). Basic Books.
- Gilbert, J. K., & Treagust, D. V. (Eds.). (2009). Multiple representations in chemistry education. Springer.
- Golafshani, N. (2003). Understanding reliability and validity in qualitative research. The Qualitative Report, 8(4), 597–607. http://www.nova.edu/ssss/QR/QR8-4/golafshani.pdf
- Goulart, M. I., & Roth, W.-M. (2010). Engaging young children in collective curriculum design. Cultural Studies of Science Education, 5(3), 533–562. https://doi.org/10.1007/s11422-009-9196-3
- Graneheim, U. H., & Lundman, B. (2004). Qualitative content analysis in nursing research: Concepts, procedures and measures to achieve trustworthiness. Nurse Education Today, 24(2), 105–112. https://doi.org/10.1016/j.nedt.2003.10.001
- Harrison, A. G., & Treagust, D. F. (1996). Secondary students’ mental models of atomic and molecules: Implications for teaching chemistry. Science Education, 80(5), 509–534. https://doi.org/10.1002/(SICI)1098-237X(199609)80:5<509::AID-SCE2>3.0.CO;2-F
- Harrison, A. G., & Treagust, D. F. (2002). The particulate nature of matter: Challenges in understanding the microscopic world. In J. K. Gilbert, O. De Jong, R. Justi, D. F. Treagust, & J. H. Van Driel (Eds.), Chemical education: Towards research-based practice (pp. 189–212). Kluwer Academic Press.
- Henderleiter, J., Smart, R., Anderson, J., & Elian, O. (2001). How do organic chemistry students understand and apply hydrogen bonding? Journal of Chemical Education, 78(8), 1126–1130. https://doi.org/10.1021/ed078p1126
- Hendrix, R., Eick, C., & Shannon, D. (2012). The integration of creative drama in an inquiry-based elementary program: The effect on student attitude and conceptual learning. Journal of Science Teacher Education, 23(7), 823–846. https://doi.org/10.1007/s10972-012-9292-1
- Hewson, M. (2010). Agency. In A. Mills, G. Durepos, & E. Wiebe (Eds.), Encyclopedia of case study research (pp. 13–17). SAGE.
- Hwang, S., & Roth, W.-M. (2009). Language and experience of self in science and transnational migration. In W.-M. Roth (Ed.), Science education from people for people: Taking a stand(point) (pp. 1–15). Routledge.
- Johnstone, A. (1982). Makro and mikrochemistry. School Science Review, 64(227), 377–379.
- King, D. (2012). New perspectives on context-based chemistry education: Using a dialectical sociocultural approach to view teaching and learning. Studies in Science Education, 48(1), 51–87. https://doi.org/10.1080/03057267.2012.655037
- Kozma, R. B., & Russel, J. (1997). Multimedia and understanding: Expert and novice responses to different representations of chemical phenomena. Journal of Research in Science Teaching, 34(9), 949–968.https://doi.org/10.1002/(SICI)1098-2736(199711)34:9
- Larsson, S. (2009). A pluralist view of generalization in qualitative research. International Journal of Research & Method in Education, 32(1), 25–38. https://doi.org/10.1080/17437270902759931
- Lemke, J. L. (1990). Talking science: Language, learning and values. Alex Publishing Corporation.
- Manneh, I., Hamza, K. M., Rundgren, C. J., & Eriksson, L. (2019). The role of anthropomorphisms in students’ reasoning about chemical structure and bonding. Asia-Pacific Forum on Science Learning and Teaching, 19(2), Article 4.
- Mortimer, E., & Scott, P. (2006). Meaning making in secondary science classroom. Open University Press.
- Nahum, T. L., Mamlok-Naaman, R., Hofstein, A., & Kronik, L. (2008). A new “bottom-up” framework for teaching chemical bonding. Journal of Chemical Education, 85(12), 1680–1685. https://doi.org/10.1021/ed085p1680
- Nahum, T. L., Mamlok-Naaman, R., Hofstein, A., & Taber, K. S. (2010). Teaching and learning the concept of chemical bonding. Studies in Science Education, 46(2), 179–207. https://doi.org/10.1080/03057267.2010.504548
- Newton, P., & Burgess, D. (2008). Exploring types of educational action research: Implications for research validity. International Journal of Qualitative Methods, 7(4), 18–30.
- Nicoll, G. (2001). A report of undergraduates’ bonding misconceptions. International Journal of Science Education, 23(7), 707–730. https://doi.org/10.1080/09500690010025012
- Ødegaard, M. (2003). Dramatic science. A critical review of drama in science education. Studies in Science Education, 39(1), 75–101. https://doi.org/10.1080/03057260308560196
- Ødegaard, M. (2015). Science theater/drama. In R. Gunstone (Ed.), Encyclopedia of science education (pp. 928–930). Springer.
- Osama, H. A. (2016). Drama-based science teaching and its effect on students’ understanding of scientific concepts and their attitudes towards science learning. International Education Studies, 9(10), 163–173. https://doi.org/10.5539/ies.v9n10p163
- Osterkamp, U. (1999). On psychology, ideology and individuals’ societal nature. Theory & Psychology, 9(3), 379–392. https://doi.org/10.1177/0959354399093007
- Othman, J., Treagust, D. F., & Chandrasegaran, A. L. (2008). An investigation into the relationship between students’ conceptions of the particulate nature of matter and their understanding of chemical bonding. International Journal of Science Education, 30(11), 1531–1550. https://doi.org/10.1080/09500690701459897
- Peterson, R. F., & Treagust, D. F. (1989). Grade-12 students’ misconceptions of covalent bonding and structure. Journal of Chemical Education, 66(6), 459–460. https://doi.org/10.1021/ed066p459
- Roth, W.-M. (2007). Theorizing passivity. Cultural Studies of Science Education, 2(1), 1–8. https://doi.org/10.1007/s11422-006-9045-6
- Schmidt, H.-G., Kaufmann, B., & Treagust, D. F. (2009). Students’ understanding of boiling points and intermolecular forces. Chemistry Education Research and Practice, 10(3), 219–226. https://doi.org/10.1039/b914501j
- Seifeddine Ehdwall, D., & Wickman, P.-O. (2018). Hur lärare kan stödja andraspråkselever på gymnasiet att tala kemi. Nordic Studies in Science Education, 14(3), 299–316. https://doi.org/10.5617/nordina.5870
- Sewell, W. H. (1992). A theory of structure: Duality, agency and transformation. American Journal of Sociology, 98(1), 1–29. https://doi.org/10.1086/229967
- Swedish National Agency of Education. (2011). Upper secondary education. Curriculum. Retreived from the Internet March 27, 2019. https://www.skolverket.se/undervisning/gymnasieskolan/laroplan-program-och-amnen-i-gymnasieskolan/gymnasieprogrammen/amne?url=1530314731%2Fsyllabuscw%2Fjsp%2Fsubject.htm%3FsubjectCode%3DKEM%26tos%3Dgy&sv.url=12.5dfee44715d35a5cdfa92a3
- Swedish National Agency of Education. (2020). Upper-secondary school – student statistics. Retrieved June 29, 2020, from https://www.skolverket.se/skolutveckling/statistik/sok-statistik-om-forskola-skola-och vuxenutbildning?sok=SokA&vform=21&kommun=0180&ar=2017&flik=s&one=29423248&run=1
- Swedish Research Council. (2017). Good research practice.
- Taber, K. S. (2002). Chemical misconceptions-prevention, diagnosis and cure: Theoretical background (Vol. 1). Royal Society of Chemistry.
- Taber, K. S., & Coll, R. K. (2002). Bonding. In J. K. Gilbert, O. De Jong, R. Justi, D. F. Treagust, & J. H. Van Driel (Eds.), Chemical education: Towards research-based practice (pp. 213–234). Kluwer Academic Press.
- Taber, K. S., Tsaparlis, G., & Nakiboglu, C. (2012). Student conceptions of ionic bonding: Patterns of thinking across three European contexts. International Journal of Science Education, 34(18), 2843–2873. https://doi.org/10.1080/09500693.2012.656150
- Venkataraman, B. (2009). Visualization and interactivity in the teaching of chemistry to science and non-science students. Chemistry Education Research and Practice, 10(1), 62–69. https://doi.org/10.1039/B901462B
- Waldrip, B., Prain, V., & Carolan, J. (2010). Using multi-modal representations to improve learning in junior secondary science. Research in Science Education, 40(1), 65–80. https://doi.org/10.1007/s11165-009-9157-6
- Watts, M., & Bentley, D. (1994). Humanizing and feminizing school science: Reviving anthropomorphic and animistic thinking in constructivist science education. International Journal of Science Education, 16(1), 83–97. https://doi.org/10.1080/0950069940160106
- Wu, K., Krajcik, E., & Soloway, E. (2001). Promoting understanding of chemical representations: Students’ use of a visualization tool in the classroom. Journal of Research in Science Teaching, 38(7), 821–842. https://doi.org/10.1002/tea.1033
- Yoon, H.-G. (2006). The nature of science drama in scientific education. The 9th International Conference on Public Communication of Science and Technology. Chun-cheon national University of Education, Korea.
- Zhang, Z. H., & Linn, M. C. (2011). Can generating representations enhance learning with dynamic visualizations? Journal of Research in Science Teaching, 48(10), 1177–1198. https://doi.org/10.1002/tea.20443