 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Cubic rocksalt structured transition-metal carbides, nitrides (TMC/Ns), and related alloys, are attractive for a wide variety of applications, notably as hard, wear-resistant materials. To-date, valence electron concentration (VEC) is used as a good indicator of stability and mechanical properties of these refractory compounds. In this perspective, we argue for the need of electronic descriptors beyond VEC to explain and predict the mechanical behaviour of the cubic TMC/Ns. As such, we point out that descriptors that highlight differences between constituents, along with semi-empirical models of mechanical properties, have been underused. Additionally, it appears promising to partition VEC into contribution to ionic, covalent, and metallic bonds and we suggest that such partition could provide more insights into predicting mechanical properties in the future.
Transition-metal carbides and nitrides (TMC/Ns), especially those made of group 4, 5, and 6 elements, possess remarkable properties: they are some of the hardest (tens of GPa), high moduli (>300 GPa), and highest melting point solids (see ) with high-temperature mechanical strengths, high electrical and thermal conductivities, and excellent wear, ablation, and corrosion resistance [Citation1–4]. These hard refractory ceramics, owing to a mixture of ionic, covalent, and metallic bonding, form a technologically important class of materials widely used as protective coatings on cutting tools [Citation5] and as structural components in aerospace vehicles, hypersonic jets, and other systems operating in extreme environments [Citation6–8]. These materials are also of interest as catalysts [Citation9,Citation10], energy storage materials [Citation11], and as diffusion barriers in electronics [Citation12]. Continued research in this class of materials stems from the necessity to discover and develop new materials with improved mechanical properties for a variety of structural applications.
Table 1. Melting point, elastic modulus E, and hardness H of a few selected TMC/Ns, from Refs. [Citation3,Citation4,Citation13,Citation14].
Alloying is probably the most commonly used approach to tailor the mechanical properties of materials. Classical examples of alloying include gold jewellry, bronze, brass, and stainless steel. The role of alloying elements on mechanical behaviour of metallic materials [Citation15], as well as simple descriptors of structural stability, such as the valence electron concentration (VEC), have been widely reported in the literature [Citation16,Citation17]. The VEC, a simple but fundamental parameter, describes well the effects of alloying on the structure and mechanical properties of metals. The insights gained from these studies on metal alloys are often transferred to ceramic materials [Citation18,Citation19], even though the chemical bonding and crystal structures, which are fundamental parameters behind the mechanical behaviour of materials, are quite different in these compounds compared to metals. The effect of alloying on the mechanical properties of TMC/Ns is still not well understood since systematic studies are scarce in comparison to those on metals. Therefore, our ability to predict and design TMC/Ns alloys with desired mechanical properties has been rather limited. Only recently, Brenner, Curtarolo, Sangiovanni and co-workers [Citation20–30] have developed strategies for predicting the stability and properties of multicomponent, high-entropy TMC alloys.
Over the past decades, a major research goal has been to enhance the hardness of protective coatings for various applications. Among the TMC/Ns, probably group 4 and group 4 based alloys (e.g. TiAlN) have been the most extensively studied [Citation31–35]. In quest of ultra-hard coatings, initial studies focused on incorporating smaller atoms (B, Al, and Si) into the TMC/N lattices to promote stronger bonding and, hence, further enhance the hardness of these ceramics [Citation5,Citation6,Citation35–38]. However, the increase in hardness is also followed by an increase in brittleness. Therefore, while high hardness is essential, focusing on increasing it alone at the expense of decreasing the ductility is not sufficient for most applications. For example, in the cutting tool and aerospace industries, where the materials are exposed to high thermo-mechanical stresses, increasing the degree of plastic deformation upon yielding (in addition to increasing the strength) is crucial to avoid brittle failure. Therefore, many applications require materials with high modulus, high strength, and large plastic strain in order to increase the lifetime of any structural component. Efforts have aimed at enhancing the ductility of these refractory compounds [Citation39–42] leading to the fabrication of tough ceramics. These approaches include the incorporation of ductile phases and nanoscale grains, or the fabrication of multilayered structures [Citation40,Citation43,Citation44]. However, most of the methods developed to improve the ductility of hard ceramics are based on trial-and-error approaches with very few reports focusing on the electronic origins of hardness vs ductility [Citation18,Citation19,Citation32,Citation45–56]. Attaining both, high strength and large plastic strain ceramics, is a long-standing challenge. Therefore, despite their exceptional strength, the use of TMC/Ns coatings, for instance, in low-temperature structural applications has been relatively limited. If we can increase their ductility while retaining their high strength, their applications could be extended. Thus, gaining insight into the effect of alloying at the electronic level is crucial to design hard-yet-tough ceramics.
For the B1-structured TMC/Ns, existing reports [Citation13,Citation14,Citation29,Citation57–59] have already provided some guidelines on how mechanical stability and properties such as elastic and shear moduli, and hardness, vary with the electron density in the d-t2g orbitals. In these compounds, the strong p(N,C)-d-eg(metal) first-neighbour bonds are responsible for the material's strength, while the relatively weaker metallic metal–metal d-t2g second-neighbour interactions are behind the material's ductility/plasticity. Since the occupation of the d-t2g states is related to the VEC, most studies have used VEC to compare and contrast the mechanical properties of different TMC/N alloys [Citation4,Citation19,Citation45,Citation47]. Holleck [Citation6] and Jhi et al. [Citation19,Citation60,Citation61] reported that maximum hardness in cubic TMC/Ns is obtained at a VEC of ∼8.4 electrons, due to the complete filling of the shear-resistive p-deg orbitals. At higher VEC, the shear sensitive d-t2g orbitals begin to be filled reducing the shear-resistance of the material and, consequently, reducing their hardness. Sangiovanni et al. [Citation47,Citation59] and others [Citation45,Citation62] used a similar approach to study how toughness can be enhanced by increasing the VEC. For clarity, we state that the ductility region is often specified by a Pugh’s ratio, the ratio of shear modulus G and bulk modulus B, G/B below 0.5 and a Poisson ratio ν above 0.28 [Citation4]. Following these density functional theory (DFT) predictions, Kindlund and co-workers [Citation14,Citation57] performed an experimental study and investigated the mechanical behaviour of pseudobinary B1-structured group 5 + 6 transition-metal alloy nitrides, VMoN and VWN [Citation13,Citation63–65]. They demonstrated that the introduction of group 6 elements (Mo and W) in group 5 nitride (VN) increases the ductility, while retaining the strength, i.e. these alloys exhibited enhanced toughness. VMoN (with VEC ∼ 10.5) is tougher than the parent binary compound VN and the reference group 4 nitride, TiN (VEC = 9). The combination of theoretical predictions and DFT-inspired experimental work laid the foundation for rational design of refractory TMNs with the most sought-after combination of mechanical properties in ceramic materials: the combination of high hardness and high ductility, i.e. toughness.
DFT computational work carried out over a wider range of pseudobinary TMC/N and carbonitrides (see data in ) [Citation4,Citation19,Citation59] concluded that these alloys are stable in the B1-structure if the VEC < 10.6, ductility increases with increasing VEC (but hardness decreases), and maximum toughness is achieved for alloys with VECs in the range 9.5–10.5. While VEC serves as a good initial indicator of mechanical properties, it alone is not sufficient to explain and predict the mechanical behaviour of TMC/Ns, in which the primary bonds are non-metallic. Iso-VEC TMC/N alloys (i.e. alloys with the same VEC) may exhibit very different mechanical behaviours. Worthy of note are, for example, the scattered values of the hardness H and elastic moduli E for the different alloys with a VEC of 10.5 (highlighted in ). TMC/N alloys with a VEC of 10.5 may be attained with alloys of group 5 + 6 nitrides (e.g. V0.5Cr0.5N, Ta0.5W0.5N, etc.) or group 6 carbonitrides (e.g. CrC0.5N0.5, MoC0.5N0.5, & WC0.5N0.5). H values for these iso-VEC alloys vary by over a factor of 3 from ∼2 GPa for Ta0.5W0.5N to ∼7 GPa for CrC0.5N0.5, while the E values vary two-fold from ∼150 GPa to ∼300 GPa for the same set of compounds. That is, the mechanical properties of TMC/Ns with the same VEC vary with cation and anion composition and are different for unrelated TMC/N alloys that share the same VEC. Zhang et al. [Citation66] address this issue by taking into account core electron count as a new descriptor.
Figure 1. Trends in DFT-calculated elastic modulus E and hardness H vs. VEC of B1-structured pseudobinary TMC/Ns (adapted with permission from Ref. [Citation4]). The scatter in E and H values of isoVEC alloys with VEC = 10.5 is highlighted using dashed orange ellipses.
![Figure 1. Trends in DFT-calculated elastic modulus E and hardness H vs. VEC of B1-structured pseudobinary TMC/Ns (adapted with permission from Ref. [Citation4]). The scatter in E and H values of isoVEC alloys with VEC = 10.5 is highlighted using dashed orange ellipses.](/cms/asset/c36d4054-cb9f-4d40-a633-ec3d2d2c7034/tphl_a_2358205_f0001_oc.jpg)
Furthermore, the trends predicted by DFT do not always seem to agree with the experimentally observed behaviour, see for example, data in from Refs. [Citation13,Citation65]. The observed increase in hardness of V1-xWxN(001) alloy films with increasing VEC (due to increasing W content) is not consistent with the DFT predicted trend. Similar inconsistencies between experimental results and predictions have also been observed in V1-xMoxN(001) alloys [Citation65] and group 4 carbonitrides [Citation67].
Figure 2. V1–xWxNy(001) film hardness H vs. x; data from Ref. [Citation13]. The increase in H with increasing W content x, i.e. increasing VEC is in direct contrast with the DFT predicted trend of hardness vs VEC in Ref. [Citation4].
![Figure 2. V1–xWxNy(001) film hardness H vs. x; data from Ref. [Citation13]. The increase in H with increasing W content x, i.e. increasing VEC is in direct contrast with the DFT predicted trend of hardness vs VEC in Ref. [Citation4].](/cms/asset/7bbb3941-ed93-491f-b4af-0e4a32a6f135/tphl_a_2358205_f0002_oc.jpg)
As justified below, research in the field in the near future will have to focus on the following question: given a set of transition-metal carbides and/or nitrides with the same valence electron concentration (iso-VEC alloys or compounds), can we fundamentally understand their properties (e.g. strength, ductility) based on their cation and anion compositions?
An alternative approach to understanding the mechanical behaviour of a material is to look at its structural stability. In any structural alloy, including high-entropy alloys, the increase in ductility and accompanying decrease in strength with increasing VEC are attributed to change in the material's crystal structure, for example, from body-centred cubic (bcc) to face-centred cubic (fcc) [Citation68]. In TMC/N, alloying can lead to the coexistence of several energetically equivalent phases, which can suppress dislocation motion and, thus, increase the material's strength [Citation26]. For 4d TM nitrides, such multiphase structures are expected to form at VECs around 9.6 [Citation27]. Clearly, it is necessary to go beyond simple descriptors such as VEC to understand the mechanical behavior in this class of materials with a mixture of ionic, covalent, and metallic bonding. We suggest below several approaches aimed at the development of beyond-VEC models for understanding the mechanical properties of B1-phase TMC/Ns, specifically those with iso-VEC.
As a step towards fundamental understanding of what controls the mechanical properties of rocksalt TMC/Ns that have the same value of VEC, one can start investigating ‘easy' parameters of the anions and cations that make up the alloy’s composition. These easy parameters (also called features in machine learning lingo) are usually computed from tabulated quantities specific to each anion or cation, and can be readily considered as potential descriptors of most properties of any complex alloys (not necessarily rocksalt-structured), ranging from the stability of single phases [Citation69] to the catalytic activity [Citation70]. From the ionic radii, atomic radii, electronegativities, Lewis acid strengths, lattice constants, cell volumes, etc. associated with each cation or with each parent structure (end member) that makes up the TMC/N alloy, we can derive various quantities associated with the alloy [Citation71], for example average and standard deviation for ionic radius of the cations; ionic radius for the anions; electronegativity of anions; electronegativity of cations; cell volume of end members, etc. We illustrate in some of these descriptors which have recently been tested for predicting the stability of single-phases of TMCs with multiple cations [Citation69].
Figure 3. Feature selection for designing single-phase high-entropy carbides (From Ref. [Citation69]). ΔHmix, ΔVmix, and ΔSmix are enthalpy, volume change, and entropy of mixing per formula unit, respectively. σi is the deviation of constituent TMCs in i = average volume V, mass m, density ρ, electronegativity χ, and VEC. The values in the squares are the Pearson correlations, and they are listed so that highly correlated features can be avoided when establishing machine learning models.
![Figure 3. Feature selection for designing single-phase high-entropy carbides (From Ref. [Citation69]). ΔHmix, ΔVmix, and ΔSmix are enthalpy, volume change, and entropy of mixing per formula unit, respectively. σi is the deviation of constituent TMCs in i = average volume V, mass m, density ρ, electronegativity χ, and VEC. The values in the squares are the Pearson correlations, and they are listed so that highly correlated features can be avoided when establishing machine learning models.](/cms/asset/846d832d-c2d3-4fbb-93b3-69bef51dc842/tphl_a_2358205_f0003_oc.jpg)
A closer look at shows that VEC is strongly correlated with the average electronegativity of the TMC constituents (end-members), and that the standard deviation of the VEC of constituents is also correlated reasonably well with the standard deviation of the electronegativities,
. These observations suggest that for isoVEC TMC/Ns, the discriminant factor that leads to the spread in mechanical properties () can very likely be related to the standard deviation of VEC, standard deviation of electronegativity, or some other measure of the variation (or spread) of VEC or variation of electronegativity of the constituent TMC/Ns. While the purpose of this perspective article is merely to point out possible ways in which the use of VEC can be enhanced by looking at other descriptors of mechanical properties (as opposed to computing the performance of these descriptors), in we plot the hardness of TMC/Ns as computed by Balasubramanian et al. [Citation4] versus
the difference in the average electronegativities of the cations and anions of the constituent TMC/Ns. As shown in , a reasonable case can be made that for iso-VEC TMC/N alloys, the hardness is controlled by
.
Figure 4. Correlation of isoVEC hardness H with electronegativity difference, The hardness data is from Ref. [Citation4], while
is defined as the difference between the weighted average of the cation and anion electronegativities.
![Figure 4. Correlation of isoVEC hardness H with electronegativity difference, Δχ¯. The hardness data is from Ref. [Citation4], while Δχ¯ is defined as the difference between the weighted average of the cation and anion electronegativities.](/cms/asset/d2ff7d3b-445d-4a68-9996-b1b89e5d5583/tphl_a_2358205_f0004_oc.jpg)
It is our hope that many other spread-type of descriptors (i.e. descriptors that highlight the differences between the constituent TMC/Ns, rather than average over them) would be tested in the near future. As shown in , those are likely to account for the variation of properties for iso-VEC TMC/Ns, with the caveat that perhaps different spread-type descriptors would perform differently for various mechanical properties. Before machine learning approaches, there were certain empirical formulations that showed significant value in designing hard materials. For example, Simunek [Citation72] designed a semiempirical model based on coordination numbers, interatomic distances, valence electrons, volume containing valence electrons, etc. of each atom in the solid, and proposed a bond strength model and a hardness model based on these quantities. The approach [Citation72] was not only simple and insightful, but also remarkably accurate, and was used by others to propose a range of super tough materials for further investigations at the level of DFT [Citation59]. Therefore, in addition to the use of spread-type descriptors mentioned above, we encourage the exploration of semiempirical formulations for mechanical properties other than hardness, in particular for the Pugh’s ratio, which can be used to classify the brittle versus ductile behaviour of materials [Citation73].
Lastly, we posit as a central hypothesis for future work that the mechanical behaviour of transition-metal carbides and nitrides can be understood based on the specific contributions of the VEC to different types of bonding: ionic, covalent, and metallic as distinguishing factors between alloys with the same VEC but different compositions and mechanical properties. Similar to the concept of the spread-type of descriptors discussed above, it is intuitively reasonable to expect that for isoVEC materials the partition of the valence electrons among ionic, covalent, and metallic bonding becomes important. However, this partition is not of the ‘easy' type described above, in the sense that it cannot be derived from tabulated properties of cations or anions. Rather, it has to be computed from DFT calculations, and we outline below a tentative procedure for such determination; improvements, validation, and testing would hopefully come from future work.
In refractory ceramics, the primary bonds are not metallic, as they occur between the TM and the C (or N) atom. In other words, it is not just about how many electrons are packed in a unit cell, but also how exactly they contribute to bonding and to mechanical properties. A TM atom is not surrounded by other 12 TM atoms (as it would in an fcc metal), but rather it is coordinated with 6 carbon or nitrogen atoms with which it exchanges or shares electrons. To illustrate the difference between fcc metals and B1 structured carbides/nitrides more concretely, we use the electron localisation function (ELF) [Citation74,Citation75], which represents the probability of finding a pair of electrons in the same region in space, and is related to the electron density and the curvature of the electron–electron correlation function [Citation76,Citation77].
While ELF is different from the electron density , it qualitatively exploits and amplifies spatial variations of n to provide a clearer picture of the bonding. For example, in covalent materials such as silicon, ELF shows maxima between atoms, in metals it shows maxima in the interstitial sites, while in ionic compounds localisation occurs mainly on the anion.
shows the DFT-calculated ELF for a metal (Ti) and a transition-metal carbide (TMC), B1-structured TiC. For metallic Ti, each atom has 12 other Ti atoms surrounding it (a), while for B1-structured TiC in b – other rocksalt TMC/Ns are similar – each Ti atom has 6 C atoms as nearest neighbours (NN) and 12 Ti atoms as next-nearest neighbours (NNN). As a result, the ELFs for the two cases are vastly different. All atoms in the metal are neutral, while in the TMC, the C acquired electrons and has a net negative charge; necessarily, the TM atom in TMC will have a positive charge equal in absolute value to that on the C atom.
Figure 5. Electron localisation function (ELF, isosurfaces) for (a) pure metal Ti in fcc structure and (b) for a transition-metal carbide, TaC in the B1 structure. For the elemental Ti, the bond is metallic, with no net charge on any atom and with electron localisation primarily in the interstitial sites. The fcc Ti structure was chosen for ease of site comparison with TiC, but the hcp ground state of Ti also shows localisation of electrons in the interstitial sites. For TiC in (b), the electron localisation occurs around the C atom. (c) Schematic view of the Bader volumes around two atoms (black dots) in the lattice, with the number of electrons evaluated over these volumes.
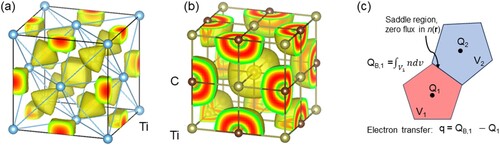
c shows how electron charges can be ‘ascribed' to an atom i using the Bader approach [Citation78], in which a volume is constructed for atom i based on planar boundaries placed at ‘zero flux' regions, i.e. at the saddle points of the electron density
. Such boundaries surround each ion with a so-called Bader volume, leading to a clear, unambiguous way to assign charges to the atoms/ions in a solid (c). As stated, we aim to partition the total number of electrons in the cell, VEC, into electrons that contribute to the three different types of bonding
where
with b = ionic, covalent, or metallic is the number of electrons that are committed to ionic, covalent, or metallic bonds, respectively. From the Bader procedure [Citation78], we readily obtain
, the electron transfer from TM to C (or N).
and
have distinctive signatures in ELF plots (a and b). ELF plots of electrons in covalent bonds show maxima exactly between two covalently bonded atoms, and the electrons contributing to metallic bonding show ELF maxima in the interstitial sites formed between 4 atoms (a). In order to define
in TMC/Ns, we would have to compute the electronic charge located inside the smallest polyhedra with the TM at corners, or more specifically, inside the (maximal) spheres inscribed in the tetrahedra created by TM atoms in the B1 lattice (these are NNN). Such choice leaves the edges of these tetrahedra to be surrounded by covalent electron distributions associated with the TM-C or TM-N bonds. With this definition of
the remaining balance of VEC will be the number of electrons committed to the covalent bond,
This partition procedure naturally offers now several descriptors beyond VEC: one can use () as independent descriptors, or use (
). In other words, at the same VEC, the parameters that lead to different mechanical and structural properties would be
, both of which are computable from the Bader charge analysis. This procedure differs from past attempts [Citation79] to separate the electronic density into bonds (bond partition charge) in that it does not require empirical parameterisations of the overlap regions between the atoms. Any shortcoming of the bond-specific electron partitioning described above would also be a shortcoming of the Bader method itself. It is our hope that this procedure to discriminate among the electrons associated with different types of bonding will enable the use of (VEC,
,
) as independent variables to analyse most mechanical properties, e.g. Young’s modulus, bulk modulus, shear modulus, hardness, Pugh’s ratio, Poisson ratio, etc. In particular, the (VEC,
,
) analysis of the Pugh’s ratio and Poisson ratio could emerge as a promising avenue to discover hard and ductile TMC/N alloys.
To summarise, the quest for appropriate descriptors of mechanical properties in B1-structured refractory carbides, nitrides, and their alloys is a long standing problem, both partially alleviated and confounded by the use of VEC as a predictor of mechanical behaviour. While providing a brief review of the approaches to understand and design materials with desired mechanical behaviour, we proposed that spread-type of descriptors can be used to understand the different properties of isoVEC TMC/Ns and illustrated the correlation between hardness and electronegativity difference between cations and anions. Furthermore, we proposed a way to ascertain the electron ‘content' responsible for the ionic, metallic, and covalent bonding, based on available tools in analysis of charge distribution. This partitioning between types of bonding is unambiguous, parameter-free, and as such will be usable to bonding in other crystalline structures that are mechanically stable (not necessarily ground states), in particular carbides, nitrides, oxides, oxycarbides in which bonding is not solely ionic. The partitioning of VEC between the different types of bonds is particularly useful for simple structures such as rocksalt, as there only a few types of cation–anion and (possibly) cation-cation second neighbour bonds.
The development and validation of relationships between simple bonding descriptors beyond VEC and mechanical behaviour will shed light on the physical origins of alloying for creating multiple and potentially opposing functionalities (e.g. strength and ductility) in refractory carbide or nitride alloys. Given that this class of materials, cubic transition-metal compounds, are of the form MX, where X = C, N, O, or other elements with equi-atomic cations and anions, we expect that the set of descriptors outlined here should be applicable to assess the stability and mechanical behaviour of multi-cation as well as multi-anion alloys, including high-entropy alloys. The ability to predict and hence design materials with properties of interest for certain applications, and the evaluation of the fundamental limits or ranges of mechanical properties for use in various applications has been a major quest for materials scientists. The approaches presented in this perspective can extend our current knowledge and provide new insights into the role of alloying on the mechanical behaviour of TMC/Ns with iso-valent cation composition.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Hanna Kindlund
Hanna Kindlund is a Collegiate Assistant Professor in the department of Materials Science and Engineering at Virginia Polytechnic Institute and State University (Virginia Tech). Kindlund obtained her doctoral degree in materials science from Linköping University, Sweden, where she worked in the thin film physics group focusing on the growth and characterization of epitaxial pseudobinary transition-metal nitride thin films with the purpose of enhancing material’s toughness. She demonstrated that it is possible to design and develop tough ceramic coatings. As a postdoctoral researcher at Lund University, Sweden, she developed methods to engineer crystal phases in group 3-5 semiconductor core-shell nanowires. Kindlund has extensive experience with both physical vapor deposition (PVD) and chemical vapor deposition (CVD) techniques (e.g., magnetron sputter deposition and metalorganic vapor phase epitaxy - MOVPE) and with structural, compositional, and nanomechanical (e.g., nanoindentation and nano-wear) characterization techniques. Her research interests include the growth and characterization of advanced structural and multifunctional materials. Her recent contributions include nanomechanical studies of transition-metal carbide single-crystals, high-entropy transition-metal nitride thin films and nanostructures.
Theodora Ciobanu
Theodora Ciobanu conducted research for this project as an undergraduate student at University of Notre Dame. She was involved in other materials science projects through Research Experiences for Undergraduates (2021), completed a study-abroad semester at John Cabot University in Rome (2022), as well an internship for PwC in Boston (2023). She enjoyed extracurricular activities such as Mock Trial and Student International Business Council. Theodora completed her honors thesis and graduated from University of Notre Dame in May 2024, majoring in Economics and in Applied Mathematics.
Suneel Kodambaka
Suneel Kodambaka is a Professor in the Department of Materials Science and Engineering (MSE) at Virginia Tech (VT), where he also served as the department head of MSE for two years. Prior to joining VT in 2022, Suneel was a professor in the Department of Materials Science and Engineering and the Area Director for Structural Materials for master of science in Engineering Online Program at the University of California Los Angeles (UCLA). Suneel graduated with a bachelor of technology (B.Tech.,) from the Indian Institute of Technology, Madras, M.S., from Southern Illinois University at Carbondale (SIUC), and Ph.D. from the University of Illinois, Urbana-Champaign (UIUC). Suneel is a recipient of the 2010 Alumni Achievement award from the SIUC College of Engineering, 2009 AVS Thin Film Division's Paul Holloway Young Investigator Award, and 2008 Best Paper award from the IBM Materials Research Community. His research relies on in situ microscopy (SEM, TEM, LEEM, and STM) studies to develop fundamental understanding of the nucleation and growth kinetics and thermo- chemical and mechanical stabilities of crystalline solids. Suneel can be reached at [email protected].
Cristian V. Ciobanu
Cristian V. Ciobanu is a Professor in the Department of Mechanical Engineering and in the Materials Science Program at Colorado School of Mines. Prior to joining the School of Mines in 2004, he was a postdoctoral fellow in the Division of Engineering at Brown University (2001-2004). He holds degrees in Physics from University of Bucharest (B. Sc., 1995) and Ohio State University (M.S., 1998 and Ph.D., 2001). His research interests are in computational materials science and mechanics, specifically in structure-property relationships, nanoscale/nanomaterials problems, two-dimensional materials, materials for renewable energy applications, developments of evolutionary algorithms for computational materials design and optimization of atomic structures, self-organized nano and bio structures on crystal surfaces, among others. His research work in these areas has led to over 100 co-authored journal articles, two patents, and a book. In addition to his teaching and research, Dr. Ciobanu carries out significant professional service in various editorial capacities for several journals, as well as technical paper and proposal reviewer and session chair/organizer for certain professional conferences.
References
- W.S. Williams, Cubic carbides. Science 152 (1966), pp. 34–42.
- G.E. Hollox, Microstructure and mechanical behavior of carbides. Mater. Sci. Eng. 3 (1968), pp. 121–137.
- L.E. Toth, Transition Metal Carbides and Nitrides, Academic Press, New York, 1971.
- K. Balasubramanian, S.V. Khare, and D. Gall, Valence electron concentration as an indicator for mechanical properties in rocksalt structure nitrides, carbides and carbonitrides. Acta Mater. 152 (2018), pp. 175–185.
- P.H. Mayrhofer, C. Mitterer, L. Hultman, and H. Clemens, Microstructural design of hard coatings. Prog. Mater. Sci. 51 (2006), pp. 1032–1114.
- H. Holleck, Material selection for hard coatings. J. Vac. Sci. Technol. A Vac. Surf. Films 4 (1986), pp. 2661–2669.
- W.G. Fahrenholtz, G.E. Hilmas, I.G. Talmy, and J.A. Zaykoski, Refractory diborides of zirconium and hafnium. J. Am. Ceram. Soc. 90 (2007), pp. 1347–1364.
- F. Monteverde, A. Bellosi, and L. Scatteia, Processing and properties of ultra-high temperature ceramics for space applications. Mater. Sci. Eng. A 485 (2008), pp. 415–421.
- S. Ramanathan, and S. Oyama, New catalysts for hydroprocessing: Transition metal carbides and nitrides. J. Phys. Chem. 99 (1995), pp. 16365–16372.
- J.G. Chen, Carbide and nitride overlayers on early transition metal surfaces: Preparation, characterization, and reactivities. Chem. Rev. 96 (1996), pp. 1477–1498.
- W. Cai, G. Li, K. Zhang, G. Xiao, C. Wang, K. Ye, Z. Chen, Y. Zhu, and Y. Qian, Conductive nanocrystalline niobium carbide as high-efficiency polysulfides tamer for lithium-sulfur batteries. Adv. Funct. Mater. 28 (2018), pp. 1704865.
- F.H. Baumann, D.L. Chopp, T.D. de la Rubia, G.H. Gilmer, J.E. Greene, H. Huang, S. Kodambaka, P. O'Sullivan, and I. Petrov, Multiscale modeling of thin-film deposition: Applications to Si device processing. MRS Bull. 26 (2001), pp. 182–189.
- H. Kindlund, D.G. Sangiovanni, J. Lu, J. Jensen, V. Chirita, I. Petrov, J.E. Greene, and L. Hultman, Effect of WN content on toughness enhancement in V1-xWxN/MgO(001) thin films. J. Vac. Sci. Technol. A 32 (2014), pp. 030603.
- H. Kindlund, D.G. Sangiovanni, L. Martinez-de-Olcoz, J. Lu, J. Jensen, J. Birch, I. Petrov, J.E. Greene, V. Chirita, and L. Hultman, Toughness enhancement in hard ceramic thin films by alloy design. APL Mater. 1 (2013), pp. 042104.
- W.F. Gale, and T.C. Totemeier, Smithells Metals Reference Book, Elsevier Science, Oxford, 2003.
- S. Guo, C. Ng, J. Lu, and C.T. Liu, Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 109 (2011), pp. 103505.
- F.Y. Tian, L.K. Varga, N.X. Chen, J. Shen, and L. Vitos, Empirical design of single phase high-entropy alloys with high hardness. Intermetallics 58 (2015), pp. 1–6.
- J.C. Grossman, A. Mizel, M. Côté, M.L. Cohen, and S.G. Louie, Transition metals and their carbides and nitrides: Trends in electronic and structural properties. Phys. Rev. B 60 (1999), pp. 6343–6347.
- S.H. Jhi, J. Ihm, S.G. Louie, and M.L. Cohen, Electronic mechanism of hardness enhancement in transition-metal carbonitrides. Nature 399 (1999), pp. 132–134.
- S. Divilov, H. Eckert, D. Hicks, C. Oses, C. Toher, R. Friedrich, M. Esters, M.J. Mehl, A.C. Zettel, Y. Lederer, E. Zurek, J.-P. Maria, D.W. Brenner, X. Campilongo, S. Filipović, W.G. Fahrenholtz, C.J. Ryan, C.M. DeSalle, R.J. Crealese, D.E. Wolfe, A. Calzolari, and S. Curtarolo, Disordered enthalpy–entropy descriptor for high-entropy ceramics discovery. Nature 625 (2024), pp. 66–73.
- M. Lim, and D.W. Brenner, Predicting properties of high entropy carbides from their respective binaries. Comput. Mater. Sci. 226 (2023), pp. 112255.
- C. Toher, C. Oses, M. Esters, D. Hicks, G.N. Kotsonis, C.M. Rost, D.W. Brenner, J.-P. Maria, and S. Curtarolo, High-entropy ceramics: Propelling applications through disorder. MRS Bull. 47 (2022), pp. 194–202.
- M.D. Hossain, T. Borman, C. Oses, M. Esters, C. Toher, L. Feng, A. Kumar, W.G. Fahrenholtz, S. Curtarolo, D. Brenner, J.M. LeBeau, and J.-P. Maria, Entropy landscaping of high-entropy carbides. Adv. Mater. 33 (2021), pp. 2102904.
- M. Esters, C. Oses, D. Hicks, M.J. Mehl, M. Jahnátek, M.D. Hossain, J.-P. Maria, D.W. Brenner, C. Toher, and S. Curtarolo, Settling the matter of the role of vibrations in the stability of high-entropy carbides. Nat. Commun. 12 (2021), pp. 5747.
- M.D. Hossain, T. Borman, A. Kumar, X. Chen, A. Khosravani, S.R. Kalidindi, E.A. Paisley, M. Esters, C. Oses, C. Toher, S. Curtarolo, J.M. LeBeau, D. Brenner, and J.P. Maria, Carbon stoichiometry and mechanical properties of high entropy carbides. Acta Mater. 215 (2021), pp. 117051.
- T.J. Harrington, J. Gild, P. Sarker, C. Toher, C.M. Rost, O.F. Dippo, C. McElfresh, K. Kaufmann, E. Marin, L. Borowski, P.E. Hopkins, J. Luo, S. Curtarolo, D.W. Brenner, and K.S. Vecchio, Phase stability and mechanical properties of novel high entropy transition metal carbides. Acta Mater. 166 (2019), pp. 271–280.
- P. Sarker, T. Harrington, C. Toher, C. Oses, M. Samiee, J.-P. Maria, D.W. Brenner, K.S. Vecchio, and S. Curtarolo, High-entropy high-hardness metal carbides discovered by entropy descriptors. Nat. Commun. 9 (2018), pp. 4980.
- D.G. Sangiovanni, K. Kaufmann, and K. Vecchio, Valence electron concentration as key parameter to control the fracture resistance of refractory high-entropy carbides. Sci. Adv. 9 (2023), pp. eadi2960.
- D.G. Sangiovanni, W. Mellor, T. Harrington, K. Kaufmann, and K. Vecchio, Enhancing plasticity in high-entropy refractory ceramics via tailoring valence electron concentration. Mater. Des. 209 (2021), pp. 109932.
- D.G. Sangiovanni, F. Tasnádi, T. Harrington, M. Odén, K.S. Vecchio, and I.A. Abrikosov, Temperature-dependent elastic properties of binary and multicomponent high-entropy refractory carbides. Mater. Des. 204 (2021), pp. 109634.
- L. Chen, J. Paulitsch, Y. Du, and P.H. Mayrhofer, Thermal stability and oxidation resistance of Ti-Al-N coatings. Surf. Coat. Technol. 206 (2012), pp. 2954–2960.
- R. Rachbauer, D. Holec, M. Lattemann, L. Hultman, and P.H. Mayrhofer, Electronic origin of structure and mechanical properties in Y and Nb alloyed Ti-Al-N thin films. Int. J. Mater. Res. 102 (2011), pp. 735–742.
- L. Chen, D. Holec, Y. Du, and P.H. Mayrhofer, Influence of Zr on structure, mechanical and thermal properties of Ti-Al-N. Thin Solid Films 519 (2011), pp. 5503–5510.
- H. Willmann, P.H. Mayrhofer, P.O.A. Persson, A.E. Reiter, L. Hultman, and C. Mitterer, Thermal stability of Al-Cr-N hard coatings. Scr. Mater. 54 (2006), pp. 1847–1851.
- P.H. Mayrhofer, A. Horling, L. Karlsson, J. Sjolen, T. Larsson, C. Mitterer, and L. Hultman, Self-organized nanostructures in the Ti-Al-N system. Appl. Phys. Lett. 83 (2003), pp. 2049–2051.
- C. Mitterer, J. Komenda-Stallmaier, P. Losbichler, P. Schmölz, W.S.M. Werner, and H. Störi, Sputter deposition of decorative boride coatings. Vacuum 46 (1995), pp. 1281–1294.
- J. Patscheider, Nanocomposite hard coatings for wear protection. MRS Bull. 28 (2003), pp. 180–183.
- K.P. Budna, J. Neidhardt, P.H. Mayrhofer, and C. Mitterer, Synthesis-structure-property relations for Cr-B-N coatings sputter deposited reactively from a Cr-B target with 20 at% B. Vacuum 82 (2008), pp. 771–776.
- M. Rühle, and A.G. Evans, High toughness ceramics and ceramic composites. Prog. Mater. Sci. 33 (1989), pp. 85–167.
- W.J. Clegg, K. Kendall, N.M.N. Alford, T. Button, and J. Birchall, A simple way to make tough ceramics. Nature 347 (1990), pp. 455–457.
- I.L. Shabalin, Y. Wang, A.V. Krynkin, O.V. Umnova, V.M. Vishnyakov, L.I. Shabalin, and V.K. Churkin, Physicomechanical properties of ultrahigh temperature heteromodulus ceramics based on group 4 transition metal carbides. Adv. Appl. Ceram. 109 (2010), pp. 405–415.
- F.D. Minatto, P. Milak, A. De Noni, Jr., D. Hotza, and O.R.K. Montedo, Multilayered ceramic composites – a review. Adv. Appl. Ceram. 114 (2014), pp. 127–138.
- S. Zhang, H.L. Wang, S.E. Ong, D. Sun, and X.L. Bui, Hard yet tough nanocomposite coatings–present status and future trends. Plasma Processes Polym. 4 (2007), pp. 219–228.
- J. Karch, R. Birringer, and H. Gleiter, Ceramics ductile at low temperature. Nature 330 (1987), pp. 556–558.
- Z.G. Wu, X.J. Chen, V.V. Struzhkin, and R.E. Cohen, Trends in elasticity and electronic structure of transition-metal nitrides and carbides from first principles. Phys. Rev. B 71 (2005), pp. 214103.
- D.V. Suetin, I.R. Shein, and A.L. Ivanovskii, Elastic and electronic properties of hexagonal and cubic polymorphs of tungsten monocarbide WC and mononitride WN from first-principles calculations. Phys. Status Solidi B 245 (2008), pp. 1590–1597.
- D.G. Sangiovanni, V. Chirita, and L. Hultman, Electronic mechanism for toughness enhancement in TixM1−xN (M = Mo and W). Phys. Rev. B 81 (2010), pp. 104107.
- D. Holec, R. Franz, P.H. Mayrhofer, and C. Mitterer, Structure and stability of phases within the NbN-AlN system. J. Phys. D Appl. Phys. 43 (2010), pp. 145403.
- Z. Sun, R. Ahuja, and J.E. Lowther, Mechanical properties of vanadium carbide and a ternary vanadium tungsten carbide. Solid State Commun. 150 (2010), pp. 697–700.
- D. Holec, R. Rachbauer, L. Chen, L. Wang, D. Luef, and P.H. Mayrhofer, Phase stability and alloy-related trends in Ti-Al-N, Zr-Al-N and Hf-Al-N systems from first principles. Surf. Coat. Technol. 206 (2011), pp. 1698–1704.
- H. Li, L.T. Zhang, Q.F. Zeng, K. Guan, K.Y. Li, H.T. Ren, S.H. Liu, and L.F. Cheng, Structural, elastic and electronic properties of transition metal carbides TMC (TM = Ti, Zr, Hf and Ta) from first-principles calculations. Solid State Commun. 151 (2011), pp. 602–606.
- Z. Gao, and S. Kang, First-principles investigation of the elastic, electronic, and thermodynamic properties of a nitrogen-doped (Ti0.75W0.25)C solid solution. Solid State Commun. 156 (2013), pp. 25–30.
- X.W. Sun, X.Y. Zhang, Y. Zhu, S.H. Zhang, J.Q. Qin, M.Z. Ma, and R.P. Liu, First-principles study of ZrCxN1-x alloys with electron concentration modulation. J. Mater. Sci. 48 (2013), pp. 7743–7748.
- L. Wu, Y. Wang, Z. Yan, J. Zhang, F. Xiao, and B. Liao, The phase stability and mechanical properties of Nb–C system: Using first-principles calculations and nano-indentation. J. Alloys Compd. 561 (2013), pp. 220–227.
- L. Wu, T. Yao, Y. Wang, J. Zhang, F. Xiao, and B. Liao, Understanding the mechanical properties of vanadium carbides: Nano-indentation measurement and first-principles calculations. J. Alloys Compd. 548 (2013), pp. 60–64.
- X.-X. Yu, G.B. Thompson, and C.R. Weinberger, Influence of carbon vacancy formation on the elastic constants and hardening mechanisms in transition metal carbides. J. Eur. Ceram. Soc. 35 (2015), pp. 95–103.
- H. Kindlund, D.G. Sangiovanni, J. Lu, J. Jensen, V. Chirita, J. Birch, I. Petrov, J.E. Greene, and L. Hultman, Vacancy-induced toughening in hard single-crystal V0.5Mo0.5Nx/MgO(001) thin films. Acta Mater. 77 (2014), pp. 394–400.
- H. Kindlund, D.G. Sangiovanni, I. Petrov, J.E. Greene, and L. Hultman, A review of the intrinsic ductility and toughness of hard transition-metal nitride alloy thin films. Thin Solid Films 688 (2019), pp. 137479.
- D.G. Sangiovanni, L. Hultman, and V. Chirita, Supertoughening in B1 transition metal nitride alloys by increased valence electron concentration. Acta Mater. 59 (2011), pp. 2121–2134.
- S.-H. Jhi, S.G. Louie, M.L. Cohen, and J. Ihm, Vacancy hardening and softening in transition metal carbides and nitrides. Phys. Rev. Lett. 86 (2001), pp. 3348–3351.
- S.H. Jhi, S.G. Louie, M.L. Cohen, and J. Morris, Jr., Mechanical instability and ideal shear strength of transition metal carbides and nitrides. Phys. Rev. Lett. 87 (2001), pp. 75503.
- K. Chen, L.R. Zhao, J. Rodgers, and J.S. Tse, Alloying effects on elastic properties of TiN-based nitrides. J. Phys. D Appl. Phys. 36 (2003), pp. 2725–2729.
- H. Kindlund, J. Lu, J. Jensen, I. Petrov, J.E. Greene, and L. Hultman, Epitaxial V0.6W0.4N/MgO(001): Evidence for ordering on the cation sublattice. J. Vac. Sci. Technol. A 31 (2013), pp. 040602.
- G. Greczynski, H. Kindlund, I. Petrov, J. Greene, and L. Hultman, Sputter-cleaned epitaxial VxMo(1-x)Ny/MgO(001) thin films analyzed by X-ray photoelectron spectroscopy: 2. single-crystal V0.47Mo0.53N0.92. Surf. Sci. Spectra 20 (2013), pp. 74–79.
- H. Kindlund, G. Greczynski, E. Broitman, L. Martínez-de-Olcoz, J. Lu, J. Jensen, I. Petrov, J.E. Greene, J. Birch, and L. Hultman, V0.5Mo0.5Nx/MgO(001): Composition, nanostructure, and mechanical properties as a function of film growth temperature. Acta Mater. 126 (2017), pp. 194–201.
- R. Zhang, X. Gu, K. Zhang, X. Gao, C. Liu, and C. Chen, Core electron count as a versatile and accurate new descriptor for sorting mechanical properties of diverse transition metal compounds. Adv. Mater. 35 (2023), pp. 2304729.
- Q. Yang, W. Lengauer, T. Koch, M. Scheerer, and I. Smid, Hardness and elastic properties of Ti(CxN1−x), Zr(CxN1−x) and Hf(CxN1−x). J. Alloys Compd. 309 (2000), pp. L5–L9.
- D.B. Miracle, and O.N. Senkov, A critical review of high entropy alloys and related concepts. Acta Mater. 122 (2017), pp. 448–511.
- J. Zhang, B. Xu, Y. Xiong, S. Ma, Z. Wang, Z. Wu, and S. Zhao, Design high-entropy carbide ceramics from machine learning. NPJ Comput. Mater. 8 (2022), pp. 5.
- S. Zhai, H. Xie, P. Cui, D. Guan, J. Wang, S. Zhao, B. Chen, Y. Song, Z. Shao, and M. Ni, A combined ionic Lewis acid descriptor and machine-learning approach to prediction of efficient oxygen reduction electrodes for ceramic fuel cells. Nat. Energy 7 (2022), pp. 866–875.
- J. Zhang, X. Xiang, B. Xu, S. Huang, Y. Xiong, S. Ma, H. Fu, Y. Ma, H. Chen, Z. Wu, and S. Zhao, Rational design of high-entropy ceramics based on machine learning – a critical review. Curr. Opin. Solid State Mater. Sci. 27 (2023), pp. 101057.
- A. Šimůnek, How to estimate hardness of crystals on a pocket calculator. Phys. Rev. B 75 (2007), pp. 172108.
- S. Pugh, XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Lond. Edinb. Dublin Philos. Mag. J. Sci. 45 (1954), pp. 823–843.
- A. Savin, O. Jepsen, J. Flad, O.K. Andersen, H. Preuss, and H.G. Vonschnering, Electron localization in solid-state structures of the elements – the diamond structure. Angew. Chem. Int. Ed. 31 (1992), pp. 187–188.
- U. Haussermann, S. Wengert, P. Hofmann, A. Savin, O. Jepsen, and R. Nesper, Localization of electrons in intermetallic phases containing aluminum. Angew. Chem. Int. Ed. Engl. 33 (1994), pp. 2069–2073.
- A.D. Becke, and K.E. Edgecombe, A simple measure of electron localization in atomic and molecular-systems. J. Chem. Phys. 92 (1990), pp. 5397–5403.
- U. Haussermann, S. Wengert, and R. Nesper, Unequivocal partitioning of crystal-structures, exemplified by intermetallic phases containing aluminum. Angew. Chem. Int. Ed. Engl. 33 (1994), pp. 2073–2076.
- W. Tang, E. Sanville, and G. Henkelman, A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condes. Matter 21 (2009), pp. 084204.
- M. Mizuno, I. Tanaka, and H. Adachi, Chemical bonding in titanium-metalloid compounds. Phys. Rev. B 59 (1999), pp. 15033–15047.
