To start with, people need a fairy tale. Maybe that's unfair, but they need a story line that's relatively simple to understand … (Condic, Citation2007).
At the same time ‘over-hyping’ the new therapeutic promises of this rapidly expanding sector of bio-innovation is assessed as a major risk (Braude et al., Citation2005). ‘Hope not hype?’ is the interrogative slogan of the Medical Research Council (MRC), the UK's principal public funding body for stem cell science. And to make sure that investors also get the message right, an industrial network meeting on commercialization of stem cell therapies was recently held under the banner ‘The hype has gone!’ Hope, hype and promise are structuring scientific innovation; these stories are also organizing the responses of its many public audiences.
Closely related to these themes is the narrative character of scientific discovery. As analyzed in an earlier special issue of Science as Culture, entitled ‘Procreation Stories’, narrative structures animate the unfolding of events by aligning the future and the past with value-laden expectations (Franklin & McNeil, Citation1993). Especially prominent is the belief in scientific progress, with its accompanying moral economy of health enhancement, manifest destiny and new frontiers to be conquered. Likewise, a recent special issue analyzes ‘biofutures’ as a self-fulfilling prophecy: a specific biofuture is promoted as rational in scenarios, policy and practice—while others are not (Birch, Citation2006, p. 173). Discovery stories render a particular point in time—the ‘moment’ of scientific ‘breakthrough’—as the basis to build hopes and expectations for the future. For the field of stem cell research, as well as for the stem cell enterprise more generally, this particular moment came in 1998.
Injecting hope. Credit: Bernd Haberl, Email: [email protected], http://www.illustrationen.at
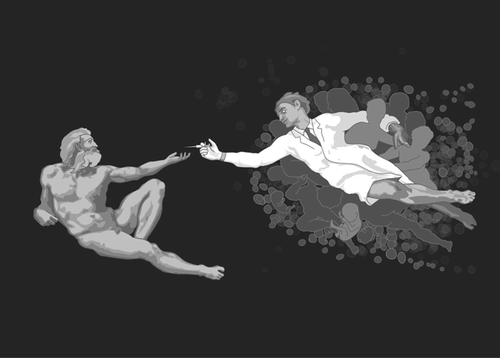
1998 Thomson Paper: Creation of Immortals—Or an Immortal Creation?
It's no longer in the realm of science fiction; I really believe that within my lifetime I will see diseases treated by these therapies (James Thomson quoted in Marshall, Citation1998, p. 1014).
Looking back 10 years later, it is worth asking: what is the significance of this ‘breakthrough’? What has become of ‘human blastocyst-derived pluripotent cell lines’?
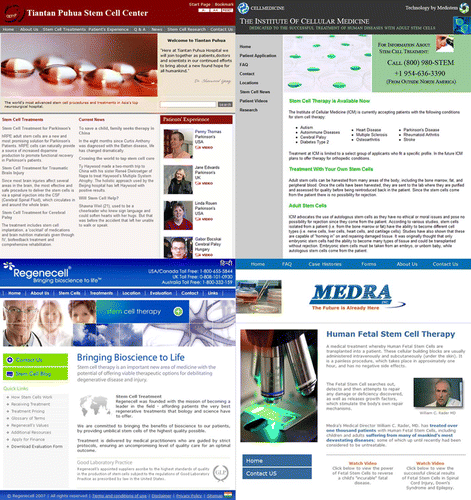
Selling hope - companies offering stem cell therapies online (2007) Clockwise from left corner: Tiantan Puhua Stem Cell Center. People's Republic of China www.stemcellspuhua.com. Cell Medicine. United States of America http://www.cellmedicine.com/. Medra Inc. United States of America, Georgia, Germany, Dominican Republic http://www.medra.com/. RegeneCell. United States, Seychelles and other locationas http://www.regenecell.com/
The standard answer has become a well-known mantra: stem cells possess a remarkable potential to develop into many different cell types in the body. With the theoretical potential to divide without limit, these cells are seen to offer a means for renewing tissue throughout an individual's life. The potentially most powerful stem cells are found in the early stage embryo and are believed to be pluripotent—i.e. able to develop into many different cell types (Smith, Citation2006). In addition, human embryonic stem cells (hESCs) are thought to be immortal (capable of dividing indefinitely without losing their genetic structure) and malleable (able to be manipulated without losing cell function).
These three attributes have stimulated the imagination of patients, politicians, and media consumers alike. If we find out how to stimulate hESCs to differentiate into the ‘right’ kind of tissue, we will be able to alleviate (or even cure) injuries or diseases as diverse as Alzheimer's and Parkinson's disease, heart and kidney failure, diabetes, traumatic spinal cord injury, vision loss and hearing loss. However, more recent reports from scientists in Japan and the US reprogramming adult skin cells ‘back’ into the stem cell stage claim that those cells possess similar pluripotent functions as their embryonic counterparts (Yu et al., Citation2007; Takahashi et al., Citation2007; Vogel & Holden, Citation2007). These discoveries have raised doubts about whether stem cells are a different category of cells in the body, or whether they merely represent a particular stage (see also Zipori, Citation2004).
Regardless of how one assesses the therapeutic promise of hESCs, there is one prediction that Thomson et al.'s Citation(1998) publication in Science doubtlessly fulfilled: it kicked off a heated scientific and public debate. Some would claim it marked the birth of Regenerative Medicine, often described as ‘a new era of medicine’ and a ‘paradigm shift’ in research. Somewhat more unexpectedly, it gave rise to what US commentators later called the ‘stem cell wars’ (Kass, Citation2005).
As one explanation for the almost instant ‘hype’ after the publication, some commentators argued that the paper had caught by surprise not only the larger public but also most of the scientific community (Holland et al., Citation2001, p. xv). Other narratives regard the eventual derivation of stem cells from the inner cell mass of early human embryos by Thomson et al. as the logical next step in a long tradition of basic scientific research. Critics in support of this argument tone down the novelty factor by referring to existing therapeutic applications of stem cells, especially regarding those obtained from bone marrow, which has been a standard treatment course for cancer since 1968. Furthermore, the successful derivation of embryonic stem cells from mice had already been reported in 1981 by recent Nobel laureate Sir Martin Evans and his team (Evans & Kaufman, Citation1981) and by another group of researchers (Martin, Citation1981). In 1995, Thomson et al. celebrated the successful isolation of an embryonic stem cell line in primates.
It has been claimed that the isolation of an embryonic stem cell line in humans had been within reach—and therefore was less of a serendipitous surprise than some made it seem afterwards. Indeed, another research project had success in isolating human embryonic germ cell lines from foetal tissue in the same year as Thomson et al. reported theirs. Shamblott et al. Citation(1998) had used a different culturing methodology and source, but their findings were considered by many as equally worthy of celebration. Consequently, the novelty of Thomson's work could be seen as
neither the existence of stem cells as such, nor of embryonic stem cells or even human embryonic stem cells, but merely the successful ‘derivation’ of human embryonic stem cells, which consequently was presented as technical and practical knowledge more than as scientific and systematic knowledge (Nielsen, Citation2005, p. 32).
But Thomson et al.'s paper touched upon more than just technical and practical insight. This becomes evident from a glance at the aforementioned Science Editorial, in a special issue on the question of publishing controversial research. There, rhetorical questions were posed: whether the publication of ethically controversial research was ‘harmful’, and whether journals should ‘draw a moral line in the sand’ (Miller & Bloom, Citation1998, p. 1045). A news article in the same issue discussed the implications of Thomson's research in terms of a ‘versatile stem cell line’ raising ‘scientific hope’ and ‘legal questions’ (Marshall, Citation1998).
In other words, in retrospect, the Thomson et al. paper might have been a ‘breakthrough’ in terms of new ethical, social, and regulatory questions—at least to the same extent that it charted new waters scientifically. Put differently, the Thomson et al. publication has come to represent a crucial turning point in scientific understandings of stem cell biology, yet the more significant implications are better understood as social, ethical and political. The paper marked a shift in the promissory economies aligned with a new understanding of biological development (Thompson, Citation2005).
Regulatory Landscape
Society is not simply ‘reacting’ to the impact of new scientific developments. As the stem cell saga illustrates, a scientific activity has been influenced by public debates and expectations. There is a deep connection between stem cell biology and the prospect of improved (or worsened) medical and economic prospects for the future, as well as moral politics.
When Thomson and his colleagues had been involved in the work leading to the publication of their 1998 landmark paper, the legal situation surrounding their activities had been uncertain. Above all, it had been unclear whether Thomson's stem cell lines could be used under US Federal law. Federal funds there could be used neither for ‘the creation of a human embryo’ for research purposes, nor for ‘research in which a human embryo or embryos are destroyed, discarded or knowingly subjected to risk of injury or death’. Most of Thomson's research was privately funded, via biotech company Geron, and via a grant from the Wisconsin Alumni Research Foundation, the university's patent agent. Nevertheless Federal restrictions were anticipated because the cells used to create his lines had come from embryos donated by couples undergoing IVF treatment in clinics in Wisconsin and Israel (Marshall, Citation1998).
In the same month as Thomson et al.’s paper was published, US President Bill Clinton ordered a review of the issues associated with human stem cell research by the National Bioethics Advisory Commission. The NBAC subsequently reported on ethical issues related to the sources of human embryonic stem cell research and included arguments of federal funding and oversight of research (NBAC, Citation1999, Citation2000). As such, hESCs colonized political arenas and venture capitalist agendas alike, thereby presenting a dominant framing in which science and biopolitics, technology and biocapital were shown to be intrinsically interwoven.
Notably, the Pontifical Academy for Life of the Vatican issued a declaration commenting on Thomson et al.'s findings:
The results of these experiments had a great impact on the world of both science and biotechnology … no less than the world of business and the mass media. There were high hopes that the application of this knowledge would lead to new and safer ways of treating serious diseases, something which had been sought for years. But the impact was greatest in the political world (Pontifical Academy for Life, Citation2000).
A Self-Fulfilling Prophecy?
As the discoveries of modern science create tremendous hope, they also lay vast ethical mine fields. As the genius of science extends the horizons of what we can do, we increasingly confront complex questions about what we should do. We have arrived at that brave new world that seemed so distant in 1932, when Aldous Huxley wrote about human beings created in test tubes in what he called a ‘hatchery’ (White House: President Discusses Stem Cell Research, 2001).
Stem cell research offers unprecedented opportunities for developing new medical therapies for debilitating diseases and a new way to explore fundamental questions of biology (NRC/IoM, 2002).
Although the number of human embryonic stem cell lines has increased considerably in the past two years, few of these have been well characterised, and large hurdles still need to be overcome to ensure safety and efficacy. These will require substantial further investment and research (Braude et al., 2005, p. 1159).
If scientists can reliably direct the differentiation of embryonic stem cells into specific cell types, they may be able to use the resulting, differentiated cells to treat certain diseases at some point in the future (‘Stem Cell Basics’, website National Institutes of Health resource for stem cell research, 2007).
Gradually, the curative and regenerative potential that lies in harnessing stem cells is being realized (Regenerative Medicine, 2007, Aims & Scope section).
Don't be fooled by stem cell hype (Editorial title, New Scientist, 24 November 2007).
Immortal Promises?
Stem cell stories are usefully illuminated by concepts in the sociology of expectations, such as the organizing power of hope (Brown, Citation2003; Brown & Michael, Citation2003; Brown et al., Citation2000; Moreira & Palladino, Citation2005). Earlier biomedical innovations, such as IVF, were described as ‘hope technologies’ (Franklin, Citation1997) or as a ‘political economy of hope’ that drives forward biomedicine (Good et al., Citation1990). Likewise, hope provides both the fuel and the trajectory for future scientific progress, while also allowing a flexible roadmap for both. For example, reports in the mass media describing ‘a shift in stem cell hopes’ away from therapies and near-term expectations function to reconstitute stem cells as research tools rather than instant cures (Wade, Citation2006).
By such means, the therapeutic promises of stem cell research can be expanded to encompass a broad range of diseases and conditions for which there is at present only partial treatment or none at all. Even if their many promissory applications have not materialized, this gap becomes less relevant than the ability to maintain, and to manage, the shape and direction of imagined futures—at the same time ensuring not to ‘overhype’ such scenarios. Such narratives carefully stage anticipatory futures that will unfold along predicted lines of development. As Brian Salter observes: ‘Embedded in these imaginations are hopes and expectations of what the future might bring and, if the faith is sufficiently strong, a commitment to support the allocation of the resources required to enable that imagined future to become reality’ (Salter, Citation2007, p. 4).
Narrative alignments of hope and expectation thus serve to create a self-fulfilling prophecy orientated toward commercial markets and therapeutic applications. Charis Thompson's Citation(2005) model of ‘promissory capital’ offers an important analysis of this process. Likewise Franklin Citation(2001) has described the ways in which stem cells have been ‘cultured up’ to become forms of biocapital with in-built expectations. Hope narratives enable the co-production of stem cell technologies, political culture and biocapital investment—which in turn makes these innovations so powerful:
Asking careful questions about the promissory work of new life forms brings us into direct contact with the generative power of representations as animating technologies themselves. At risk in the effort to separate the reality from the hype are all of the dense reciprocities and economies of co-production through which they emerge and perform in tandem (Franklin & Lock, Citation2003, p. 15).
This Special Issue: ‘Stem Cell Stories’
Much public discussion of stem cells emphasizes their future potential. ‘Stem cell talk’ has analogies with the ‘gene talk’ described by Evelyn Fox Keller Citation(1995). So too have the social sciences and cultural studies turned their attention to questions of hope, expectations, promises and ‘progress’.
Complementing such analyzes, this special issue focuses on how these expectations for a particular vision of stem cell futures are driven by science and capital. We explore their ‘futurity’—both as an imaginary domain of speculative promise, and as an instrumental process of ‘realizing’ their potential. Commentators on these experimental cells often attempt to distinguish between their realistic potential and merely speculative hope or hype. Yet this distinction can be deceptive. Potential futures are being shaped by various investments—be they in the form of expert promises, elusive hopes and prayers or venture capital. So we explore how investments in stem cells entangle their futures in the present and its history.
We analyze the following:
-
how stem cells as objects attracting investment relate to stem cells as projects building a new future;
-
how scientific, political, and commercial discourses represent their potential, such as immortalization;
-
how scientific understandings constitute the objects they describe, especially for a non-scientific audience; and
-
how science classifies and characterizes cells in action.
A subsequent special issue, focusing on controversy over stem cell research, will be published in late 2008.
Overview of Papers
The building and meaning of the therapeutic promise is analyzed by Beatrix Rubin in her article ‘Therapeutic Promise in the Discourse of Human Embryonic Stem Cell Research’. In the recent past, biomedical research has been repeatedly promoted on the grounds that it will lead to novel cures. Drawing on the Foucauldian notion of a dispositif as well as the concept of the ‘therapeutic promise’, Rubin discusses the important role of medical proposals in the discourse on hESC research. She offers an alternative ‘story’ of the emergence of this particular research domain. In particular the quest for therapies has rendered the human embryo accessible first as an object of experimental manipulation, then of public debate, and finally as the subject of regulation. This therapeutic promise ‘at work’ has fostered an alliance between bioethics and science ensuring the continuation of hESC research.
Paul Martin, Nik Brown and Alison Kraft analyze expectation and ‘communities of promise’ shaping emerging technologies exemplified by haematopoietic stem cells (HSCs). Their article ‘From Bedside to Bench?’ discusses the changing relationships between basic science and the clinic. Covering several decades of development, they show how the relationship between basic science and clinical research communities has been based on a two-way flow of knowledge, where clinical innovation has played a key role in the translation process.
Lena Eriksson and Andrew Webster analyze efforts at ‘Standardizing the Unknown’. As the authors argue, standardizing hESCs is an exercise in taming different kinds of unknowns, while simultaneously changing understandings of what a stem cell is. Scientists exchange research materials and data across institutions and national borders to increase both competition and cooperation. Recognizing ‘known unknowns’, scientists are equally aware of a different epistemic currency than the types of unknowns that could lead to scientific fame and fortune. The notion of ‘pluripotency’ provides a discursive resource when demarcating the capacities of embryonic stem cells from those of adult stem cells, yet it can also present a practical problem. A more flexible definition allowing for different stem cell ‘niches’ could render the cell lines less malleable but more potent. The reconfiguration of pluripotency may serve to transport hESCs into a clinical, do-able future.
Neil Stephens, Paul Atkinson and Peter Glasner show how the UK Stem Cell Bank links future visions with past and present strategies. The Bank takes donations of ethically approved stem cell lines, tests them, grows larger stocks, and re-distributes the material internationally. As such the Bank has an important guardianship role in the international movement of human embryonic stem cell lines. It also enacts a particular future vision of stem cell science. Its strategies involve a complex temporal and spatial interplay: securing accounts of the past (both technical and social), while validating the regulatory legitimacy of the present. The authors analyze the centrality of trust, social networks, and wider public legitimacy in the Bank's work. It is important to recognize the ways in which the Bank makes these social relationships tangible, and in some cases durable, through their embodiment in documentary form. These practices are essential to the Bank's particular vision of the future of stem cell science.
Inna Kotchetkova, Rob Evans and Susanne Langer contribute to the long-standing debates about method and meaning of public participation in highly contested techno-scientific fields. In their paper ‘Articulating Contextualized Knowledge: Focus Groups and/as Public Participation?’ they reflect on calls for increased public participation in science and technology policy for social scientists and policy-makers alike. The authors analyze how a particular choice of method in assessing ‘public’ opinions can bear upon public participation. They contrast findings obtained from various focus groups on perceptions of stem cell research, on the one hand, and more conventional survey-based representations of public opinion, on the other. By contrast to the ‘pro’ and ‘anti’ positions in survey research, focus groups highlight participants' uncertainty and ambivalence. By providing alternative representations of public concerns that resist polarization, social science can inform a more broad-ranging ‘upstream’ debate about the social purposes that science should serve.
Margaret Sleeboom-Faulkner likewise takes a fresh approach to the role and meaning of public debate in her article ‘Debates on Human Embryonic Stem Cell Research in Japan: Minority Voices and their Political Amplifiers’. Debate on the status of the embryo is said to be hardly relevant to Japanese culture because this country has no cultural canons that forbid hESC research. Nevertheless Japan has a ‘public’ debate, which is considered crucial to science policy-makers, though monopolized by the voices of only a few social groups. Sleeboom-Faulkner describes how the views of different stakeholders are quoted and used by various political interest groups, including how these groups capitalize on raised expectations of hESC research. The past experiences of the three social groups with Japanese politics on health are linked to promises, risk perception and doubts about the future of hESCR.
Acknowledgements
The idea for this special issue on stem cell stories, and a forthcoming one on controversies in the stem cell landscape, arose during the EASST conference in August 2006 in Lausanne, Switzerland. The guest editors would like to thank all participants in the panel on ‘Global Governance of Stem Cell Therapies: Policies, Practices and Moral Systems’ for their input and insights. Also the many referees for these special issues did a great job by providing us with critical and detailed feedback, sometimes upon our rather last-minute requests. We thank all authors for their patience and enthusiasm while working towards tight deadlines. We are especially grateful to Les Levidow for his outstanding intellectual and moral support in making these special issues happen. Last but not least, we thank the Genomeresearch in Austria (GEN-AU) programme of the Austrian Federal Ministry for Science and Research and the UK Economic and Social Research Council (ESRC grant number PTA-037-27-0079) for their support.
Correspondence is welcome: please send comments to the email addresses on the title page.
References
- Birch , K. 2006 . Introduction: biofutures/biopresents, Special Issue . Science as Culture , 15 ( 3 ) : 173 – 181 .
- Braude , P. , Minger , S. and Warwick , R. 2005 . Stem cell therapy: hope or hype? (Editorial) . British Medical Journal , 330 21 May : 1159 – 1160 .
- Brown , N. 2003 . Hope against hype: accountability in biopasts, presents and futures . Science Studies , 16 ( 2 ) : 3 – 21 .
- Brown , N. and Michael , M. 2003 . A sociology of expectations: retrospecting prospects and prospecting retrospects . Technology Analysis and Strategic Management , 15 ( 1 ) : 3 – 18 .
- Brown , N. , Rappert , B. and Webster , A. 2000 . Contested Futures: A Sociology of Prospective Technoscience , Edited by: Brown , N. , Rappert , B. and Webster , A. Aldershot : Ashgate .
- Committee on the Biological and Biomedical Applications of Stem Cell Research, Commission on Life Sciences, National Research Council, Board on Neuroscience and Behavioral Health, Institute of Medicine . 2002 . Stem Cells and the Future of Regenerative Medicine , Washington, DC : National Academy Press .
- Condic , M. L. 2007 . What we know about embryonic stem cells . First Things: A Journal of Religion, Culture and Public Life , January Available at: http://www.firstthings.com/article.php3?id_article = 5420
- Editorial . 2007 . Don't be fooled by stem cell hype . New Scientist , 24 November : 3
- Evans , M. J. and Kaufman , M. H. 1981 . Establishment in culture of pluripotential cells from mouse embryos . Nature , 292 ( 5819 ) : 154 – 156 .
- Franklin , S. 1997 . “ Embodied Progress: a cultural account of assisted conception ” . London and New York : Routledge .
- Franklin , S. 2001 . Culturing biology: cell lines for the second millennium . Health , 5 ( 3 ) : 335 – 354 .
- Franklin , S. and Lock , M. 2003 . “ Animation and cessation: the remaking of life and death ” . In Remaking Life and Death: Toward an Anthropology of the Biosciences , Edited by: Franklin , S. and Lock , M. 3 – 22 . Santa Fe, NM : School of American Research Press .
- Franklin , S. and McNeil , M. 1993 . Procreation stories (Editorial Introduction) . Science as Culture , 3 ( 4 ) : 477 – 482 .
- Good , M. , Good , B. , Schaffer , C. and Lind , S. E. 1990 . American oncology and the discourse on hope . Culture, Medicine and Psychiatry , 14 : 59 – 79 .
- Holland , S. , Lebacqz , K. and Zoloth , L. 2001 . The Human Embryonic Stem Cell Debate: Science, Ethics, and Public Policy , Edited by: Holland , S. , Lebacqz , K. and Zoloth , L. Cambridge, MA : MIT Press .
- Kass , L. R. 2005 . A way forward on stem cells . Washington Post , 12 July : 21 Available at: http://www.washingtonpost.com/wp-dyn/content/article/2005/07/11/AR2005071101415.html
- Keller , E. F. 1995 . Reconfiguring Life: Metaphors of Twentieth-Century Biology , New York : Columbia University Press .
- Marshall , E. 1998 . A versatile cell line raises scientific hopes, Legal Questions . Science , 282 ( 5391 ) 6 November : 1014 – 1015 . Available at: http://www.sciencemag.org/cgi/content/full/282/5391/1014?ck = nck
- Martin , G. R. 1981 . Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells . Proceedings of the National Academy of Sciences of the USA , 78 ( 12 ) December : 7634 – 7638 .
- Miller , L. J. and Bloom , F. E. 1998 . Editorial: publishing controversial research . Science , 282 ( 5391 ) 6 November : 1045 Available at: http://www.sciencemag.org/cgi/content/summary/282/5391/1045
- Moreira , T. and Palladino , P. 2005 . Between truth and hope: on Parkinson's disease, neurotransplantation and the production of the ‘self’ . History of the Human Sciences , 18 : 55 – 82 .
- National Bioethics Advisory Commission NBAC . 1999 . Ethical Issues in Human Stem Cell Research, vol. 1. Report and Recommendations Rockville, MD Available at: www.bioethics.gov/reports/past_commissions/nbac_stemcell1.pdf
- National Bioethics Advisory Commission NBAC . January 2000 . Ethical Issues in Human Stem Cell Research, vol. 2. Commissioned Papers January , Rockville, MD Available at: www.bioethics.gov/reports/past_commissions/nbac_stemcell2.pdf
- Nielsen , T. H. 2005 . Five framings—one entity? The political ethics of human embryonic stem cells . Science Studies , 18 ( 1 ) : 30 – 51 .
- Pontifical Academy for Life . 25 August 2000 . Declaration on the Production and the Scientific and Therapeutic Use of Human Embryonic Stem Cells 25 August , Vatican City Available at: http://www.vatican.va/roman_curia/pontifical_academies/acdlife/documents/rc_pa_acdlife_doc_20000824_cellule-staminali_en.html
- Regenerative Medicine . 2007 . Aims & Scope of Journal , Available online at: http://www.futuremedicine.com/page/journal/rme/aims.jsp
- Salter , B. 2007 . State strategies and speculative innovation in regenerative medicine: the global politics of uncertain futures . Working Paper 20 , July Available at: http://www.kcl.ac.uk/content/1/c6/03/03/65/wp20.pdf
- Shamblott , M. J. , Axelman , J. , Wang , S. , Bugg , E. M. , Littlefield , J. W. , Donovan , P. J. , Blumenthal , P. D. , Huggins , G. R. and Gearhart , J. D. 1998 . Derivation of pluripotent stem cells from cultured human primordial germ cells . Proceedings of the National Academy of Sciences of the USA , 95 ( 23 ) 10 November : 13726 – 13731 .
- Smith , A. 2006 . A glossary for stem-cell biology . Nature , 441 ( 7097 ) : 1060
- Takahashi , K. , Tanabe , K. , Ohnuki , M. , Narita , M. , Ichisaka , T. , Tomoda , K. and Yamanaka , S. 2007 . Induction of pluripotent stem cells from adult human fibroblasts by defined factors . Cell , 131 30 November : 861 – 872 .
- Thompson , C. 2005 . Making Parents: The Ontological Choreography of Reproductive Technology , Cambridge, MA : MIT Press .
- Thomson , J. A. , Itskovitz-Eldor , J. , Shapiro , S. S. , Waknitz , M. A. , Swiergiel , J. J. , Marshall , V. S. and Jones , J. M. 1998 . Embryonic stem cell lines derived from human blastocysts . Science , 282 ( 5391 ) 6 November : 1145 – 1147 . Available at: http://www.sciencemag.org/cgi/content/full/282/5391/1145?ck = nck
- Thomson , J. A. , Kalishman , J. , Golos , T. G. , Durning , M. , Harris , C. P. , Becker , R. A. and Hearn , J. P. 1995 . Isolation of a primate embryonic stem cell line . Proceedings of the National Academy of Sciences of the USA , 92 ( 17 ) 15 August : 7844 – 7848 .
- Vogel , G. 1999 . Breakthrough of the year: capturing the promise of youth . Science , 286 ( 5448 ) 17 December : 2238 – 2239 .
- Vogel , G. and Holden , C. 2007 . Developmental biology: field leaps forward with new stem cell advances . Science , 318 ( 5854 ) 23 November : 1224 – 1225 .
- Wade , N. 2006 . Some scientists see shift in stem cell hopes . The New York Times , 14 August Available at: http://www.nytimes.com/2006/08/14/washington/14stem.html?fta = y
- White House Office of the Press Secretary . 9 August 2001 . President Discusses Stem Cell Research , 9 August , Crawford, Texas : The Bush Ranch . Available at: http://www.whitehouse.gov/news/releases/2001/08/20010809-2.html
- Yu , J. , Vodyanik , M. , Smuga-Otto , K. , Antosiewicz-Bourget , J. , Frane , J. , Tian , S. , Nie , J. , Jonsdottir , G. , Ruotti , V. , Stewart , R. , Slukvin , I. and Thomson , J. 2007 . Induced pluripotent stem cell lines derived from human somatic cells . Science , published online 20 November (in Science Express Reports). Available at: http://www.sciencemag.org/cgi/content/abstract/1151526v1
- Zipori , D. 2004 . The nature of stem cells: state rather than entity . Nature Reviews Genetics , 5 November : 873 – 878 .