?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Recently, the development of smart materials and the study of their properties has provided an innovative approach to the field of tissue engineering. Piezoelectrics, which are able to generate electric charge in response to mechanical stress or strain have been utilised in the stimulation of electrophysiologically responsive cells , including those found in bone, muscle, and the central and peripheral nervous systems. This area of study has experienced tremendous growth in the past decade in terms of both the array of piezoelectric materials and analytical methods by which they are evaluated in relation to specific tissue types. This review provides a critical and comprehensive overview of the most recent advances in this emerging field. Furthermore, it will extend the scope to examine the most recent developments in piezoelectric biomedical devices that extract energy from physiological processes to either power biomedical implants or act as biomedical sensors .
Introduction
Background and context
The global observatory on donation and transplantation (GODT), the most comprehensive source of data on organ donation and transplantation worldwide, reported 153,863 annual organ transplants in 2019 at a rate of 17.5 organ transplants every hour [Citation1]. This level is projected to increase in the short term at 6.25% between 2019 and 2024 [Citation2]. Typically, the only treatment for organ failure is transplantation (World Health Organisation) [Citation3], therefore, there is a significant need for biologically compatible sources of organs and tissues. Furthermore, many tissues, such as those found in the central nervous system (CNS), have no natural ability to regenerate [Citation4,Citation5]. Therefore, the production and restoration of damaged tissues and organs is a highly pertinent area of research.
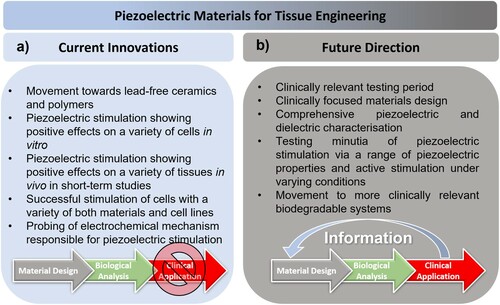
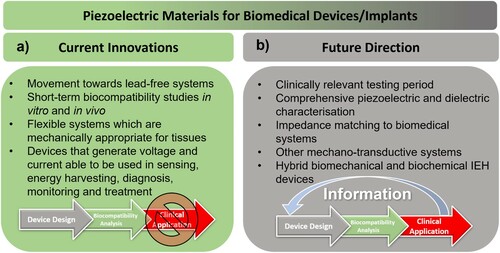
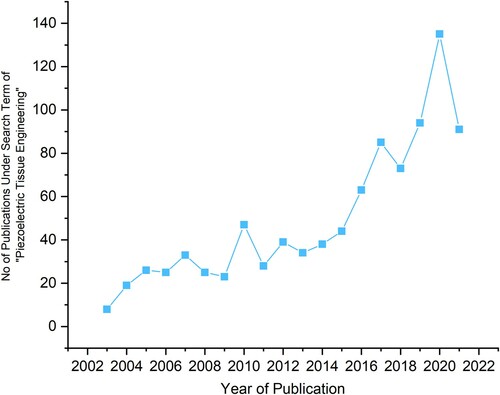
Tissue engineering has been a paradigm shift in the field of regenerative medicine. The ability to construct systems which improve and encourage the restoration and maintenance of damaged tissues has the potential to save and improve the lives of hundreds of thousands of people worldwide. Within tissue engineering, the study of smart piezoelectric scaffolds has been a burgeoning field over the past decade, where there is a peak in publication frequency in the last five years, according to publications indexed by the Web of Science (). While many recent reviews have provided in-depth synopses, they tend to be either tissue-specific [Citation6–8] or focus on applications in the wider context of biomedical devices [Citation9]. The most comprehensive reviews to date were by Ribeiro et al. [Citation10] and Hossein et al. [Citation11] in 2015, which provide overviews of piezoelectric materials and biomaterials, respectively, and provide an excellent introduction to both the background and the state-of-the-art in the field. Since the publication of these reviews there has been resurgence in research activity on these materials, along with the development in the techniques that demonstrate their promise and potential in the field of tissue engineering.
Figure 1. Most recent papers found after a search for ‘Piezoelectric Tissue Engineering’ on Web of Science, by year.
This review will not only focus on this recent resurgence in research activity but will take a deeper examination of the analytical techniques employed for characterisation and the biomedical devices which utilise these emerging technologies for clinical applications. Since tissue engineering is a multidisciplinary field that combines the collective knowledge of biology, chemistry and engineering, this review presents a collective combination of piezoelectric biomaterials, tissue types and characterisation methods to bridge the gap between the areas of expertise.
Piezoelectricity
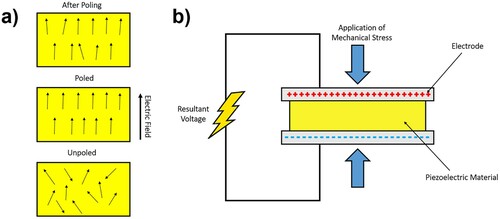
Piezoelectric materials are a class of materials that develop an electrical polarisation when strained through applied stress; this is termed the direct piezoelectric effect. Ferroelectric materials are a sub-class of piezoelectric materials whose polarisation direction can be switched by an externally applied electric field. Typically, to activate this response in a ferroelectric-based piezoelectric, the material has to be poled, by the application of a high electric field to align the dipoles (unit cells with inherent equal and opposite charges separated by a given distance). Scaffolds and biomedical devices fabricated from ferroelectric materials are, therefore, subjected to such a poling process before application. This is achieved by subjecting the materials to an electric field above the material’s coercive field (Ec) and typically at elevated temperatures to facilitate dipole and domain alignment in the electric field direction, as in (a), to overcome the multiplicity of randomly oriented domains within the material [Citation12]. The electric field is maintained while the material is cooled down to room temperature and the poling field is then removed. This poling process leads to bulk polarisation and a net piezoelectric response. Following poling, the application of strain induces a charge and a transient current that can flow in an external circuit as a result of its change in polarisation [Citation12], see (b).
Figure 2. Schematic of (a) alignment of piezoelectric dipoles in a ferroelectric material after application of an electric field above its coercive field (Ec), a remnant polarisation (Pr) after poling is achieved, (b) representation of the direct piezoelectric effect, where a charge is generated from an applied mechanical load.
Since the Curie temperature (Tc) represents the upper temperature limit and tends to be over 100°C for typical ferroelectric ceramics, the loss of polarisation and piezoelectric properties at biological temperatures in not a significant concern; for example, for BaTiO3 Tc ∼ 120°C, potassium sodium niobate (KNN) Tc ∼ 420°C and lead zirconate titanate (PZT) Tc ∼ 490°C. For non-ferroelectric piezoelectric materials, such as ZnO, the polarisation direction cannot be switched by an electric field; these materials are therefore typically used in single crystal or highly aligned form, such as nanowires (NWs), to achieve a net polarisation of the material.
The charge generated by a piezoelectric material can be defined by the coefficient dij and is characterised by the change of polarisation (C m−2) with applied stress (N m−2). This coefficient will be used throughout the review and used as a direct measure of a material’s piezoelectric potential, since it is a measure of the charge generated by an applied mechanical load, making it a useful parameter for quantifying the surface charge of the scaffolds in direct contact with tissue. In addition to the charge generated by the piezoelectric effect, the open-circuit voltage generated from applied stress can be determined by the gij coefficient, where gij = dij/ϵij where ϵij is the permittivity of the material, making it a useful parameter when discussing the potential of biomedical sensing and energy harvesting devices.
Fundamentals of tissue engineering
Tissue engineering is defined as ‘an interdisciplinary field that applies the principles of engineering and the life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function’ [Citation13] and is characterised by a triad of engineering disciplines: cell-based technologies, biomolecules/growth factors and extracellular environments/matrices. graphically illustrates this triad. In brief, cells are extracted and isolated via biopsy from a donor, they are then cultured in vitro, seeded onto a scaffold, typically with growth factors, proliferated (rapid cell number expansion by cell division) to form tissues and transplanted back into the host [Citation14]. A key aspect of engineering cells in an extracellular environment is to create an optimum cell scaffolding using substrate materials which support cell attachment, proliferation and development.
Figure 3. Pictographic overview of the key areas of research covering tissue engineering: cell-based technologies focused on improving bodies acceptance of neo-tissues through autografted stem cells, biomolecules/growth factors to help facilitate cell proliferation and differentiation of cells and extracellular environment/matrices optimisation as a platform to support cells and tissues in their growth.
Classical scaffolds
An extracellular matrix (ECM) is a four-dimensional organisation of extracellular macromolecules that provide both physical scaffolding and dynamic biomechanical and biochemical guidance [Citation15]. The best scaffolds are mimetic of the native environment of cells or ECM, mirroring its local topology, mechanical properties, physiochemical properties, local pH and the specific properties which are dependent on cell/tissue type and stage of cell damage [Citation16–18]. The range of potential scaffolds are as diverse as the tissues they support, and include polymers, biopolymers [Citation19–21], hydrogels [Citation22–24], ceramics [Citation25,Citation26] and composites [Citation27–29], with an abundance of literature over recent decades that support each type [Citation30,Citation31].
Smart scaffolds
Smart scaffolds are able to modulate their properties, in a controlled manner, in response to external stimuli [Citation32] such as temperature, pH, electric or magnetic field [Citation33] to actively enhance regeneration by mimicking the dynamic nature of the extracellular environment. The influence of electrical stimulation (ES) on cell response has been well documented in the literature through the activation of bioelectrical cues, which act as instructive signals that mediate changes in the proliferation, differentiation and migration of cells [Citation34–36]. ES has been shown to provide a variety of benefits. This includes the ability to regulate muscle cell behaviour, enhance differentiation and proliferation [Citation37–39], promote attachment and neurite outgrowth in neurons [Citation40–42], enhance adhesion, proliferation and differentiation of osteoblasts [Citation43] as well as osteogenic differentiation of stem cells [Citation44,Citation45]. This has primarily been achieved using electrically conductive scaffolds such as polypyrrole [Citation46], polyaniline [Citation47], poly(3,4-ethyl-enedioxythiophene) [Citation48] or carbon nanotubes [Citation49,Citation50]. While these materials are fascinating, conductive scaffolds are hindered by their need for an external power source and, when considering scale up to in vivo biomedical applications, become less practical.
Piezoelectric materials, on the other hand (refer to section entitled Piezoelectricity), can provide an alternative autonomous approach to electrically stimulate cells, without the need to provide an external energy source. Piezoelectric materials are a sub-set of these smart scaffolds, which use the direct piezoelectric effect to generate an AC-voltage or AC-charge when subjected to mechanical deformation, in order to actively stimulate cells. The hypothesis is that these materials can utilise mild ultrasound (US), or even the movement of a human body, to create charge that can actively stimulate cells and improve cell growth, differentiation and ultimately tissue regeneration. These new scaffolds are evaluated in Piezoelectric Scaffolds to elucidate the mechanisms by which they operate and enhance cellular regeneration.
Another variety of novel smart scaffolds are electromechanically active biomaterials. Biomaterials with naturally anisotropic structures such as cellulose [Citation51–53] or proteins such as collagen [Citation54,Citation55], silk [Citation56–58] and keratin [Citation59,Citation60] are commonly used in tissue engineering. They have also been shown to exhibit electromechanical properties through intrinsic (piezoelectricity, electrostriction and flexoelectricity) and extrinsic processes (electrochemical and electrostatic effects). However, truly macroscopic piezoelectric responses are difficult to quantify in these materials and appear to originate from other potential electromechanical coupling mechanisms [Citation61]. For further information, the reader is referred to Tofail and Bauer [Citation62], who comprehensively cover polarisable biomaterials and their interactions with surrounding biological environment, and a variety of biomaterial and protein-based nanogenerators that have been documented in the literature [Citation63–66]. This review will focus exclusively on piezoelectric materials, and biomolecular piezoelectrics for biomedical applications are discussed in the section entitled Biomolecular Piezoelectrics for Biomedical Applications.
Mechanism of piezoelectric stimulation in mammalian cells
Cells have a natural difference in electric potential between the interior and exterior of the cell across the cell’s lipid bilayer, this is known as the transmembrane potential (Vmem) and is maintained by the balance of inter- and extracellular ion concentrations. These potentials can regulate cell proliferation, migration and differentiation and are characteristic of different cell types [Citation67]. The underlying hypothesis of piezoelectric-based tissue engineering is that these endogenous electric fields can be manipulated to facilitate effects that are beneficial to the regeneration of tissues.
To understand piezoelectric-based tissue engineering we must first understand the biochemical processes which facilitate the effects observed on cell behaviour. In 1972, Cone proposed a ‘unified theory for the basic mechanism of normal mitotic control’ (control of cell division and consequently ‘growth’) in which it was observed that Vmem in non-proliferating cells can act as an obstruction for mitosis, or its associated preparative events, but upon stimulation Vmem can be changed to levels appropriate for proliferation [Citation68].
Sundelacruz et al. [Citation67] outlined the bioelectric pathways which facilitate the modulation of cell proliferation and differentiation, as outlined in . In brief, the initial bioelectrical event (ion transporters at cell membrane, transfer of ions and signalling molecules between cells and/or by breakage in epithelial sheet causing a transepithelial potential) ((a)) induces a physiological response (membrane voltage, pH gradient, ion flux and/or electric field) ((b)) which results in a variety of biochemical mechanisms (Ca2+ influx, loss of intracellular K+, electro-osmosis, electrophoretic movement of signalling molecules and/or changes in membrane voltage gates) ((c)) that trigger a secondary response that activate early response genes (genes which are activated rapidly and temporarily) ((d)). This culminates in the modulation of cell behaviour whether that be cell number (proliferation or apoptosis) or cell pathway (differentiation or dedifferentiation) ((e)). These biochemical cascades can be activated by changing Vmem, and importantly they can also be monitored by observing the biochemical and biophysical effects at differing stages of the cascade using a range of analytical techniques, many of which will be presented in this review. Therefore, piezoelectric materials, through the generation of an external ES, have the potential to change Vmem and consequently induce a response in cell behaviour such as apoptosis, proliferation and differentiation.
Figure 4. Flow diagram adapted from the work of Sundelacruz et al. [Citation67] outlining bioelectronic events, resultant biochemical and genetic sequence which modulate cell behaviour (a) source of bioelectrical signal, (b) physiological process affected, (c) proximal biophysical transduction mechanism, (d) secondary gene response, amplification and transcription effectors and (e) resultant cell behaviour.
![Figure 4. Flow diagram adapted from the work of Sundelacruz et al. [Citation67] outlining bioelectronic events, resultant biochemical and genetic sequence which modulate cell behaviour (a) source of bioelectrical signal, (b) physiological process affected, (c) proximal biophysical transduction mechanism, (d) secondary gene response, amplification and transcription effectors and (e) resultant cell behaviour.](/cms/asset/67c49851-1490-46bf-9bd7-0df229b82a8d/yimr_a_1988194_f0004_oc.jpg)
Manufacture and characterisation of piezoelectric scaffold materials
In this section, we introduce the methods of manufacture and characterisation utilised for the production and evaluation of piezoelectric scaffolds for tissue engineering. This section provides a guide that can be referred for the development of future materials, by outlining the common methods utilised throughout the literature.
Manufacture
For ferroelectric piezoceramics such as lead zirconate titanate (PZT) [Citation69], barium titanate (BT) [Citation70] or barium strontium titanate (BST) [Citation71] the most common method of production is via solid-state/mechanochemical reaction. In brief, this involves two main processes, calcining and sintering. Calcining involves the reduction of precursor oxides by heat treatment, allowing ions of the reactants to inter-diffuse across their interfaces of the ceramics constituents [Citation12]. This process is typically proceded and followed by milling to reduce particle size and maximise the interfacial surface area. Sintering involves heating the ceramic before the point of liquefaction to densify it, fusing the individual particles of ceramic to create a homogenous system. The driving force for the sintering process is the high surface area of the fine ceramic particles.
While common in the literature, solid-state synthesis is generally performed at high temperatures (∼800–1400°C), over long periods of time (hours) where volatile ion species make stoichiometric control of the ceramic unreliable [Citation72]. A development that seeks to overcome this is microwave-based heating which drastically decreases calcining and sintering times [Citation73], and can be used in combination with classical solid-state techniques [Citation74–77].
The sol–gel method is another technique which features prominently in the literature in the synthesis of barium calcium zirconium titanate (BCZT) [Citation78], ZnO [Citation79] and PZT [Citation80] based materials. It is a wet technique and has the distinct advantage of, not only being able to produce dense and porous materials, but can be used to coat other materials such as thin films or structures which contain nano-periodicity [Citation81–83]. While this process has its distinct advantages, the sol–gel process is more complex than solid-state synthesis due to its larger variety of possible precursors and more intricate processing methodology. In brief, the sol–gel process involves a metal alkoxide (M[OR]n) or metal salt precursor solutions with the necessary ionic ratio undergoing hydrolysis and polycondensation, typically under acid or base catalysis (<100°C) forming a inorganic network (sol) [Citation84,Citation85]. Due to their propensity to lose their ligands during hydrolysis and thermal treatment, and their ability to produce highly pure hydrated oxide [Citation86], metal alkoxides are the most common precursors. However, nitrates, acetates, stearates and oxides have also been utilised in the sol–gel process in the formation of piezoelectric ceramics such as BT [Citation72]. The sol produced can be used to coat or cast using a mould forming a malleable wet-gel after rapid evaporation (<100°C), which then undergoes further drying to remove any remaining solvent to crystallise and shrink the gel network, thereby making the gel compact and similar to a conventionally sintered ceramic, but at low temperature (<150°C) [Citation85]. To remove any excess organic substituent, the powder undergoes further heating (<500°C) to produce a dry-gel which can be sintered for further densification (∼1000–1400°C) [Citation78,Citation85]. In addition to the lower synthesis temperature and morphological variability, another key advantage of this process is its liquid phase, which provides a better and more homogenous reaction media compared to its pure solid-phase counterpart.
The hydrothermal process is another wet synthesis method used in the tissue engineering literature, which is primarily used to produce hydroxyapatite (HA) [Citation78]; although it has been used in the production of a variety of ferroelectric piezoelectric ceramics, such as KNN [Citation87], PZT [Citation88] and BT [Citation89,Citation90]. The hydrothermal method, like the sol–gel process, utilises low processing temperatures (∼200°C) [Citation87–90] and can be used to cast on a variety of substrates, but unlike the sol–gel process can produce thick films and piezoceramics with self-aligned structures, such as NWs and nano-rods [Citation90–93]. The process involves the treatment of aqueous solutions or suspension of precursor chemicals (a mixture of inorganic powders and solutions) at an elevated temperature in a pressurised vessel, typically over 24 h [Citation94]. A variety of precursor chemicals have been used including oxides, hydroxides, phosphates, nitrates and chlorides [Citation78,Citation87,Citation90,Citation95].
For the construction of reticulated porous structures, a foam skeleton can be utilised as a backbone on which ceramics can be built around. This approach is known as foam replication [Citation96], and has been used in the literature for the construction of BT-gelatine/hydroxyapaptite (BT-Gel/HA) composites with a high volume of interconnected porosity [Citation97]; although it is not limited to BT and has been used successfully with PZT [Citation98] and HA [Citation99,Citation100]. During the foam replication of BT-Gel/HA, a polymer foam, polyurethane (PU), was coated in a ceramic slurry of BT in a polyvinyl alcohol (PVA) solution, after which the PU was burned away during heat treatment (350°C for 30 min) and sintered (1100–1400°C) [Citation97]. The disadvantage of this technique is the mechanical fragility of the highly porous ceramic system [Citation101], although the authors consolidated the ceramic by dip-coating and in situ precipitation with gelatine and HA, respectively, and cross-linking using glutaraldehyde vapour in order to improve water resistance [Citation97].
Electrospinning is a promising technique for the introduction of nanoscale properties to a scaffold in order to mimic cells local ECM. Electrospinning involves the extrusion of fibres from a variety of polymer solution or melts at a high applied voltage (1–30 kV), charging the liquid body and utilising electrostatic repulsion to overcome the droplets surface tension to elongate it into a fibre, whose diameter is dependent of this applied voltage [Citation102]. Fibre sizes can range from 3 nm to 5 μm, depending on the viscoelastic properties of the polymer and the electrostatic field applied to the extruder [Citation103].
Scaffold characterisation
Each scaffold must be tested and evaluated in variety of different ways to determine its overall performance. This includes an evaluation of topological and chemical surface characterisation, cytotoxicity, biocompatibility, cell attachment and proliferation of cells on the materials surface, as well as a range of extensive cell assays and analyses for morphology, differentiation, protein and gene expression.
Electrical characterisation
For piezoelectric materials, their ability to polarise in the presence of an electric field is a key parameter to consider for the poling process ((a)). This inherent ability to impede the flow of electrons and induce polarisation is known as its dielectric property and is characterised by the dielectric constant (or relative permittivity). In the literature, the dielectric constant has been measured with an induction (L), capacitance (C), resistance (R) (LCR) meter on composite samples of BST/β-tricalcium phosphate (TCP) [Citation71]. The dielectric constant is a ratio of the capacitance of the material compared to that of the air and dictates how easily it can be polarised in an electric field. The higher the constant the more easily the material is polarised, therefore the smaller the electrical field required to polarise the material. A low dielectric constant also tends to lead to a higher voltage for specific applied stress, since the piezoelectric voltage constant, gij = dij/ϵij.
Figure 5. Summative schematic of (a) electrical and (b) structural and (c) mechanical characterisation of piezoelectric scaffold. dxy, piezoelectric coefficient (pC/N); PFM, piezoelectric force microscopy; LCR, inductance (L), capacitance (C), resistance (R) meter; XRD, X-ray powder diffraction; FTIR, Fourier-transform infrared spectroscopy; DSC, differential scanning calorimetry; DLS, dynamic light scattering; TGA, thermogravimetric analysis; DTA, differential thermal analysis; WCA, water contact angle; SEM, scanning electron microscopy; TEM, transmission electron microscopy; CFP, capillary flow porometery; UTM, universal testing machine; UEP, ultrasound echo pulse; AFM, atomic force microscopy.
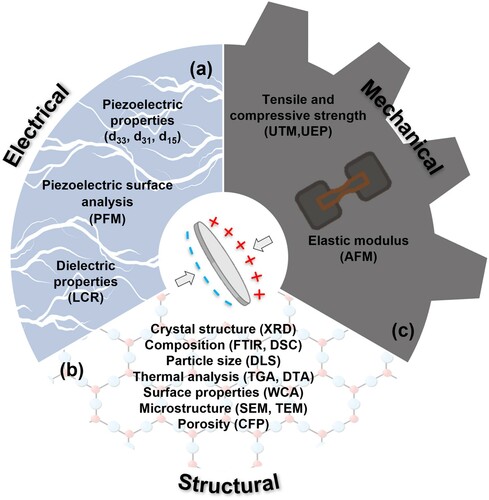
For those materials which were characterised in terms of their piezoelectric properties, this was typically performed using a d33 Berlincourt piezometer, this system operates through the application of low frequency force (<200 Hz) to a sample and comparing the charge generated by the sample under test to an in-built reference, thereby allowing the system to provide a direct d33 (C/N) measurement.
Structural characterisation
To understand the cell–scaffold interactions, it is necessary to have a detailed understanding of the material properties of scaffolds ((b)). For many piezoelectric materials, an evaluation of their crystal phase structure serves a dual purpose, first and foremost to indicate that the synthesis of the material was successful, but secondly as a means to quantify the piezoelectric response of the material as a function piezo-active phase abundance. For example, in materials such as a polyvinylidene fluoride (PVDF) polymer, the piezoelectric coefficient is directly related to the amount of ferroelectric β-phase [Citation104].
The three key techniques in identifying crystal phases are X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR) and differential scanning calorimetry (DSC). XRD utilises X-rays to reveal the structural orientation of the crystal phases based on the diffracted X-ray pattern reflected by atoms in a crystalline solid. FTIR utilises infrared (IR) radiation to study molecular vibrations of a material to provide information on the chemically relevant bonds present in the material under evaluation. This technique is utilised throughout the literature for materials such as PVDF [Citation105], BT [Citation97] and ZnO [Citation79], to identify the bonds relevant to the piezo-active materials and in some cases their surrounding composites, but also the phases these bonds belong to [Citation105]. DSC is a thermoanalytical technique used to monitor and derive the phase changes in a material and its heat capacity. The endothermic peaks observed during measurement are not only indicative of a material phase, but also the phase purity [Citation106].
Similarly, thermogravimetric analysis is a thermoanalytical technique which measures the mass of a sample with temperature and exploits the disparity between the high decomposition temperature of ceramics and the relatively low decomposition temperature of their surrounding organic composites to determine the constitution of a composite system. This can be used in tandem with DSC for full thermogravimetric-differential thermal analysis.
Water contact angle (WCA) measurements are used to determine the hydrophilicity and surface energy of a sample. This is of particular importance to tissue engineering since biointerfaces between cells and scaffolds require a balance of hydrophilic and hydrophobic surface properties, as cells are mostly water. This is achieved by measuring the angle between a water droplet and the material’s surface, where a high contact angle indicates both a low affinity for water and low surface energy. Excessively hydrophobic surfaces enhance cell affinity but reduce biocompatibility, while extremely hydrophilic surfaces prevent intracellular interactions which are vital for tissue formation [Citation107]. Another facet of bio-interface characterisation is surface roughness, which is typically determined by atomic force microscopy (AFM) and utilises a tip of <10 nm to probe the topology of a material surface.
Scanning electron microscopy (SEM) is a highly versatile microscopic technique which utilises the scattering of electrons produced by a focused beam to build an image of a scanned surface and thus can be used to not only visualise scaffold structure and porosity, but also the morphology of fixed cells grown on it. Transmission electron microscopy (TEM) works similarly but derives its image from electrons that have passed through the sample. TEM has a much higher, near atomic, resolution of <50 pm (while SEM is limited to ∼50 nm) [Citation108] and is therefore especially useful for imaging nanoparticles [Citation108,Citation109].
Dynamic light scattering (DLS) can be used to determine hydrodynamic diameter distribution of piezoelectric nanoparticles in dispersions. DLS uses the scattering of monochromatic light in a dispersion to determine particle size as a function of change in signal intensity over time, where larger particles exhibit slower intensity fluctuations with time due to their slower motion. From the measured data, a translational diffusion coefficient (D) can be derived and applied to the Stokes–Einstein equation along with other dispersion information, such as viscosity and absolute temperature to determine particles hydrodynamic diameter. This is typically achieved with a masteriser, but can also be performed on a zetasiser which determines the zeta potential (mV) of a dispersion; this is a measurement of a stability of a colloidal dispersion by comparing the difference in potential between the dispersion media and stationary media surrounding the particle. In terms of typical values of dispersion stability, 0 to ±5 mV is indicative of coagulation or high instability, ±10 to ±30 mV shows low stability, ±30 to ±40 mV shows moderate stability, ±40 to ±60 mV shows good stability and <60 mV shows excellent stability [Citation110].
Capillary flow porometry is used to quantify a variety of pore parameters. In this methodology, the pore size distribution is measured by saturating pores with a wetting liquid and blowing out the liquid with an inert gas at increasing pressure. The pressure needed to evacuate the pores is proportional to the pore size and this relationship can be quantified by the Young–Laplace equation (Equation (1)), where pc is the capillary pressure, σ is the surface tension and R is the radii of curvature (pore dimension) [Citation110–112].
(1)
(1)
Mechanical characterisation
The large variation in the mechanical properties of tissues with respect to its position in the body [Citation113] and the condition of a patient [Citation114,Citation115] imply that scaffolds for tissue engineering applications have to be equally versatile. Any scaffold being developed must therefore undergo mechanical characterisation to determine their suitability for a given tissue, as well as its ability to withstand the stresses and strains applied following implantation into the human body ((c)).
The universal testing machine (UTM) is a highly versatile tool utilised to evaluate the mechanical properties of materials by applying a load (N) at a specific rate (mm min−1) using a variety of testing fixtures. The tensile strength is the ability of a material to withstand a load under tension and compressive strength is its ability to withstand being pushed together under a compressive load [Citation116]. A similarly useful method for the analysis of the strength of brittle ceramics is the ball on three balls test; in this test a disc is supported by three balls and then axially loaded from the opposite side via a fourth ball [Citation117].
The ultrasound echo pulse method is a non-destructive technique which is highly useful for testing brittle ceramics and has been utilised in the literature to measure the elastic properties for KNN ceramics [Citation118]. The method determines the elastic properties by the transmittance of acoustic energy pulses through a sample and measurement of the pulses transit time as it reflects back through the sample with a given acoustic impedance.
In this section, we provide a summary of the cell culture assays used to assess the piezoelectric materials within the literature. Due to the interdisciplinary nature of the field, this section can be used to reference terminology, but is not considered an exhaustive list of all the methods that can be used to evaluate the applicability of materials as scaffolds for tissue engineering.
Cell internalisation
In the case of piezoelectric nanoparticles, cells must uptake the particles into their cytoplasm to allow stimulation to occur, this internalisation must therefore be confirmed and characterised. TEM is the primary technique used to visualise nanoparticles in the cytoplasm of (fixed) cells. In the literature, the resultant micrograph is described for boron nitride nanotubes (BNNT) as a ‘noncellular electron-dense material with cytoplasmatic vesicle localization, compatible in terms of appearance and size with the BNNTs’ [Citation119] discerning the nanoparticles from the surrounding cell body.
Electron energy loss spectroscopy (EELS) can be used to identify the individual (light) chemical elements of internalised nanoparticles. EELS is an analytical technique which can determine the chemical or structural properties of a sample by exposing it to electrons of known kinetic energies in a given range, the change in kinetic energy due to elastic and inelastic scattering is measured and is indicative of the specimen’s properties.
Inductively coupled plasma mass spectrometry (ICP-MS) is a type of mass spectroscopy which utilises an argon plasma to ionise the material being tested, atomising the sample and producing polyatomic ions which can be detected and characterised by mass and charge. For materials internalised by cells, they must first go through the process of cell lysis, nitric acid treatment (to completely disrupt any organic component) and freeze drying.
Cell viability and proliferation
Cytocompatibility tends to be defined by two main factors in the literature, cell viability and activity/proliferation in the presence of the material. The most common cells viability test in the literature is a LIVE/DEAD assay. The LIVE/DEAD assay is a fluorescence-based assay technique that stains cells with two different colours to differentiate living and dead populations from each other. The most common of these is based on calcein acetoxymethyl (calcein-AM) as the LIVE stain, counterstained with ethidium bromide (EtBr) [Citation119,Citation120] or propidium iodide (PI) as the DEAD stain [Citation121]. Calcein-AM is a non-fluorescent compound which can be transported through a cell’s membrane via diffusion into the cytoplasm of living cells where it can be hydrolysed by intracellular esterases to produce calcein, a strongly fluorescent compound retained in cells cytoplasm, appearing green [Citation122]. In contrast, EtBr and PI are both non-membrane-permeable DNA intercalating agents which can only permeate the membranes of dying or dead cells that have undergone rupture or degradation of their cell membrane [Citation123]; these dyes bind to nucleic acids in DNA and are thus indicative of cell death typically appearing red [Citation122].
4′, 6-diamidino-2-phenylindole (DAPI) is another example of DNA bind staining, and is primarily used in cell counting. DAPI is a fluorescent probe which is typically used once cells are fixed, therefore excluding the need to duplicate samples by allowing multiple uses of the same cells [Citation124].
Tetrazolium reduction assays such as MTT, MTS and WST-1 assays are a set of simple metabolic, colourimetric assays typical of those seen in the literature as a technique to derive the cells proliferation rate and viability through the measurement of its metabolic rate. These fall into two categories; the first which is a positively charged membrane-permeable tetrazolium salts such as MTT, these are converted to coloured formazans (absorbance ∼570 nm) by mitochondrial enzymes present in the living cell [Citation125]. The second, such as MTS and WST-1, are negatively charged tetrazolium salts which cannot readily infiltrate cells, except in the presence of an intermediate membrane-permeable electron acceptor, where they can be reduced in the cells by mitochondrial enzymes [Citation125]. Principally these all work in the same way, the reagents work colourimetrically and develop colour in response to cell activity (enzymatic reduction of the tetrazolium salt) that is directly proportional to the amount of viable and proliferating cells. This can then be measured by a spectrophotometer [Citation126].
Apoptotic phenomenona, a form of programmed cell death, have been investigated using fluorescein isothiocyanate-labeled annexin V (annexin V-FITC) with PI, a red fluorescent DNA-intercalator. During early-stage apoptosis, most mammalian cells migrate phosphatidylserine from the inner face of the plasma membrane to the cell surface. Once at the surface, the phosphatidylserine can be stained with annexin V-FITC by the strong binding between the protein annexin and phosphatidylserine [Citation127]. Necrotic cells are stained red, apoptotic cells in their early stages green, apoptotic cells both red and green and normal cells remain unstained.
Another important phenomenon to consider when understanding the longevity of cell life is the production and build-up of reactive oxygen species (ROS), which can be responsible for the damage of DNA and proteins and early cell apoptosis [Citation128]. Fluorescent assays have been used to confirm ROS production and apoptotic phenomenon in the exposure of cells to a BNNT material. ROS production was detected using an assay based on 5-(and-6)- carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2 DCFDA), which is a commonly used fluorogenic marker for the detection of ROS in living cells [Citation129]. The non-fluorescent stain permeates live cells and, when in contact with nonspecific ROS that are produced during oxidative stress, it oxidises the fluorescein compound and fluoresces green [Citation129].
Finally, the wound-healing assay is a useful tool to understand the coordinated migration of a population of cells across a ‘wounded area’ and is specifically used to monitor the proliferation of fibroblasts (NIH/3T3) [Citation105]. In brief, a cell-free area (wound) is created in a culture either mechanically, thermally or chemically, and cells then migrate into the gap and a sequence of representative images is taken over several hours or until a confluent layer is produced [Citation130].
General cell differentiation
Cellular differentiation is the process in which cells gain features are specialised to their purpose, these specialisations are unique to individual cell types and therefore must be tested and quantified with an equally wide range of methodologies. Below, we outline those methodologies which specifically apply to the methods which characterise the differentiation of the cell-lines identified.
Reverse transcriptase polymerase chain reaction (RT-PCR) is a technique used to measure gene expression by reverse transcription of RNA to a complementary cDNA strand [Citation131]. This can then be used as a template for PCR to produce millions/billions of copies of that specific strand for further study.
Western blot analysis, sometimes referred to as protein immunoblot analysis, is used to detect the expression of specific proteins. In brief, an extract of a protein source is taken from cell culture, this extract then undergoes gel electrophoresis to separate proteins by weight to provide an initial indication of the protein, although many others could be in the same weight band. The sample is then treated with monoclonal antibodies (antibodies are large Y-shaped proteins which binds to a specific protein/antigen), and this new antigen–antibody complex can be detected colourmetrically using chemiluminescence or fluorescence to quantify its abundance in the sample.
Skeletal Muscle Cells
Skeletal muscle undergoes a variety of morphological changes which can be observed to quantify differentiation, see (a). Skeletal muscle can also undergo immunofluorescence staining to determine its degree of differentiation. One way to achieve this is to analyse the presence of myosin heavy chains (MHC), a functional unit of myotubes in skeletal muscle that regulates the operation of muscle [Citation132]. Cells can be stained with an MHC-specific antibody stain (red, IgG-NL557 secondary antibody) and DAPI to determine the degree of maturation fusion of cells and therefore differentiation.
Figure 6. Summative schematic of quantification methods for differentiation in (a) skeletal muscle, (b) bone and (c) neuronal cells utilised in the literature on piezoelectric scaffolds. MHC, myosin heavy chains; ALP, alkaline phosphatase; MAP 2, microtubule-associated protein 2.
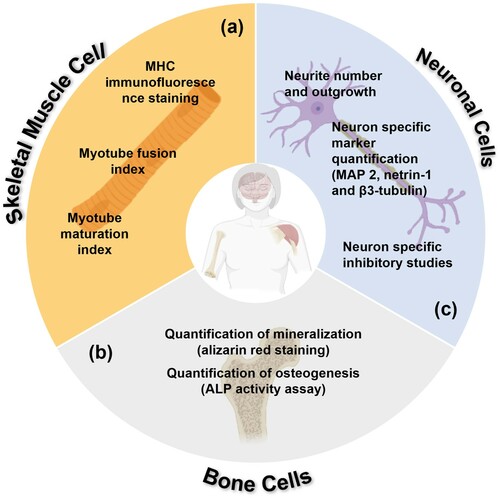
Individual myoblasts fuse during differentiation to form multinucleated myotubes; this is therefore the basis for quantification of myogenesis. The fusion index (Equation (2)) quantifies the initial fusion of myoblasts to multinucleated systems, while the maturation index (Equation (3)) quantifies the late stage development of myoblasts into myotubes, which form the base units for muscle fibres following differentiation [Citation133].
(2)
(2) Studies on average myotube length, diameter and density have also been undertaken, as myotube maturation index and length are closely associated. Myotube size is closely related to contractile force generation of muscle tissue, a key parameter for normal muscle tissue function [Citation134].
(3)
(3)
Bone cells
In the study of osteogenic differentiation, there are two key methods that are used consistently, alizarin red staining and alkaline phosphatase (ALP) activity assays, both of which help to quantify crucial aspects of osteogenic differentiation; see (b). Mineralisation, a process by which calcium phosphate is produced by bone-forming cells as part of formation of the osteoblast bone matrix, is a key phase in late stage osteoblast differentiation and is therefore a good indicator of bone cell development [Citation135]. Alizarin red is an anthraquinone dye which chelates to free ionic calcium forming a precipitate which can be visualised immediately as an intense red colour [Citation136]. ALP is a well-known marker for hard tissue cell differentiation and in osteogenesis its expression inevitably leads to the mineralisation of neo-tissues [Citation137]. ALP is critical in the degradation of phosphate compounds to release phosphate ions that react with free calcium and form hydroxyapatite in bones cells ECM [Citation135], and is therefore a pertinent marker of differentiation and maturation.
Neurons
Neuronal differentiation, like the differentiation of all cells, is characterised by many biochemical and morphological changes. In order to determine the extent of a given cultures differentiation these must be identified and quantified, see (c).
Axonal growth is an important morphological marker which can be quantified following immunofluorescent staining of specific neuronal markers. In biochemistry, immunostaining uses antibody-based methods to detect specific proteins in a sample. Wen et al. [Citation138] used this technique when attempting to characterise the expression of neuron-specific markers, MAP2 (microtubule-associated protein 2), netrin-1 and its corresponding receptor, DCC, when exploiting highly piezoelectric PZT to modulate axonal growth in rat cortical neurons. MAP2 is involved with microtubule assembly during neuritogenesis (the process of forming of new neurites which will develop into axons and dendrites) and is exclusively expressed in neuronal cell’s dendrites [Citation139]; netrin-1 is a protein involved in axonal guidance and cell migration [Citation140]. Cells were stained with protein-specific antibodies to identify axons via immunohistochemistry allowing the axonal length and cell densities to be determined from images obtained via fluorescence microscopy.
Another neuron-specific marker utilised is β3-tubulin, a key isotype of tubulin found in the neurites and cytoplasm of differentiating neuroblastoma [Citation141]. Similarly, β3-tubulin-containing cells can undergo immunofluorescent staining and the percentage of immunopositive β3-tubulin cells is derived by dividing by the total number of cell nuclei observed via a DAPI stain. Finally, to investigate the underlying mechanism of stimulation (refer to Mechanism of Piezoelectric Stimulation in Mammalian Cells), inhibitory studies can be undertaken alongside immunofluorescent staining. Inhibitory factors can be used to block certain biochemical pathways, this can be utilised to investigate which pathways are likely to be responsible for the effects induced by piezoelectric stimulation. The examples which have been primarily used are K252a, which blocks neural growth factor (NGF)-specific receptors [Citation142] and LaCl3 which blocks calcium ion channels [Citation143].
Cell bioactivity
As outlined in Mechanism of Piezoelectric Stimulation in Mammalian Cells, a key mechanism through which piezoelectric stimulation occurs is the modulation of Vmem, which is primarily achieved through influencing the dynamic of intracellular ions (Ca2+ and Na+). Intracellular Ca2+ dynamics have been monitored using fluorescence imaging in response to BNNT and ultrasound (US) stimulation [Citation109]. Cells can be incubated with Fluo-4 AM, a non-fluorescent dye which cleaves to its fluorescent counterpart Fluo-4 inside cells and can bind to Ca2+ in situ. This dye can pass the cell membranes while being attached to Ca2+ and permeate ion channels [Citation144], where the fluorescence can be measured in comparison to background fluorescence (ΔF/F0). This can similarly be performed for Na+ activity using CoroNa Green AM, a sodium ion-indicating dye [Citation145].
Ciofani et al. also performed inhibitor studies, similar to those described in the subsection entitled Skeletal Muscle Cells, to block the mechano-sensitive ion channels of neuron-like SH-SY5Y cells using gentamicin, a mechano-sensitive cation channel blocker [Citation146]. Another method to study the electrophysiology of cells is a patch clamp assay, a method used to study ionic currents in individual cells. The principle of this technique involves a glass pipette containing an electrolytic solution which seals and isolates a section of the cell membrane, and ion currents flowing through the channel can be recorded by an electrode connected to a highly sensitive amplifier [Citation147].
In vivo Implantation
In vivo implantation of scaffolds serves as a model to monitor the effect of a scaffold on living tissues and therefore provides a useful insight into its power as a biomedical implant. Many studies have been performed on Sprague–Dawley (SD) and Wistar rats (WRs), which are species of rat specifically bred for scientific research. In general, in vivo implantation involves the introduction of a defect into a given specimens tissues, followed by the introduction of a scaffold to the damaged area and monitoring its regeneration over time.
Ribeiro et al. [Citation148] performed prototypical in vivo studies on the femurs of four WR using electrospun PVDF. Two 3 mm large bone defects were made in each WR’s femur, after which the scaffolds were inserted, the wounds were sutured and the animals were returned to their cages. After four weeks, the animals were euthanised, and the femurs exhumed. The femurs were then prepared, fixed with formalin overnight, decalcified, dehydrated, embedded in paraffin to infiltrate the tissues and underwent histological analysis. The histological analysis involves a cross-sectional study of the given tissue for signs of disease by examination under a microscope. Haematoxylin and eosin (H&E) stain is typically used in this examination as the ‘gold standard’ in the pathological analysis [Citation149].
On examination of the field as a whole, extensive studies have been undertaken into the effects of piezoelectric scaffolds on cells, which include a variety of cell and tissue types, and a range of piezoelectric materials. Due to the interdisciplinary nature of the field, there is a need for standardisation when assessing materials effectiveness. First and foremost is understanding the material properties of the scaffold. There is a distinct lack of dielectric characterisation (polarisation-electric field, impedance and piezoelectric coefficients) of these scaffolds, which provides a fundamental understanding of how these materials generate charge and voltage, especially in vitro or in vivo. Consequently, this results in a lack of electrochemical characterisation of the materials surface, which would provide a more fundamental understanding of the interface between cell and scaffold. In addition, while there are some reports on the piezoelectric charge coefficients (dij) of the materials employed, few evaluate other relevant coefficients (such as gij voltage coefficients) resulting in a lack of a cohesive trend between the piezoelectric properties of the materials and improved cell and tissue outcomes.
Piezoelectric scaffolds
Having established the key characterisation methodologies across the field, both with respect to materials and cells (), we can now proceed to review the wide range of piezoelectric materials and their effects on cells. While there is no clear correlation between piezoelectric material type, its piezoelectric coefficient (such as charge, dij, or voltage, gij) and how the material is used in tissue engineering, see , it is worth inspecting the variety on materials in the field, which are summarised in the proceeding sections and in .
Table 1. Tabulation of piezoelectric materials found in the tissue engineering literature, their piezoelectric coefficeints and characterisation methods.
Lead zirconate titanate (PZT)
Lead zirconate titanate (PZT) is among the most extensively studied ferroelectric/piezoelectric materials for energy harvesting devices and wireless sensors due to its high piezoelectric coefficient (d33), which range from d33 ∼ 200–620 pC/N [Citation146–149]. Despite the high toxicity of PZT, due to the presence of Pb, the material has been investigated in tissue engineering to help elucidate the mechanisms which underpin piezoelectric stimulation.
Wen et al. [Citation138] used a poled PZT ceramic with a piezoelectric coefficient of d33 ∼ 480 pC/N to modulate axonal growth in rat cortical neurons. Cells cultured on PZT slides showed a significant increase of more than 100% in axonal extension, compared to the control material but there was also a decrease of 20–40% in cell density. In a patch clamp assay, as described in Cell Bioactivity, PZT was observed to significantly increase both the frequency and amplitude of excitatory postsynaptic currents associated with increased influx of positively charged ions (Ca2+) compared to the control; thereby suggesting that piezoelectricity could be attributed to neuronal activity. Ca2+ ions are present in neurons and are involved in the growth of dendritic spines and axons [Citation163–165]. Furthermore, immunocytochemistry confirmed a clear activation of the netrin–DCC interaction, which has been linked with neurite outgrowth [Citation166]. However, the toxicity of PZT due to lead (Pb) can lead to serious health problems, even in low doses, and has prevented further studies. Nevertheless, this work has provided new insights and increased attention to developing lead-free systems, which will be described below.
Barium titanate (BT)
BT, another ferroelectric piezoceramic, provides a lead-free alternative with a relatively high piezoelectric coefficient of d33 ∼ 330 pC N−1 [Citation167] and low cytotoxicity [Citation168]. Ciofani et al. feature prominently in the BT literature, utilising unpoled barium titanate nanoparticles (BTNP) to stimulate neuroblastoma (SH-SY5Y) both individually [Citation109] and in a composite with P(VDF-TrFE) ferroelectric polymers [Citation151]. Their earliest work in 2012 involved the stimulation of mesenchymal stem cells (MSCs) in order to enhance proliferation and differentiation, as well as elucidating the electrophysiological mechanism [Citation169].
Barium titanate nanoparticles (BTNP)
In their work, Ciofani et al. [Citation169] initially demonstrate the excellent cytocompatibility of their BTNP with MSCs up to 100 μg/mL in culture using viability (Live/Dead), especially early apoptotic phenomena (annexin V-FITC) and production of ROS (carboxy-H2 DCFDA), and proliferation assays (WST-1). Interestingly, to stabilise the BTNP from forming large aggregates, due to their hydrophobicity, they were wrapped in glycol-chitosan (GC) which is a commonly used water-soluble encapsulating polymer in the burgeoning field of theranostics [Citation170–173] and is frequently used due to its low toxicity, cytocompatibility and fast uptake by a variety of endocytic pathways [Citation174].
The primary focus of their work was to study the effects of BTNP uptake on MSCs. After confirming NP internalisation by SEM and TEM, they monitored the influence of BTNP on the arrangement and mechanical properties of the cell’s cytoskeletons via staining for the microfilament monomeric subunit f-actin. These microfilaments are a system of protein filaments that constitute a cell’s cytoskeletons, and provide cells with mechanical strength, control of shape and a means to drive motion [Citation175]. They used the staining of f-actin and AFM to determine the increased amounts of BTNP internalisation, which result in more cytoskeletal rearrangement and a stiffer cytoplasmic cell region. This was then correlated with an increase in HA deposition (quantified by OsteoImage), which is a clear indicator of osteogenesis. Furthermore, it has been reported that the regulation of the differential fate of MSCs to either adipocytes (fat cells) or osteoblasts is contingent on cell shape, more specifically its cytoskeletal tension and surrounding mechanical cues [Citation176].
Figure 7. Scheme summarising all characterisation methods utilised to test piezoelectric scaffolds with respect to (a) cell viability and proliferation, (b) in vivo implantation, (c) material properties, (d) cell internalisation, (e) cell bioactivity and (f) differentiation.
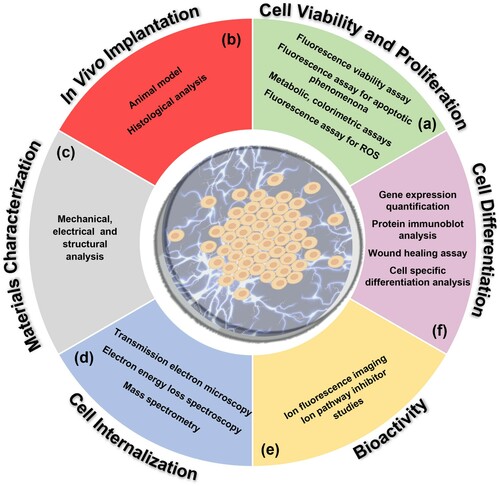
Figure 8. Graph showing the trend of piezoelectric materials, their piezoelectric coefficients and their uses in the tissue engineering literature. PZT was utilised primarily on neuronal cell lines [Citation138]. BT was utilised on bone [Citation150,Citation97] and neuronal cell lines [Citation151]. BCZT was utilised primarily on bone cell lines [Citation78,Citation152]. PVDF was utilised on bone [Citation148,Citation156,Citation153], skin [Citation105], neuronal [Citation157,Citation158] and skeletal muscle [Citation154,Citation155] cell lines. BNNT was utilised on neuronal [Citation119] and skeletal muscle [Citation159] cell lines. ZnO was utilised on neuronal [Citation160], skin [Citation79] and cell lines and non-specifically [Citation161]. BST was utilised primarily on bone cell lines [Citation71]. KNN and LN were utilised primarily on skin [Citation118] and bone [Citation162] cell lines, respectively.
![Figure 8. Graph showing the trend of piezoelectric materials, their piezoelectric coefficients and their uses in the tissue engineering literature. PZT was utilised primarily on neuronal cell lines [Citation138]. BT was utilised on bone [Citation150,Citation97] and neuronal cell lines [Citation151]. BCZT was utilised primarily on bone cell lines [Citation78,Citation152]. PVDF was utilised on bone [Citation148,Citation156,Citation153], skin [Citation105], neuronal [Citation157,Citation158] and skeletal muscle [Citation154,Citation155] cell lines. BNNT was utilised on neuronal [Citation119] and skeletal muscle [Citation159] cell lines. ZnO was utilised on neuronal [Citation160], skin [Citation79] and cell lines and non-specifically [Citation161]. BST was utilised primarily on bone cell lines [Citation71]. KNN and LN were utilised primarily on skin [Citation118] and bone [Citation162] cell lines, respectively.](/cms/asset/17f6e074-fb7d-4101-a9e2-095baa0a9bc3/yimr_a_1988194_f0008_oc.jpg)
Ferroelectric BTNP with an asymmetric tetragonal crystal structure have been utilised to electrically stimulate neuron-like SH-SY5Y cells through the application of ultrasonic (US) stimulation to observe the effects on calcium and sodium influx [Citation109]. CFM showed strong associations between BNTP (red) and SH-SY5Y’s plasma membrane (green), with no internalisation ((a)). Under ultrasonic stimulation a high influx of Ca2+ and Na+ transience moving through ion channels was observed in cultures with BTNP when compared to cultures with no NP’s, with no transience under similar conditions ((b)). A deeper investigation of this pathway performed with Ca2+ and Na+ ion channel inhibitors under stimulation shows that the transience in the uninhibited channel was greatly reduced in the presence of both inhibitors, with and without ultrasonic stimulation. The origin of Ca2+ was found to be stored in the endoplasmic reticulum (ER). The observation of high Ca2+ peaks (ΔF/F0) in the presence of gentamicin, a mechano-sensitive cation channel blocker [Citation146], when compared to the lower peaks without BTNP, indicates that the effects observed under dynamic conditions are a result of piezoelectric stimulation of BT. This agrees with the lack of an observed response for non-ferroelectric and non-piezoelectric cubic BT crystals, whose transience was the same as ultrasound on its own. Furthermore, higher frequency ultrasonic stimulation was shown to increase local temperature [Citation177] which causes the ER to release Ca2+ [Citation178]. When tested, with an ER-specific heat-sensitive fluorescent dye, the application of ultrasonic stimulation was shown to increase the temperature of the ER by 1.66 ± 0.30°C and 1.68 ± 0.31°C with and without BTNP respectively, but no significant differences in the two conditions were seen. Ciofani et al. provided an interesting insight into the effects of piezoelectric stimulation, with their studies indicating that ferroelectric domains from the piezoelectrically active tetragonal phase of BT provide the means for stimulation of neuron-like cells. This raises a question for piezoelectric stimulation as to the distinction of ferroelectric and non-ferroelectric states vs poled/unpoled conditions and how they individually contribute stimulation of cells.
Figure 9. (a) Barium titanate nanoparticles (BTNP) characterised by confocal fluorescence microscopy of BTNPs associating with SHY5Y cells and cell's neurites. (b) Calcium imaging of SH-SY5Y-derived neurons in response to ultrasonic stimulation at 0.8 W cm−2 without BTNP and with BTNP [Citation109]. (Reproduced from Ref [Citation109] with permissions of American Chemical Society (Copyright 2018)).
![Figure 9. (a) Barium titanate nanoparticles (BTNP) characterised by confocal fluorescence microscopy of BTNPs associating with SHY5Y cells and cell's neurites. (b) Calcium imaging of SH-SY5Y-derived neurons in response to ultrasonic stimulation at 0.8 W cm−2 without BTNP and with BTNP [Citation109]. (Reproduced from Ref [Citation109] with permissions of American Chemical Society (Copyright 2018)).](/cms/asset/7a0c081c-91fe-4fb6-b54f-3c6771bfcea1/yimr_a_1988194_f0009_oc.jpg)
BT scaffolds
Liu et al. [Citation150] tested well-dispersed poled polycaprolactone/barium titanate (PCL/BT) composites with a d33 ∼ 0.5–3.9 pC/N and a BT volume fraction of 15–40% as scaffolds for MG63 osteoblast cells. The MTT assay shows that the PCL/BT composite scaffolds (15 vol.% BT) have an adverse effect on cell activity compared to cells without scaffolds, although the authors claim there is no obvious cytotoxicity. As both BT and PCL are biocompatible with a variety of cell types [Citation164,Citation174–176], this discrepancy could be a product of undesirable surface roughness [Citation179].
Ehterami et al. [Citation97] studied porous BT-gelatine/hydroxyapaptite (BT-Gel/HA) composite scaffolds to determine the effects of polarisation on the viability, adhesion and proliferation of MG63 osteoblast-like cells via MTT assay and SEM. Gel/HA-coated porous BT scaffolds were prepared by foam replication. Hydroxyapatite, an essential inorganic component of normal bone [Citation178,Citation180], was added to improve biocompatibility and a gelatine coating was added to improve water resistance. Piezoelectric coefficients of d33 ∼ 1.5–4.5 pC/N were measured [Citation181]. The Gel/HA coating was also shown to significantly increase the compressive strength, compared to the uncoated scaffolds, which increased with increasing sintering temperature.
In all groups tested, the cell density of both the coated and uncoated was consistently higher than the control, with the coated scaffold showing a higher cell density than the uncoated, see (a). Cell morphology was also studied (SEM), where it was initially observed that the cells attached to the pore walls for all scaffolds. Slender cytoplasmic projections, which are indicative of cell sensing, movement and intracellular interaction (filopodia) [Citation182], were also observed. For the uncoated scaffolds, those which were poled showed not only improved cell attachment but far more filopodial extensions. The authors hypothesised that cations can attract proteins (integrin and fibronectin) which mediate cell adhesion and proliferation through electrostatic interactions [Citation183,Citation184]. In the case of both poled and unpoled Gel/HA-coated scaffolds, cell adhesion and proliferation were far greater with a greater amount of cytoplasmic projections (lamellipodia and filopodia extensions), with both growing a confluent layer, although no clear difference was seen between the polarised and non-polarised variants.
Figure 10. (a) MTT assay of MG-63 cell culture on uncoated and Gel/nHA coated BT scaffolds after 1, 3 and 7 days [Citation97]. (b) Calcium imaging analysis of SH-SY5Y neuroblastoma cells differentiated on P(VDF-TrFE) and P(VDF-TrFE)/BTNP films following US stimulation. (c) Percentages of β3-tubulin positive cells. (d) Neurite lengths are expressed as median values [Citation151]. (Reproduced from Ref [97] and [151] with permissions of Elsevier (Copyright 2018) and John Wiley and Sons (Copyright 2016) respectively).
![Figure 10. (a) MTT assay of MG-63 cell culture on uncoated and Gel/nHA coated BT scaffolds after 1, 3 and 7 days [Citation97]. (b) Calcium imaging analysis of SH-SY5Y neuroblastoma cells differentiated on P(VDF-TrFE) and P(VDF-TrFE)/BTNP films following US stimulation. (c) Percentages of β3-tubulin positive cells. (d) Neurite lengths are expressed as median values [Citation151]. (Reproduced from Ref [97] and [151] with permissions of Elsevier (Copyright 2018) and John Wiley and Sons (Copyright 2016) respectively).](/cms/asset/d82592c0-aefd-491d-bddf-7936d4ae105c/yimr_a_1988194_f0010_oc.jpg)
Genchi et al. [Citation151] prepared homogenous P(VDF-TrFE) (70/30 copolymer) and P(VDF-TrFE)/BTNP (60 wt-% BTNP) films for stimulation of SH-SY5Y. Surface properties were quantified by AFM and piezoelectric force microscopy (PFM), showing the BTNP composites had a rougher surface (212 vs 63 nm) and a 4.5-fold higher local piezoelectric coefficient (d31 ∼ 11.8 pm V−1 compared to d31 ∼ 53.5 pm V−1). Moreover, XRD confirmed that both the fraction of ferroelectric and piezoelectric β-phase of the films increased between the P(VDF-TrFE) and P(VDF-TrFE)/BTNP from 30% to 50%, and that the non-ferroelectric α-phase decreased from 30% to 15%. However, a consequence of doping BTNP with P(VDF-TrFE) was a lower ultimate tensile strength, extension at maximum load and at break, indicating the doped scaffolds were more brittle and less flexible compared to pure P(VDF-TrFE).
For viability and proliferation (LIVE/DEAD and PicoGreen, respectively), scaffolds were compared to cell culture plastic (CCP). Although all scaffolds were highly comparable and showed no dead cells, there was a clear difference in cell density. Both P(VDF-TrFE) and P(VDF-TrFE)/BTNP performed worse than CCP after 24 and 72 h, with P(VDF-TrFE)/BTNP having the lowest cell density. Ca2+ transients were measured in ultrasound (US)-stimulated cell culture (calcium imaging analysis). The CCP showed no significant increase in transients, indicating US stimulation alone cannot facilitate an increase in transients, thereby consolidating conclusions found in the research of the Ciofani group on BTNP in SH-SY5Y cell culture [Citation109]. Both scaffolds showed considerable peaks in Ca2+ transients upon US stimulation, although the BTNP-doped scaffolds produced a much higher response (ΔF/F0 = 7.16 ± 0.51 vs ΔF/F0 = 3.54 ± 0.35) ((b)). Neurite median length and percentage of β3-tubulin positive cells were visualised and quantified with and without ultrasonic stimulation by staining (β3-tubulin antibody and DAPI stain). Ultrasonic stimulation significantly increased the amount of β3-tubulin positive cells on both the plain and BTNP-doped films compared to the other test groups ((c)). The same trend was observed in neurite outgrowth with the ultrasound stimulated, the plain and doped P(VDF-TrFE) films providing the best performance ((d)).
Barium strontium titanate (BST)
BST is a ferroelectric ceramic and is particularly interesting since Sr2+ has been shown to stimulate proliferation of osteoblasts, while supressing the differentiation of osteoclasts, a type of bone cell which breaks down bone tissue [Citation185]. Tariverdian et al. [Citation71] integrated BST into 0–50 wt-% β-tricalcium phosphate (β-TCP), which is an osteoconductive matrix material that biodegrades into calcium and phosphorus ions that can facilitate osteoblast activity [Citation186]. BST/β-TCP scaffolds were either pressed into disks or underwent 3D-printing by injecting the solution to produce a 3D interconnected macroporous structure. Density and compressive strength testing (uniaxial pressing) of the disks showed that a higher density correlated to increased compressive strength. However, the density varied in a non-linear manner due to the interaction of β-TCP on the composite grain boundaries. Finally, the dielectric constant/relative permittivity (εr) of the composites (measured by an LCR meter) increased from εr ∼3 to εr ∼104 as the BST content increased from 60 to 100 wt.%, in agreement with previous research [Citation182,Citation183].
The scaffold biodegradation rate was monitored under dynamic conditions in simulated body fluid by measuring the change in scaffold mass and the surrounding solution pH. Although pure BST showed a diminutive mass loss, the β-TCP composites showed not only steady degradation but an increase in degradation rate with an increase in β-TCP content. As the samples decreased in mass, there was an increase in pH as a result of the release of Ca2+ ions from the β-TCP (Ca3[PO4]2) matrix. Galow et al. [Citation187] reported that an alkaline pH (pH ∼ 8.0–8.4) increased the proliferation of osteoblasts, therefore an increase in alkalinity due to degradation could play a beneficial role in further enhancing bone regeneration. In addition, the surface bioactivity of scaffolds was characterised by the presence of HA deposition using SEM and energy-dispersive X-ray spectroscopy (EDX). Both methods showed the presence of apatite crystals and clear peaks in the EDX associated with crystals on the surface of all samples, except 100% BST. The amount of HA on the surface increased as the amount of β-TCP in the composite increased.
An MTT assay was performed on human bone marrow-derived mesenchymal stem cells (BM-hMSC) to assess scaffold cytotoxicity. Although all composites had a lower cytocompatibility compared to the control, the difference was not sufficiently large to regard the scaffolds are cytologically toxic. Light microscopy was then used with alizarin red staining on BM-hMSC to quantify mineralisation. It was observed that as the β-TCP level increases so does the intensity of the red stain, indicating an increase in mineralisation with the highest intensity observed for BST with 60 wt-% β-TCP. Finally, an ALP assay was performed on MG63 cells to determine osteoblast activity. Following testing, the BST with 60 wt-% β-TCP was observed to have the greatest ALP activity. This is likely due to the material having the highest β-TCP content as phosphate and calcium ions are important for ALP (section entitled Bone Cells). It would be of interest in further work to compare these scaffolds to pure β-TCP and probe both ends of the compositional extremes.
Barium calcium zirconium titanate (BCZT)
BCZT has also been evaluated due to its ferroelectric and piezoelectric properties (d33 ∼ 650 pC/N) [Citation188]. Poon et al. [Citation189] prepared BCZT by a solid-state reaction and tested cell cytotoxicity and proliferation with primary human osteoblast (HOB) cells and primary human umbilical vein endothelial cells (HUVEC). Initially, the piezoelectric properties of BCZT were tested and a polarisation-electric field hysteresis loop was derived ((a)) with piezoelectric coefficients of d33 ∼ 280 pC/N. The cytotoxicity and morphology of HOB and HUVEC were observed using a LIVE/DEAD assay and compared to polystyrene (PS), a widely accepted polymer surface for cell culture [Citation190]. For all scaffolds, both cell lines presented well-spread filopodia, but significantly more HOB cells were detected with BZCT scaffolds compared to a PS control. Following this, cell viability was determined by the WST assay where consistently high cell viability was seen for the BCZT scaffolds for both cell lines compared to PS ((b)). Cell proliferation was measured using a cell counter (Scepter™ 2.0) ((c)). HUVECs and HOB cells both showed consistent proliferation over the initial 7 days, but this decreased towards the end of the study at 10 days, while PS showed a consistent cell number increase over the whole testing period for both cell types.
Figure 11. (a) d33 hysteresis loop for BCZT, (b) proliferation and (c) absorbance of mitochondrial dehydrogenase activity of HUVECs and HOB cells on BCZT and PS as a control [Citation189]. (d) MC3T3 cell proliferation observed for the various HA-BCZT nanocomposites [Citation78]. (Reproduced from Ref [189] and [78], licensed by John Wiley and Sons and De Gruyter respectively, open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/)).
![Figure 11. (a) d33 hysteresis loop for BCZT, (b) proliferation and (c) absorbance of mitochondrial dehydrogenase activity of HUVECs and HOB cells on BCZT and PS as a control [Citation189]. (d) MC3T3 cell proliferation observed for the various HA-BCZT nanocomposites [Citation78]. (Reproduced from Ref [189] and [78], licensed by John Wiley and Sons and De Gruyter respectively, open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/)).](/cms/asset/d7065ab0-a96c-4921-872a-1d5f3fd42d9b/yimr_a_1988194_f0011_oc.jpg)
Manohar et al. [Citation78] produced BCZT-HA composites of varying compositions (10–50 wt.% BCZT) via hydrothermal and sol–gel synthetic routes for HA and BCZT, respectively. The composites were poled, but only pristine BCZT (d33 ∼ 304 pC/N) and 50:50 HA-BCZT (d33 ∼ 7 pC/N) showed measurable d33 values. The effect of the composites on proliferation was quantified colourimetrically by an MTT assay test using an MC3T3 cell line on unpoled scaffolds of varying weight ratios. For all weight ratios, the composites showed greater cell proliferation compared to pristine HA, with the highest proliferation observed at 10 wt-% BCZT, see (d). It would be of interest to repeat these studies on poled composites at both extremes of high and low d33 values to explore the link between piezoelectric activity (d33 magnitude) and cell response. It would also be of critical importance to perform dielectric characterisation to determine why composites with up to 90% BCZT loading showed no measurable d33 coefficients, when similar composites made with BT showed high piezoelectric coefficients of d33 ∼ 57.8 ± 10.8 pC/N [Citation191].
Polyvinylidene fluoride (PVDF)
Discovered by Professor Heiji Kawai in 1969 [Citation192], PVDF is a flexible, non-toxic ferroelectric polymer [Citation193] that has found prominence in the biomedical field for sensing [Citation194–198] and as a scaffold for tissue engineering [Citation199–201]. Although PVDF does not have the high piezoelectric d33 charge coefficient of the ferroelectric ceramics, it has the advantage of being a polymer, and is therefore easily processable [Citation202] and can be flexible and formed into a variety of 3D structures [Citation203]. It is therefore adaptable for use on different kinds of tissues that require different mechanical and topological properties. The low permittivity of the material also leads to relatively high voltage coefficients, gij = dij/ϵij. Ribeiro et al. have assessed PVDF both in vitro and in vivo, demonstrating its capabilities for tissue engineering for bone and muscle cell lines.
Ribeiro et al. [Citation153] monitored the cellular response of MC3T3-E1 on poled and unpoled PVDF (d33 ∼ −32pC N−1), with and without a titanium electrode layer (∼ 30 nm). They grew cells on the positively poled (+) PVDF surface, as studies have shown that osteoblast cells show better adhesion to and proliferation on the positively charged surfaces of hydroxyapatite, a naturally occurring mineral found in bones [Citation204,Citation205]. Surface characterisation (AFM and WCA) showed the titanium layer increased surface roughness and decreased wettability, while (+) poling proved to produce the most hydrophilic scaffolds. MC3T3-E1 cell viability was validated (LIVE/DEAD assay) and MTT assay was performed under both static and dynamic conditions (1 Hz). Following the testing period, the poled (+) PVDF under static conditions had the greatest proliferation rate with and without a titanium electrode. However, throughout the initial stages of the study during days 1–3, the (+) PVDF that was subjected to dynamic stimulation showed significantly improved proliferation compared to all other groups tested.
Similarly, Ribeiro et al. [Citation154] also reported the response of C2C12 myoblast cells on poled and unpoled PVDF of varying morphologies such as films, aligned fibres and randomly oriented fibres. The LIVE/DEAD assay showed high cell viability on all scaffolds and cells clearly aligned on scaffolds that were fibrous. Moreover, fluoresce microscopy (DAPI) showed that the poled scaffolds showed the highest cell adhesion and proliferation, with the (−) poled scaffold performing the best. These results were mirrored following MTS activity assay (see Cell Viability and Proliferation), as shown. Both the unpoled aligned and randomly oriented fibres showed elongated cell morphologies along their fibre length under SEM. This degree of directionality is highly useful for muscle tissue growth. However, they also showed the lowest proliferation and activity compared to the glass control. It is of interest to note that WCA measurements showed the (–) and (+) poled scaffolds to be the most hydrophilic with contact angles of 45.0° and 51.3°, respectively.
Ribeiro et al. [Citation155] subsequently undertook further in vitro studies with PVDF (d33 ∼ −32 pC/N) on C2C12 myoblasts using immunofluorescence stain in characterisation to analyse the presence of MHCs and determine the degree of cell maturation in two different growth media. They used basal medium (BM), a general cell growth medium that supports cell growth in mammalian cells, and differentiation media (DM) to enhance myoblast differentiation. All samples showed a clear presence of MHC, indicating that even without DM the PVDF was sufficient to promote myogenic differentiation. This differentiation was further quantified as a function of fusion index and maturation index, where the poled PVDF performed the best in respective measurements. The positively poled PVDF had the highest fusion index in both media, while both positively and negatively poled surfaces showed similar maturation indices. Studies on average myotube length, diameter and density were also undertaken since the myotube size is closely related to the contractile force generation of muscle tissue, which is a key parameter for good muscle tissue function [Citation134]. Overall, the poled scaffolds led to the longest myotubes, with and without DM in respective studies, and the myotube diameter was generally the same, except for a large mean diameter in the control cultures.
Stem cells have a great potential in the field of tissue engineering but one of their key drawbacks is their scarcity in the human body, since they are typically derived from bone marrow MSCs which are only 1/34,000 of the cells population [Citation155,Citation206]. Ribeiro et al. [Citation156] attempted to overcome the scarcity of this typical stem cell source by stimulating adipose-derived stem cells (ADSC) which occur at a higher frequency of 1/50 [Citation207]. Ribeiro et al. [Citation156] also utilised PVDF (d33 ∼ −32 pC/N)-coated with fibronectin (both covalently and non-covalently) to stimulate human adipose stem cells (hASC) and promote osteogenic proliferation. Osteogenic differentiation was quantified by ALP activity assays under both static and dynamic conditions in a bioreactor ((a)) (1 Hz, 1 mm amplitude) in both BM and an osteogenic medium (OM). The highest ALP activity was seen in those cells grown in OM under static and dynamic conditions, in most cases and conditions the (−) poled scaffolds performed the best, although this was difficult to assess due to the lack of a control material. This is particularly important in the context of previous studies by Ribeiro et al. when PVDF has been shown to perform less well than glass during MTS studies performed on MC3T3-E1, a pre-osteoblast cell line [Citation208] and to have performed similarly to glass during MTT studies on MC3T3-E1, an osteoblastic cell line [Citation209].
Figure 12. (a) Differentiation of hASCs on different PVDF films conditions in different media quantified by ALP assay over 15 days [Citation156]. WR specimens underwent (b) implantation of PVDF under a variety of conditions, (c) non-poled PVDF, (d) poled PVDF, (e) randomly oriented PVDF fibres and (f) no scaffold as a control scaffolds, into their femurs, after 4 weeks the femurs were exhumed and underwent H&E [Citation148]. (Reproduced from Ref [156] and [148] with permissions of John Wiley and Sons (Copyright 2014) and Elsevier (Copyright 2017) respectively).
![Figure 12. (a) Differentiation of hASCs on different PVDF films conditions in different media quantified by ALP assay over 15 days [Citation156]. WR specimens underwent (b) implantation of PVDF under a variety of conditions, (c) non-poled PVDF, (d) poled PVDF, (e) randomly oriented PVDF fibres and (f) no scaffold as a control scaffolds, into their femurs, after 4 weeks the femurs were exhumed and underwent H&E [Citation148]. (Reproduced from Ref [156] and [148] with permissions of John Wiley and Sons (Copyright 2014) and Elsevier (Copyright 2017) respectively).](/cms/asset/dda8f4fb-50c8-4240-bbea-af0045a2fcd7/yimr_a_1988194_f0012_oc.jpg)
This investigation was in agreement with Li et al. [Citation210] who proposed a PVDF scaffold for pre-differentiated ADSC for peripheral nervous system damage repair. Conductive scaffolds have been shown to enhance neural differentiation of stem cells [Citation211–213], more importantly, this has recently been shown on ADSC [Citation214,Citation215] and well as differentiation into other cell lines such as osteoblasts [Citation216].
Ribeiro et al. [Citation148] performed in vivo studies on four WRs ((b)) using electrospun PVDF (fibre diameters of 500 nm with a d33 ∼ −24 pC/N). Upon defect introduction, the degree of regeneration was quantified by obtaining cross-section images through the site of the defect. Non-poled PVDF showed no regeneration around the defect ((c)) and was worse than that of the control with no scaffold ((d)). In comparison, the poled PVDF scaffolds ((e)) showed the formation of bone marrow and trabecular bone, which almost filled the whole defect. Randomly oriented PVDF fibres showed advanced regeneration with cell infiltration of the porous structure of the spun system, but with some inflammatory cell infiltration ((f)). However, both spun and unspun (poled and unpoled) materials were shown to have improved regeneration compared to no scaffold.
Hoop et al. [Citation157] studied the effect of PVDF (d33 ∼ −30 ± 2 pC/N) and ultrasound stimulation on neuron-like PC12 cells in order to elucidate the mechanism of piezoelectric stimulation. β-PVDF was compared to non-ferroelectric and non-piezoelectric α-PVDF and tissue culture plastic (TCP) as controls, and all were treated in poly-L-lysine to increase cell adhesion. PC12 cell cultures were exposed to ultrasound and compared to unstimulated cultures as a negative control and NGF was used as a positive control. A phase-contrast microscopy was used to monitor neurite outgrowth. In these studies, only neurites 10 μm or larger were considered differentiated neurites. The only significant outgrowth was seen for the cells grown with NGF (34.5± 9.5 μm) and on poled and ultrasonically stimulated β-PVDF (22.9 ± 6.8 μm), see (a).
Figure 13. (a) Diagrammatic representation of neuronal stimulation on piezoelectric scaffold via ultrasound and neurite length (µm) measured under both US stimulate and unstimulated condition. Illustration of intracellular pathways affecting PC12 differentiation being impeded by (b) K252a, (c) LaCl3 and (d) RV. Inhibitor studies using K252a,RV and LaCl3 showing change in average neurite length of PC12 with (e) NGF, (f) β-PVDF and (g) both β-PVDF and NGF in vitro [Citation157]. (Reproduced from Ref [157] licensed by Springer Nature, open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/)).
![Figure 13. (a) Diagrammatic representation of neuronal stimulation on piezoelectric scaffold via ultrasound and neurite length (µm) measured under both US stimulate and unstimulated condition. Illustration of intracellular pathways affecting PC12 differentiation being impeded by (b) K252a, (c) LaCl3 and (d) RV. Inhibitor studies using K252a,RV and LaCl3 showing change in average neurite length of PC12 with (e) NGF, (f) β-PVDF and (g) both β-PVDF and NGF in vitro [Citation157]. (Reproduced from Ref [157] licensed by Springer Nature, open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/)).](/cms/asset/3aa6ec06-06a4-454d-8021-ffa9ae4fc51a/yimr_a_1988194_f0013_oc.jpg)
Inhibitory studies were then performed to determine the mechanism by which piezoelectric stimulation occurs. PC12 cultures were treated with three inhibitors K525a and roscovitine (RV), to block NGF-specific pathways ((b,c)) and LaCl3, a nonspecific calcium ion channel blocker ((d)) that has been observed to completely inhibit electrically induced differentiation [Citation143]. K252a and RV inhibited NGF-treated PC12 cells, decreasing the average neurite length. K252a completely inhibited any differentiation and RV mainly reduced neurite growth, while LaCl3 partially decreased neurite length ((e)). For the stimulated PC12s, K252a and RV had no effect on neurite growth, but LaCl3 significantly reduced average neurite length, indicating the positive effects of piezoelectric stimulation are likely to be due to the activation of calcium channels ((f)). Finally, Hoop et al. tested ultrasonically stimulated β-PVDF with NGF to determine if any synergistic effects occur, but they found no significant increase in neurite length compared to NGF alone ((g)), indicating that mutual activation of these independent pathways does not affect ‘differentiation efficiency’ [Citation157].
Royo-Gascon et al. [Citation158] tested the effects of PVDF (d31 ∼ 22.6 ± 1.2 pC/N) on a mixed culture of spinal cord neurons obtained from rat embryos. Following rheological analysis, the voltage output across the 20 μm films was found to peak at 52 mV under experimental conditions, using culture plates filled with water. Proceeding cell culture, the PVDF was coated in poly-D-lysine. Poled and unpoled PVDF films were then tested with and without mechanical stimulation (via a mechanical vibration isolation platform), neurons were stained for MAP2 and underwent fluoresce microscopy. Neurons with ≥180° of spatial freedom were analysed for better identification of individual neurons and to eliminate contributions of potential cell–cell interactions. Neurons were then imaged and the branching points, terminal points and total number of neurites and neuronal density were analysed.
Although the vibrational conditions had little effect on cells grown on the non-poled films, the poled films showed an increase in branching points (116%), number of terminal points (84%) and neurites (79%) during vibration. This was similarly reflected in neuronal density measurements, while mechanical stimulation reduced the density of neurons on non-poled PVDF, it greatly increased the cell density on poled samples (115%). The neurite number as a function of branching order on the poled materials showed an increase in all orders of branching complexity measured when the vibrational stimulation was applied. Sholl analysis [Citation217] was used to quantify the number of dendric branches at the point which a neuron splits into new dendric trees (intersection points). The unpoled films, both stimulated and non-stimulated, showed few neurites after 75 μM of a dendric branch, while the piezoelectric films showed neurites significantly further up the dendric branch (100 μM) and more intersections than those on the stimulated unpoled films.
Guo et al. [Citation105] prepared polyurethane/polyvinylidenefluoride (PU/PVDF) scaffolds via electrospinning for both in vitro studies on NIH/3T3 fibroblast and in vivo studies on SD rats. The mechanical and piezoelectric properties of the scaffolds were characterised for a variety of different PU:PVDF compositions/ratios (1:3 to 3:1) and a ratio of 1:1 was determined to be the most optimal due to a balance of piezoelectric properties (d33 determined by thin/thick film piezoelectric analyzer) and mechanical properties (tensile strength and elongation at break determined by UTM). The proliferating NIH/3T3 fibroblast cells on PU/PVDF had normal cell morphology, from SEM and LSCM, indicated by secretion of the ECM, proliferation and forming of a cell layer that covered almost the whole scaffold. Cell viability and proliferation were measured colourimetrically using a WST-8 assay and were comparable to TCP. A wound-healing assay was performed to monitor cell migration under dynamic stimulation provided by a computer-regulated bioreactor and the wound healing rate was measured periodically. It was observed that the PU/PVDF scaffolds that were subjected to dynamic stimulation showed the most rapid wound healing. After 24 h, the poled and stimulated scaffolds showed a confluence of 100%, compared to the control scaffolds (unpoled and stimulated PU/PVDF, and dynamically stimulated PU) which both had a confluence ∼ 50%. Similar effects were also observed with respect to cell attachment. Under dynamic stimulation, 1.6 times as many cells adhered to the surface of the PU/PVDF scaffolds compared to the control scaffolds.
RT-PCR and WBA were used to quantify the mRNA expression levels and specific proteins relevant to cell upkeep; collagen type I (Colla1), elastin (Eln) and fibronectin 1 (Fn1). In all cases, the stimulated, poled PU/PVDF scaffolds expressed the highest levels of all proteins compared to the control scaffolds. In vivo testing was performed on SD rats where the PU and PU/PVDF scaffolds were implanted in the vertex, abdomen and the back of the rats and subjected to different degrees of bending. During testing, no signs of systematic or neurological toxicity were detected. Cells were stained with H&E and characterised by flow cytometry, which showed primarily fibroblasts in all regions. Samples taken in vivo were then compared by level of fibrosis (wound healing) where in all cases the PU/PVDF scaffolds proved superior with respect to wound healing. The fibrosis levels of PU/PVDF scaffolds implanted in the back and abdomen were comparable to those found in the vertex, which naturally has a richer blood supply but undergoes much less deformation than the other regions due to its role in supporting the cranium.
Boron nitride (BN)
Ciofani et al. have promoted the use of non-ferroelectric piezoelectric BNNT for both in vitro [Citation119–218] and in vivo [Citation219] testing and use on freshwater flatworms [Citation220]. They utilised stable dispersions of BNNT-glycol-chitosan (GC) to stimulate PC12 and SH-SY5Y cells and monitor neurite outgrowth and differentiation, respectively ((a)) [Citation221]. Following analysis and confirmation of cellular internalisation of BNNT (TEM and EELS), PC12 cytocompatibility was evaluated (using LIVE/DEAD, apoptotic analysis, ROS detection and WST-1 viability assay) showing high levels of cytocompatibility of up to 50 μg/mL.
Figure 14. (a) Wireless stimulation of cells which has internalised BNNT nanoparticles via ultrasound. (b) Trends of the differentiation during the 9 days of experiments [Citation221]. (c) Relative gene expression levels for 10 genes important for skeletal muscle differentiation. (d) Fusion index for C2C12 cells cultured on the different sample type [Citation159]. (Reproduced from Ref [221] with permissions of American Chemical Society (Copyright 2019). Reproduced from Ref [159] ,licensed by PLOS, open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/)).
![Figure 14. (a) Wireless stimulation of cells which has internalised BNNT nanoparticles via ultrasound. (b) Trends of the differentiation during the 9 days of experiments [Citation221]. (c) Relative gene expression levels for 10 genes important for skeletal muscle differentiation. (d) Fusion index for C2C12 cells cultured on the different sample type [Citation159]. (Reproduced from Ref [221] with permissions of American Chemical Society (Copyright 2019). Reproduced from Ref [159] ,licensed by PLOS, open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/)).](/cms/asset/b45af74a-2dbb-462f-bcdd-1b06b37ea3df/yimr_a_1988194_f0014_oc.jpg)
PC12 cultures were subjected to ultrasound (US) in the presence of varying amounts of BNNT (0, 5 and 10 μg/mL) to observe neurite growth, neuronal processes and the number of differentiated cells in culture. Differentiation was observed with fluorescence microscopy and both stimulated and unstimulated cultures did not differ in differentiation compared to the control. With respect to neuronal processes and neurite length, the cultures that were incubated with both BNNT and US stimulation (BNNT + US) had, on average, more and longer neurites (30% longer), see (b,c). To clarify the mechanism underlying these observations, inhibitory studies were performed using K252a and LaCl3, after which their differentiation status was evaluated. Cultures treated with K252a had poor differentiation (5–10%) until treated with a combination of BNNT + US where differentiation increased significantly (∼25%). Similarly, neurite growth exhibited a large increase (∼ 8 to 20 μm) for cultures treated with BNNT + US compared to all others conditions tested. Differentiation was lower in the cultures treated with K252a. However, the LaCl3 studies showed no significant difference with respect to number of differentiated cells or neurites; a notable increase in neurite length (∼15%) was observed when cells were treated with BNNT + US, although these neurites were much smaller than in cultures without LaCl3.
Studies were also performed on SH-SY5Y cells, which can be differentiated through a route that does not involve the NGF-activated TrkA pathway [Citation222,Citation223]. This allows further probing of the mechanism of electrically induced stimulation. All cultures tested showed similar and moderate amounts of differentiation (∼ 40%) and no difference in number of neurites. However, those treated with BNNT + US showed a large increase in neurite length (∼30%), indicating the significant effects of piezoelectric stimulation, even without the induction of the TrkA pathway.
Ricott et al. [Citation159] utilised BNNT for the stimulation of normal human dermal fibroblasts (nHDF) and skeletal muscle (C2C12 myoblasts) co-cultures on micro-grooved polyacrylamide gels. The C2C12 cells were seeded on top of the nHDF. BNNT cell internalisation was confirmed and quantified by ICP-MS, TEM and EELS where BNNT were found in both early and mature endosomes (membrane-bound vesicles that function to internalise and transport materials in the cell membrane) in C2C12, but not in the nHDF layer beneath it. In vitro genetic analysis for the expression of 10 genes associated with skeletal muscle differentiation was evaluated using RT-PCR, both at the early stages and at the end of the study. Early cultures treated with BNNT + US showed increases in expression of myogenin and MLP, which are two genes that play key roles in myogenesis and MYH2 which is a late differentiation marker indicating an acceleration in differentiation. No significant increase was seen for those genes encoding the production of ECM protein, see (d). This was mirrored by an increase in fusion index for those samples exposed by BNNT + US ((e,f)). Late stage cultures treated with BNNT + US showed a higher expression of genes that were associated with proteins that are key for motor function in muscle contraction (myosin and α-actinin) and an almost complete switching-off of genes related to early-stage differentiation, indicating that cells have reached maturity. This was performed in tandem with fluoresce immunostaining of myosin and α-actinin, thereby confirming cultures treated with BNNT + US had larger multinucleated myotubes with more peripheral nuclei, a key indicator of advanced differentiation.
Zinc oxide (ZnO)
Zinc oxide (ZnO) is a non-ferroelectric piezoelectric material that is highly prevalent in the biomedical literature [Citation224] due to its antibacterial [Citation225,Citation226], antimicrobial [Citation227,Citation228] and antifungal [Citation229,Citation230] properties. ZnO nanoparticles are used in bioimaging as photoluminescent [Citation231] as well as a pharmaceutical carrier of chemotherapeutic [Citation232,Citation233]. Furthermore, it is a non-ferroelectric material that is inherently piezoelectric, due to its non-centrosymmetric wurtzite crystal structure, and therefore does not require poling to exhibit piezoelectric properties when used in single crystal or highly textured form [Citation234]. Despite this, there are limited studies on the application of this material in the field of tissue engineering as opposed to its lead-free ferroelectric ceramic counterparts. This is possibly due to its potential cytotoxicity [Citation235–237], and that is why ZnO-based scaffolds are often encapsulated in a polymer matrix.
Seil and Webster [Citation160] utilised polyurethane, zinc oxide nanoparticle composites (ZnO-PU) of varying ZnO weight ratios to decrease astroglial cell density. Reactive astrocytes (those which reacting to CNS damage, form ‘glial scars’ during the wound healing process) are vital during healing to provide a physical and molecular barrier to inflammation, protecting the remaining fragile tissues [Citation238]. However, this scarring comes at a cost of being highly obstructive to regrowth of major axon projection pathways [Citation239]. Therefore, at the site of injury, it can be pertinent to limit astrocyte growth to better allow overall CNS regeneration. The authors hypothesise that the differing surface roughness and resulting surface energies result in differing protein surface absorption, which in turn create different environments for cells. Following surface analysis (SEM, XPS, surface energy and wettability), proliferation was quantified using fluorescence microscopy and generally showed a reduction in both astrocyte adhesion and proliferation as ZnO particle concentration increased ().
Figure 15. Astrocyte proliferation following 1, 2 and 3 day assay [Citation160]. (Reproduced from Ref [60] with permissions of Dove Medical Press (Copyright 2008)).
![Figure 15. Astrocyte proliferation following 1, 2 and 3 day assay [Citation160]. (Reproduced from Ref [60] with permissions of Dove Medical Press (Copyright 2008)).](/cms/asset/da7a8cf0-5312-42ae-b0bc-3cc12a1eb75d/yimr_a_1988194_f0015_ob.jpg)
Amna et al. [Citation79] evaluated the cytocompatibility of electrospun ZnO-PU composites using NIH 3T3 fibroblasts. Following morphological (field emission-SEM) and compositional analyses (XRD, EDX, TEM, electron probe microanalysis and FTIR), scaffold cytocompatibility was determined by the MTT assay and was considered to be non-toxic. Furthermore, cell morphology on the nanofibres, both pristine and doped, was analysed (SEM) showing that fibroblast growth was more active on the ZnO-doped composite compared to the pristine PU and healthy fibroblast proliferation, with no stress-related cells was observed on both scaffolds. This was further elucidated using LIVE/DEAD staining. While both pristine and doped scaffolds showed no dead cells , the doped scaffolds showed a much higher density of cells.
Recent work has utilised ZnO thin films sputtered on a polyethylene terephthalate (PET) polymer matrix [Citation161]. While yet to be tested on cells, the materials exhibit good piezoelectric coefficients (d33 ∼ 3.2–3.6 pC/N), uniform nucleation on the top of the substrate, high stability and good substrate adhesion.
Potassium sodium niobate (KNN)
Potassium sodium niobate (KNN) is another ferroelectric material seeking to replace lead-based piezoelectric ceramics due to increasingly strict enforced environmental regulations against the use of lead-based materials in electronic devices [Citation240,Citation241]. Kuo et al. [Citation118] prepared KNN (d33 ∼ 63 pC/N) and 6 mol-% Li-doped LKNN (d33 ∼ 98 pC/N) for evaluation of cytotoxicity and degradation (in saline solution) using L929 mouse fibroblasts. The ceramics were prepared by typical solid-state reaction and underwent analysis of mechanical properties (elastic moduli and biaxial strength) following and proceeding soaking where the elastic modulus of KNN was found to decrease (19%) and SEM revealed cracks on the materials surface, while the KNLN increased in strength (15%).
During the soaking period of LKNN’s, the d33 coefficient greatly decreased (30%), falling below that of KNN whose d33 increased (19%) halfway through the soaking period. The authors commented on this unusual change in piezoelectric behaviour, stating that LKNN’s two morphological phases (monoclinic and tetragonal), confirmed by XRD, compared to KNN’s single monoclinic phase, allow for more dipole directions and therefore a larger d33. However, the significant Li+ ion release from the LKNN, that was confirmed, results in structural distortions and a decrease in d33. Degradation was monitored as a function of pH and ion concentration in solution (ICP-AES). Generally, the pH of the surrounding environment of a cell is of great importance to cell growth. For fibroblasts, a reduction in intracellular pH inhibits cell growth via anchorage-independent growth pathways [Citation242]. Therefore, it is logical that the material which maintained the most alkaline environment would be the most beneficial. The normal saline solution decreased in pH over time (pH = 6.7–5.6) as CO2 from the air dissolved into it, while LKNN followed a similar trend to normal saline (pH = 6.4–5.6) and KNN had a higher pH (pH = 7.0–5.7) than both solutions. The ion concentrations of K+, Na+, Nb5+ and/or Li+ in the solution (ppm) were determined and initially, large amounts of K+ and Na+ were released from both KNN and LKNN. However, KNN showed a slightly higher concentration of both ions. The concentration of Nb5+ remained consistently low for both samples and while LKNN had a small percentage of Li present (6 mol-%) the amount of Li+ in the solution was an order of magnitude larger than Nb5+, indicating the high solubility of Li+.
Cytotoxic evaluation (MTT assay) was performed in accordance with the international standard for pre-market materials cytotoxicity (ISO10993-5) [Citation243] and showed that for the raw extracted cells KNN had a much higher viability (84%) than LKNN (58%) which the authors ascribe to a change in pH. Following adjustment for pH, the viability of both KNN (79%) and LKNN (72%) remains relatively high, thereby indicating both materials are not directly harmful to cell viability.
As a final example, Carville et al. [Citation162] showed ferroelectric polarisation of lithium niobate wafers improves osteoblast proliferation ((a)) and cell mineralisation ((b)) of MC3T3 on both positively (+z) and negatively (−z) charged surfaces compared to unpoled lithium niobate wafers. The next section will now focus on examples of piezoelectric-based biomedical devices.
Figure 16. (a) Osteoblast proliferation derived from fluorescence imaging of MC3T3 cell proliferation over 11 days. (b) Representative optical images alizarin red mineralisation [Citation162]. (Reproduced from Ref [162] with permissions of John Wiley and Sons (Copyright 2014)).
![Figure 16. (a) Osteoblast proliferation derived from fluorescence imaging of MC3T3 cell proliferation over 11 days. (b) Representative optical images alizarin red mineralisation [Citation162]. (Reproduced from Ref [162] with permissions of John Wiley and Sons (Copyright 2014)).](/cms/asset/f96d25de-2ae5-4472-a21f-542bd0187bcf/yimr_a_1988194_f0016_oc.jpg)
These observations indicate a range of piezoelectric materials having positive effects on a variety of cells and tissue, but there is currently no clear implication of how the magnitude of piezoelectric activity effects cells differently. While it is worth noting that reports which actively stimulate their materials (Ciofani et al. [Citation169,Citation221], Genchi et al. [Citation151], Ribeiro et al. [Citation209], Hoop et al. [Citation157], Guo et al. [Citation105] and Ricott et al. [Citation159]) all show positive outcomes for cell in vitro. These observations are particularly relevant since the application of dynamic loads leads to charge generation during each stress cycle and indicates that stimulation is needed to continually change the polarisation of the material and develop charge to piezoelectrically stimulate cells. A detailed understanding of how specific piezoelectric properties, such as dij charge coefficients or gij voltage coefficients, affect cells and tissues is currently not available. An examination of these research questions would allow the field to quantifying how, and to what extent, piezoelectric stimulation directly affects cells.
Piezoelectric biomedical devices
Wearable and implantable medical electronics (IMEs) have the potential to be a powerful real-time diagnostic and health management tool. This includes applications ranging from blood pressure monitors [Citation244–246] to pacemakers [Citation247–249] and defibrillators [Citation250–254], deep brain stimulation (DBS) [Citation255–258], cochlea [Citation259–262] and photoreceptor replacement [Citation263,Citation264] as well as a variety of biochemical and physiological factors [Citation265]. IMEs have been used in myriad of ways to monitor, treat and diagnose those suffering from chronic disease. Hwang et al. [Citation266] provide a comprehensive review of earlier developments in the field.
Although IMEs have achieved success, they are typically powered by batteries which have a limited lifetime [Citation267,Citation268] and need replacement. Real-world data on the lifespan of implantable defibrillators show device life times to be less than 6 years [Citation269] and other studies have shown a typical lithium-ion battery in physiological temperatures (37°C) was found to be less than 10 years [Citation270]. The mechano-transductive properties of a variety of piezoelectric materials provide an attractive means of overcoming this problem, by harvesting electrical energy from the body’s own movements such as muscle contraction and relaxation, lung inhalation and exhalation or vasoconstriction and vasodilation in the heart.
Lead-based implants
Lead-based ceramics feature prominently throughout these devices due to their exceptional piezoelectric properties and therefore excellent mechanical harvesting potential. Conventional PZT and doped lead titanate (PT) derivatives have shown very good piezoelectric properties (d33 ∼ 200–620 pC/N) [Citation271] and are therefore often the first choice for energy harvesting devices.
Lead zirconium titanate (PZT)
Lu et al. [Citation272] developed a flexible PZT-based system to harvest energy from cardiac contractions generated by the heart to power pacemakers which they tested in vivo on swine hearts. The device consisted of a commercial PZT wafer processed into nanoribbons (NRs) on a flexible polyimide -based film (Kapton® substrate) which has attractive dielectric properties such as low conductivity and dielectric loss across a wide range of temperatures [Citation273–275] ((a)). The resultant system was integrated into a swine heart in both the open and closed cavity of the specimen. Although the output voltage peaks produced in the open cavity were not uniform, due to lack of counteraction provided by the rib cage, the closed cavity specimen produced a peak-to-peak voltage (3 V), which is sufficiently large to power a commercially available pacemaker (Affinity DR 5330L, St. Jude Medical) [Citation268,Citation276]. The device was then evaluated for its in vivo longevity and produced a regular output voltage synchronised to the periodicity of the cardiac cycle under three conditions, open-chest (2.3 V), close-chest (2.2 V) and conscious (0.3 V). The large decrease in output voltage seen when the specimen was conscious was due to the swine suffering from a thoracotomy and pericardiotomy.
Figure 17. (a) PZT capacitor stacks fabricated on Si wafer and encapsulate with polyimide (PI) on a Kapton substrate produced by Lu et al. [Citation272]. Devices conformationally attached to human skin with biocompatible liquid bandage sensing, (b) wrist, (c) artery pulse and (d) respiration [Citation277]. (e) PZT system which lies underneath the BM of the ear proposed by Lee et al. [Citation278]. (Reproduced from Ref [272], licensed by Springer Nature, open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/)). Reproduced from Ref [277] and [278] with permissions of John Wiley and Sons (2017 and 2014 respectively)).
![Figure 17. (a) PZT capacitor stacks fabricated on Si wafer and encapsulate with polyimide (PI) on a Kapton substrate produced by Lu et al. [Citation272]. Devices conformationally attached to human skin with biocompatible liquid bandage sensing, (b) wrist, (c) artery pulse and (d) respiration [Citation277]. (e) PZT system which lies underneath the BM of the ear proposed by Lee et al. [Citation278]. (Reproduced from Ref [272], licensed by Springer Nature, open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/)). Reproduced from Ref [277] and [278] with permissions of John Wiley and Sons (2017 and 2014 respectively)).](/cms/asset/670b4494-75fc-4f44-8a90-207094721cef/yimr_a_1988194_f0017_oc.jpg)
Similar PZT NR systems have been developed to characterise soft tissue biomechanics (skin, heart and lungs), which have utility in the assessment of pathophysiologic conditions. Dagdeviren et al. [Citation279] utilised PZT NRs encapsulated in polyimide ex vivo on the skin of a patient. After proving to be cytocompatible, when tested on human epithelial keratinocytes, the devices were used for the characterisation of dermatological malignancies. On comparing healthy skin to that suffering from pathology, a clear distinction was observed in all areas tested (breast, leg, forehead, neck, nose and eye). This was also observed for conditions such as young, old, moisturised and dry skin. Ex vivo studies also quantified the state of bovine heart and lungs and found an apparent correlation between the dehydration state and elastic moduli of the device’s implantation site. Finally, the device was then used for the spatial mapping of patient’s basal cell carcinoma (skin cancer) to produce a 360° map of the skin’s local elastic moduli.
Park et al. [Citation277] produced self-powered PZT-based devices for real-time artery pulse sensing. The devices consisted of a PZT thin film on an ultrathin PET substrate which could wirelessly transmit a signal to a smartphone for instantaneous pulse monitoring. Following verification of cytocompatibility using human embryonic kidney (HEK293) and mouse cardiac muscle (HL-1) cell lines, the films were applied successfully to a human wrist ((b)) and neck for sensing of the pulse ((c)), respiratory rate and tracheal movements ((d)). The sensor also responded to the direct application of sound waves through a speaker in the ranges of 0.2–5.0 Hz and up to 240 Hz.
An additional application of interest for these technologies is biomimetic replacements for acoustically responsive hair cells in those suffering from sensorineural hearing loss, with the goal of these devices being the conversion of sound energy to electrical energy to stimulate auditory nerves. Lee et al. [Citation278] and Han et al. [Citation280], inspired by the basal membrane of the inner ear, utilised PZT as an acoustic nanosensor. Lee et al. proposed a PZT system which lies beneath the basilar membraneof the ear which responds to sound stimulation, much like hair cells in the inner ear ((e)). The devices consisted of PZT on a flexible PET substrate, bound to a silicone membrane which was able to effectively convert through nanometer scale motions, audible frequencies of sound into electrical signals at a range of audible frequencies. They performed finite element analysis, where the PZT was bent to the height where the basal membrane naturally displaces in response to sound stimulation (600 nm), resulting in a 3 V piezoresponse, which is sufficient to stimulate auditory nerves [Citation281] and function as natural hair cells. Similarly, Han et al. [Citation280] also produced successful devices, which consisted of a thick PZT film on a flexible polymer substrate. Their devices proved to be four to eight times more sensitive than the most widely commercialised reference condenser microphone. Sonmezoglu et al. [Citation282] have recently reported on the implantable and wireless deep-tissue O2 sensor that is powered by ultrasound applied to a PZT material to generate sufficient power for operation of the sensing system.
Lead magnesium niobate-lead titanate (PMN-PT)
PMN-PT is a ferroelectric material with exceptional piezoelectric properties (d33 ∼ 670–2500 pC/N) which has been utilised in biomedical implants. Hwang et al. [Citation283] developed a piezoelectric energy harvester to power cardiac pacemakers [Citation284] which operates at 3 V and 100 μA. The device consisted of a PMN-PT thin film bound to a flexible PET substrate (d31 ∼ 1102 pC/N), see (a), and achieved an output voltage and current of 8.2 V and 145 μA, respectively, upon periodic bending and unbending, with a consistent output current without degradation over 30,000 cycles (∼18 h). The device was implanted into an anesthetised rat ((b)) for direct stimulation of the heart where it was monitored using an electrocardiogram (ECG) before ((c)) and during stimulation ((d)). The device generated 2.7 μJ from one bending motion during cyclic bending which is above the minimum energy for the cardiac pacing of a human heart (0.10–0.16 μJ [Citation285]) indicating potential for use in cardiac regulation.
Figure 18. (a) Schematic of the biomedical application of the flexible PMN-PT energy harvester. (b) Photograph of the medical experiment on a living rat for stimulating the heart. The EGC of a living rat heart (c) before stimulation and (d) during stimulation from bending and unbending of the device [Citation283]. Conceptual scheme of (e) in vivo self-powered energy harvester attached to porcine heart. ECG showing (f) current and (g) voltage of porcine heat and energy harvesting device [Citation286]. (Reproduced from Ref [283] and [286] with permissions of John Wiley and Sons (Copyright 2014 and 2017) respectively).
![Figure 18. (a) Schematic of the biomedical application of the flexible PMN-PT energy harvester. (b) Photograph of the medical experiment on a living rat for stimulating the heart. The EGC of a living rat heart (c) before stimulation and (d) during stimulation from bending and unbending of the device [Citation283]. Conceptual scheme of (e) in vivo self-powered energy harvester attached to porcine heart. ECG showing (f) current and (g) voltage of porcine heat and energy harvesting device [Citation286]. (Reproduced from Ref [283] and [286] with permissions of John Wiley and Sons (Copyright 2014 and 2017) respectively).](/cms/asset/151b5658-95a9-4434-ae76-d08344fb1c5d/yimr_a_1988194_f0018_oc.jpg)
Kim et al. [Citation286] opted for a similar PMN-PZT-based system (d33 ∼ 1140 pC/N) that consisted of a PMN-PZT thin film bound to a flexible PET substrate to harvest energy from a porcine heart, a model which is widely regarded as similar in scale and physiology to the human heart [Citation287,Citation288] for a wireless data transmitter, as shown in (e). The device produced short-circuit current and open-circuit voltage (1.75 μA and 17.8 V), with no degradation over 100,000 bending and release cycles. The device proved to be both cyto- and biocompatible after testing using human embryonic kidney (HEK293), rat cardiomyoblasts (H9C2), mouse cardiac muscle (HL-1) cell lines and histological studies under the dorsal skin of a normal rat showing. Upon implantation the output current ((f)) and voltage ((g)) produced by the device were well synchronised to the ECG of the porcine heart, moreover the typical QRS peaks were expressed in both the current produced by the heart (blue and red) and the ECG directly recording the porcine heart (orange).
Lead indium niobate-lead magnesium niobate-lead titanate (PIMNT)
PIMNT exhibits excellent piezoelectric properties, ranging from d33 ∼ 435 to 925 pC/N [Citation289,Citation290]. Hwang et al. [Citation291] employed an indium-doped PMN-PT system (PIMNT) for DBS applications. The device consisted of a PIMNT thin film that was bound to a flexible PET substrate, see (a), and an electrical charge was generated through the periodic bending and unbending of the device with no degradation of current over ∼15,000 cycles (∼12 hours). The devices produced sufficient current and voltage (283 μA and 11 V) to apply to a mouse’s motor cortex (M1), see (b), and induce forearm movement in a measurable way ((c,d)). The induction of forearm contraction through primary motor cortex stimulation of mice has a threshold current of ∼30 μA [Citation292] and, even when combined with the impedance of the stimulation electrode, the device consistently generated a current of ∼45 μA.
Figure 19. (a) Illustrative representation of PIMNT energy harvesting device. (b) Schematic of DBS application using the PIMNT film. (c) Video captured image converted to binary image to tract DBS and (d) resultant 6 plotted iterations of stimulation [Citation291]. (Reproduced from Ref [291] with permissions of Royal Society of Chemistry Publishing (Copyright 2008)).
![Figure 19. (a) Illustrative representation of PIMNT energy harvesting device. (b) Schematic of DBS application using the PIMNT film. (c) Video captured image converted to binary image to tract DBS and (d) resultant 6 plotted iterations of stimulation [Citation291]. (Reproduced from Ref [291] with permissions of Royal Society of Chemistry Publishing (Copyright 2008)).](/cms/asset/e5e4fd1a-b956-46e8-9319-87bf4d04b34d/yimr_a_1988194_f0019_oc.jpg)
Lead-free biomedical devices
Despite the inherent toxicity of lead and its oxides, lead-based systems have demonstrated potential as biomedical devices due to their excellent piezoelectric properties. While long-term studies on the effects of exposure to these lead-based devices in the body are currently lacking, lead itself has been shown to be cytotoxic to an array of human cells (CNS, prostate, colon, breast and lung cells) [Citation293], a carcinogen [Citation294] and even low levels of contamination can induce oxidative stress to the brain, heart kidney and reproductive system [Citation295]. This leaves an almost insurmountable barrier between these devices and their use in a clinical environment. Consequentially, this has opened up opportunities for the development lead-free alternatives. For a more far-reaching and comprehensive study into the lead-free systems, the reader is referred to the work of Saito et al. [Citation296] and Zhang et al. [Citation297] who have outlined the important progress required for lead-free systems to flourish in the field. Wu [Citation298] has also published a key textbook in the recement advances in lead-free systems. Examples of promising lead-free-based devices are now outlined.
Zinc oxide implants
ZnO is regarded as biocompatible, lead-free and non-toxic [Citation299] and has been indicated as a safe substance by the Food and Drug Administration (FDA) [Citation300,Citation301]. In addition, it has been observed to cause oxidative stress and damage to a variety of cell lines [Citation300,Citation302], and therefore its utility in biomedical applications is a hotly debated area of research. ZnO-based piezoelectric systems have been reported with a range of piezoelectric coefficients (d33 ∼ 3–44 pC/N [Citation303–306]) which compares poorly to lead-based systems in terms of piezoelectric charge coefficients. Despite this, there are a variety of ZnO-based strain sensors in the literature [Citation307–310].
Li et al. [Citation311] utilised ZnO NWs to generate energy from the breathing and heartbeat of a live rat. The devices consisted of NWs fixed to a flexible polyimide substrate and then covered in a flexible polymer to increase durability in vivo. Initial testing on the diaphragms of anesthetised rats ((a)), whose respirator-induced periodic expansion and contraction, resulted in equally periodic peak signals of current and voltage (1 mV and 1 pA), as shown in (b). This was consistent with the rats own physiological breathing, but these signals were lower than the typical output observed by other similar devices (50 mV and 500 pA) [Citation312] even with rats whose breathing was unaided and deeper (2 mV and 4 pA). The next set of experiments sought to harvest energy from the heartbeat of a live rat ((c)), producing signals of 3 mV and 30 pA, as shown in (d). Although these output values are of interest as a sensor, they are not sufficiently high for powering devices such as pacemakers (3 V and 100 μA) and require both biocompatibility and histological analyses for a deeper understanding of the potential of devices as biological implants.
Figure 20. ZnO nanowire-based energy harvester implanted in the (a) diaphragm and (b) output current and voltage resultant and (e) heart of a rat [Citation311]. (Reproduced from Ref [311] with permissions of John Wiley and Son (Copyright 2010)).
![Figure 20. ZnO nanowire-based energy harvester implanted in the (a) diaphragm and (b) output current and voltage resultant and (e) heart of a rat [Citation311]. (Reproduced from Ref [311] with permissions of John Wiley and Son (Copyright 2010)).](/cms/asset/ccd007bd-95ec-4f75-939a-c6f88dc4dc5c/yimr_a_1988194_f0020_oc.jpg)
PVDF implants
Polyvinylidene fluoride
Chen et al. [Citation313] and Zhang et al. [Citation314] utilised PVDF for blood pressure monitoring and energy harvesting from the aortas of porcine specimens. Both devices consisted of a piezoelectric thin film ∼200 μm thick that was encased in polyimide.
Chen et al. undertook in vivo simulation of aortic expansion and contraction utilising an intra-aortic balloon pump (IABP). The piezoelectric device was fastened to the outside of a tube with varied degrees of tightness and an excellent linearity between flow pressure and voltage output was observed, with an excellent sensitivity (173 mV mmHg−1). There was a consistent voltage output for 50,000 cycles and a maximum instantaneous power output of 2.3 μW, which was higher than previously observed in the literature (circa 2016). In vivo testing consisted of implantation of the device around the right femoral aorta of a porcine specimen. Good linearity was observed between power and blood pressure, with good sensitivity (14.3 mV mmHg−1) and a maximum instantaneous power output of 40 nW. Finally, the device was attached to a battery-free liquid crystal display to serve as both a sensor and a power source. Using the same in vitro experimental conditions, a lower limit of detection was found to be 120 mmHg, thereafter different digits appeared on the screen as flow pressure increased. When implanted on the aorta of a porcine specimen, the lower detection limit decreased to 140 mmHg. Although a pressure of 140 mmHg is indicative of hypertension in the human body [Citation315], the system requires a higher sensitivity for biomedical applications.
Zhang et al. utilised similar methods to achieve equally successful results, using an IABP to mimic in vivo flow pressure. The device achieved a maximum output voltage and current of 10.3 V and 400 nA (flow pressure of 160/80 mmHg), respectively, and a maximum power output of 681 nW. Following this, in vivo studies were performed on a porcine specimen. While all signals were well synchronised with the heart rate, blood pressure and ECG of the specimen, there was a significant decrease in maximum output voltage, current (1.5 V and 300 nA) and an instantaneous output of 30 nW at 70 bpm and lood pressure of 160/105 mmHg.
Poly(vinylidenefluoride-co-trifluoroethylene) (PVDF-TrFE)
Sharma et al. [Citation316,Citation317] utilised poly(vinylidenefluoride-co-trifluoroethylene) (PVDF-TrFE) thin films for pressure sensor applications and catheters; this copolymer exhibits higher piezoelectric activity compared to PVDF. The devices consisted of PVDF-TrFE thin films on a silicon substrate and their pressure-sensing capabilities were determined in an air chamber (0–300 mmHg). The devices were integrated with a catheter and tested on a model which imitates the vascular structure and physiological flow (between 0 and 300 mmHg) of the pelvis of an average-sized male patient. In comparison to a commercial pressure sensor (Freescale Semiconductors, MPX2300DT1), the PVDF-TrFE sensor was found to be four times more sensitive (99 vs 25.3 μV mmHg−1) and fivefold more responsive (0.26 and 1.30 s) than the commercial pressure sensor.
Inaoka et al. [Citation318] sought to use PVDF-TrFE to mimic the ability of cochlear hair cells as a means of restoring auditory function. Their device consisted of a PVDF-TrFE membrane (40 μm thick), which they implanted into the basilar membrane of a guinea pig. The device was able to achieve an electrical output of 0.14–5.88 mV (100 dB SPL at 1–40 kHz), which was greater than the relatively small power input required to stimulate the human cochlea (∼14 μW) [Citation319]. Other similar and interesting technologies were developed by Kim et al. [Citation320] and Graz et al. [Citation321] who produced a PVDF-TrFE-PbTiO3 composite and PVDF-TrFE system, respectively, for skin-based pressure sensing, see (a,b). Both use piezoelectric-pyroelectric coupling, which can sense both temperature and pressure, and both showed promising results.
Figure 21. (a) Scheme of the bifunctional piezo- and pyroelectric sensor array [Citation320] and (b) bifunctional device mapping of the temperature distribution on human skin including the area with a blood vessel and comparing it with the thermal image obtained by an IR camera [Citation321]. (Reproduced from Ref [320] ,licensed by Springer Nature, open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/). Reproduced from Ref [321] with permissions of American Institute of Physics Publishing (Copyright 2009)).
![Figure 21. (a) Scheme of the bifunctional piezo- and pyroelectric sensor array [Citation320] and (b) bifunctional device mapping of the temperature distribution on human skin including the area with a blood vessel and comparing it with the thermal image obtained by an IR camera [Citation321]. (Reproduced from Ref [320] ,licensed by Springer Nature, open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/). Reproduced from Ref [321] with permissions of American Institute of Physics Publishing (Copyright 2009)).](/cms/asset/731df64d-64f5-46e4-abc6-9b7ab3463b8c/yimr_a_1988194_f0021_oc.jpg)
Potassium sodium niobate (KNN) implants
Recently, Kim et al. [Citation322] proposed the use of biocompatible ceramic KNN in biomedical energy generators. Their system has piezoelectric coefficient of d33 ∼ 50 pC/N and large voltage and current outputs (2.0 V and 40 nA) compared to other biocompatible systems. However, they have yet to be tested in a biomedical context for comparison with its lead-based counterparts.
Jeong et al. [Citation323] undertook a comprehensive study on flexible KNN-based energy harvesting devices, investigating beyond short-term in vitro and in vivo studies typically seen in the literature. In the study, in addition to studies of the cytotoxicity, cell adherence and histology of their KNN material, they quantified the dissolution of heavy metal ions against similar PZT-based systems as outlined in (a).
Figure 22. (a) Scheme illustrating the biocompatibility of the high-output lead-free KNN-based piezoelectric energy harvesting device. (b) Comparison between this study and previous studies [Citation323]. (Reproduced from Ref [323] ,licensed by American Institute of Physics Publishing, open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/)).
![Figure 22. (a) Scheme illustrating the biocompatibility of the high-output lead-free KNN-based piezoelectric energy harvesting device. (b) Comparison between this study and previous studies [Citation323]. (Reproduced from Ref [323] ,licensed by American Institute of Physics Publishing, open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/)).](/cms/asset/2b963035-8f48-4b88-9734-43d871e7894f/yimr_a_1988194_f0022_oc.jpg)
Cytotoxicity studies on human kidney (HEK-293) and rat cardiomyocyte (H9C2) cells, cell adhesion studies on human osteosarcoma (MG-63) cells and histological analysis following implantation into living rats all show no negative effects on cells or serious histological inflammation in rat specimens in both KNN- and PZT-based films. Nevertheless, Jeong et al. highlight that these ‘highly localized and short-term’ studies cannot ensure device biocompatibility to a satisfactory standard due to the detrimental effects of prolonged exposure to heavy metal ions.
With the need for long-term data, they performed studies that used inductively coupled plasma mass spectrometry to quantify heavy ion (Nb and Pb) dissolution in human serum at physiological (36.5°C) and room temperature (25°C), with agitation to mimic the motion of the body. It is worth noting that niobium oxides are neither toxic [Citation324] nor water-soluble [Citation325], while lead oxides are both soluble in blood serum [Citation326] and toxic. The concentration of Pb ions was found to be three orders of magnitude higher than Nb. While this preliminary work is not a direct measure of toxicity of PZT-based devices, Jeong et al. intend to follow these studies with long-term epidemiological studies on canine and porcine models. Clearly, it is important that more work is undertaken in this important area for the successful application of electroactive biomedical devices.
With respect to materials properties, the KNN-based films produced voltage and current outputs matching, or exceeding, other KNN-based and PZT-based devices reported in the literature, see (b). The devices were able to endure 6000 cycles with no mechanical cracks and provided an output of 5 V and 700 nA when sutured onto a living porcine heart. The output was higher during regular bending (130 V and 1.3 µA) and randomly flicking (170 V and 5.5 µA). In summary, Jeong et al. provide an interesting reflection on the current approach to determining the biocompatibility and the clinical viability of lead-free implantable energy harvesting devices, as well as providing a strong case for the use of lead-free KNN-based devices.
BT devices
Barium zirconium titanate-barium calcium titanate (BZT-BCT)
Yuan et al. [Citation327] produced BZT-BCT NW-PDMS-based composites for in vivo energy extraction, see (a). As with other BT-based piezoelectric materials, BZT-BCT shows a high piezoelectric coefficient (d33 ∼620 pC/N) [Citation328] making it a promising candidate for energy harvesting applications. Yuan et al. performed in vitro viability studies on both pristine BZT-BCT NWs (210 ± 50 nm) and devices using MTT assay on Chang liver and L929 (murine fibroblast) cells and Chang cells alone. The cells dosed with NWs decreased in viability as the dose increase, but viability remained consistently high (>96% between 1 and 10 µg mL−1 and >70% at 100 µg mL−1) with respect to Chang cell morphology studies. The mitochondrial distribution (at 48 hours) had no change compared to the control, although cell density decreased in a similar manner to viability as NW dose increased. Chang visualised cells cultured on the device were observed by optical microscopy and covered the device. In culture, during mechanical stimulation for 2 h, the NWs produced an output of 0.3 V and 15 nA.
Figure 23. (a) Schematic of BZT-BCT nanogenerator, (b) device implanted into rabbits back and resultant current output (nA) as the rabbit walks [Citation327]. Other BT-based devices produced by (c) Won et al. [Citation329], BNKS-PDMS composite film and SEM image of well-dispersed BNKS in PDMS, as well as (d) device structure and (e) SEM image of Park et al. [Citation330], BaTiO3 nanogenerator on plastic substrates with XPS spectrum of BaTiO3 and (f) MIM microstructure of the devices. Reproduced from Ref [327] and [329] with permissions of John Wiley and Sons (Copyright 2014 and 2019). (Reproduced from Ref [330] with permissions of American Chemical Society (Copyright 2010)).
![Figure 23. (a) Schematic of BZT-BCT nanogenerator, (b) device implanted into rabbits back and resultant current output (nA) as the rabbit walks [Citation327]. Other BT-based devices produced by (c) Won et al. [Citation329], BNKS-PDMS composite film and SEM image of well-dispersed BNKS in PDMS, as well as (d) device structure and (e) SEM image of Park et al. [Citation330], BaTiO3 nanogenerator on plastic substrates with XPS spectrum of BaTiO3 and (f) MIM microstructure of the devices. Reproduced from Ref [327] and [329] with permissions of John Wiley and Sons (Copyright 2014 and 2019). (Reproduced from Ref [330] with permissions of American Chemical Society (Copyright 2010)).](/cms/asset/5e217773-64f8-448f-8acc-7289bb96f494/yimr_a_1988194_f0023_oc.jpg)
Following subcutaneous implantation into the back of a rabbit specimen for 5 weeks, see (b), the implanted area showed no histological damage or immunological response. When periodically pressed, the device produced a current of 0.13 nA; when the rabbit walked slowly the device produced currents of 0.1 nA.
While yet to be tested in vitro or in vivo, there are other promising BT lead-free-based devices reported in the literature which demonstrate higher levels of power generation. Won et al. [Citation329] produced a barium sodium potassium titanate (BNKT)-PDMS device using spin-coating ((c)) which generated 100 V and 20 µA through periodic tapping. In addition, Park et al. [Citation330] produced a BT thin film device via sputtering onto a plastic substrate, see (d), which generated of a voltage output of 1 V, current density of 0.19 µA cm−2 and a power density of 7 mW cm−3.
Biomolecular piezoelectrics for biomedical applications
Biomolecular piezoelectric materials are an auspicious set of materials which have shown great promise in recent years with encouraging piezoelectric properties and excellent biocompatibility which makes them a compelling candidate for the next generation of piezoelectric biomedical devices [Citation331]. Piezoelectric biomolecules are plentiful in nature and include chitin in the shells of crustaceans [Citation332], cellulose in the wood of trees [Citation66], viruses [Citation333] and the constituent molecules that make life possible, DNA [Citation334] amino acids, peptides and proteins [Citation331]. Their piezoelectric properties arise from highly ordered, low symmetry structures which lack an inversion centre [Citation335]. The archetypal example mentioned in literature is the amino acid glycine which forms three distinct polymorphs (α,β and γ), two of which as non-centrosymmetric and show ferroelectric properties (β and γ) [Citation336]. Glycine-based devices developed by Yang et al. [Citation337] have shown a d33 of 5–6 pC/N, and a peak-to-peak voltage and a short-circuit current of 4.1 V and 360 nA, respectively. The device performance is comparable to those of inorganic piezoelectric devices discussed previously, while being both biocompatible in vitro with human fibroblast cells and in vivo with SD rat specimens, and biodegradable in both PBS buffer (5 days) and in a SD rat (1 day). Furthermore, these devices successfully detected muscle contraction in both the leg and chest of the SD rat, showing both potential as a biomedical device and a material for tissue engineering.
Glycine is not the only example of this, dl-alanine crystals have shown a d33 as high as 3.5 pC/N and a max Voc of 0.8 V [Citation338], diphenylalanine-based devices obtained a d33 of 17.9 pm V−1 via PFM and a Voc of 1.4 V [Citation65]. Systems based on spider silk developed by Karan et al. [Citation339] generated voltages and current as high as 21.3 V and 0.68 μA and could sense arterial pulse, swallowing, coughing, drinking and was even sensitive enough to distinguish between the vibrations caused by the words ‘START’ and ‘NANOGENERATOR’ when attached to the throat. Virus-based systems developed by Park et al. [Citation340] also showed a peak voltage and current of 2.8 V and 120 nA (17 N), with a five-phase-based integrated energy harvester producing an output voltage and current of 12 V and 300 nA, respectively, by pressing the device with a finger.
There is further potential to be explored in piezoelectric biomolecules, collagen fibril in the human cornea has shown d33 values of up to 2250 pC/N [Citation341] and prestin, a motor protein in inner ear hair cells, has shown a d33 of 20 mC/N [Citation342] which is orders of magnitude greater than any synthetic piezoelectric material thus far. Guerin et al. [Citation343,Citation344] have performed density functional theory modelling on glycine’s polymorphs and the amino acids that make up collagen and found β-glycine to have a d16 of 178 ± 11 pm V−1, similar in magnitude to inorganic piezoelectrics, and hydroxy-L-proline to have a d25 of 25 ± 5pC/N. The potential of these materials warrants extensive future exploration, for further information into these materials the reader is referred to the works of Xu et al. [Citation345], Kim et al. [Citation331] and Guerin et al. [Citation346] in ‘Construction of bio-piezoelectric platforms: from structures and synthesis to applications’, ‘Biomolecular piezoelectric materials: from amino acids to living tissues’ and ‘Organic piezoelectric materials: milestones and potential’, which comprehensively outline these materials rise to prominence and future potential in the field.
Future perspectives
Materials manufacture and characterisation
The multidisciplinary nature of piezoelectric tissue engineering has the distinct advantage of allowing many different approaches to the manufacture and characterisation of piezoelectric scaffolds and their effects on cells, with each publication building towards the bigger picture. Despite the wide range of developed materials there has been no direct correlation between piezoelectric material type and properties with how the material is used in tissue engineering (see ).
This leaves a myriad of questions for future research: What are the upper and lower limits of this stimulation? How does piezoelectric stimulation affect protein and growth factor attachment to the scaffold surface? And consequently, how does this affect protein and growth factor uptake? Are the same mechanisms seen in all cell-lines? As differentiation between different cell-lines is characterised by different cellular phenotypes, it is therefore logical to assume their stimulatory pathways vary in a similar manner.
The mechanism by which piezoelectric stimulation occurs is not fully understood. While electrophysiological potentials and Ca2+ ion flux has been studied and hypothesised as the initial trigger for which regenerative effects occur [Citation109,Citation138,Citation151,Citation157], all of these studies have been conducted on neuronal cell-lines. Despite being carefully studied, this phenomenon requires further elucidation into the specifics of the mechanism as both stimulatory methods and methods used to monitor ion influx have varied significantly in different studies. Further investigations will help consolidate this larger picture and provide new avenues for the development of suitable piezoelectric scaffold materials.
The interfacial chemistry between cells and polarised surfaces is another aspect which naturally plays an important role in piezoelectric stimulation. While an in-depth analysis of this phenomenon reaches outside the realm of piezoelectric materials, for a detailed understanding of this phenomenon the reader is referred to ‘Biological Interactions with Surface Charge in Biomaterials’ published by the RSC in 2011 and edited by Tofail [Citation347] and the pioneering work of Tofail et al. on electrically polarised biomaterials [Citation348], which covers interfacial phenomena in detail. Importantly, studies performed on poled PVDF which showed enhanced nerve fibre outgrowth in mouse neuroblastoma (Nb2a) in vitro was subjected to electron spectroscopy for chemical analysis and wettability studies [Citation349]. The study showed that the poled and unpoled scaffolds were indistinguishable chemically and with respect to wettability and surface adhesion; this indicates that bulk electrical properties are key parameter in enhanced cell properties. Furthermore, like any other scaffold in tissue engineering, studies have been undertaken to modify the surface properties of piezoelectric materials in order to mitigate unfavoured material surface properties [Citation350,Citation351].
Piezoelectrics in tissue engineering
A wide variety of materials have been evaluated, ranging from ferroelectric to non-ferroelectric piezoelectric materials, in bulk ceramic, polymer, composite and nanoparticle form; however, ultimately each material has its strengths and weaknesses which require independent assessment.
PVDF, arguably the most comprehensively researched material to date, is highly processable [Citation352], biocompatible and beneficial to the growth of a variety of cell types; bone in vitro [Citation209] and in vivo [Citation148], muscle [Citation155] and skeletal muscle [Citation154], neuronal [Citation157] and stem cells [Citation156]. However, PVDF suffers from the disadvantage of not being biodegradable due to its high chemical stability [Citation353]. An ideal system would degrade, acting as a template for cells and slowly being replaced by the ECM of cells over time.
The ferroelectric ceramic family of PZT, BT, BST, BCZT and KNN all show promising results due to high d33 piezoelectric coefficients but suffer from high stiffness, making them inappropriate for softer varieties of tissues such as those found in muscles and the nervous system without being combined with a polymer matrix. For BT [Citation97,Citation150], BST [Citation71] and BCZT [Citation78,Citation189], these materials are primarily proposed for use in the regeneration of bone tissues, but they tend to be incorporated into more biocompatible matrices (β- TCP, HA and PCL) to produce a more effective bio-interface with cells. Although these matrices provide an additional advantage of being bioabsorbable, β-TCP and HA [Citation354], and PCL [Citation355] allow for better infiltration and integration of cells. PZT and KNN are exceptions to this hard tissue rule but provide their own challenges since PZT contains toxic Pb, and KNN has only proven to be non-cytotoxic, and therefore requires further testing.
Finally, the nanomaterials BTNP, BNNT and ZnO show promise with excellent evidence of electrostatic manipulation of Ca2+ in the former two and cytocompatibility in the latter. Ion imagining (Ca2+ and Na+) and inhibitory studies verified the stimulatory effects these nanomaterials have had on cells, primarily of neurons and MSCs. Although this shows the direct biological effect of the nanomaterials, these effects are part of a highly complex biological system that is not yet fully understood and is difficult to compare to other systems with quantifiable piezoelectric coefficients, such as PVDF and ceramics. Moreover, precise studies in which the d33 is varied (by changing poling time, field or temperature [Citation356–358]) are necessary to study the impact of piezoelectric stimulation for a more rigorous understanding of the mechanism, as well as facilitating the design of optimum cell/tissue-specific systems for regeneration. This is best highlighted when discussing bone tissues, where the bone matrix is inherently piezoelectric [Citation359] and piezoelectric stimulation is the mechanism that allows bone cells to adapt to changes in their environment [Citation360]. While this phenomenon is well studied, its specifics with respect to the effect of different amounts of charge is less well understood, and piezoelectric materials provide a viable system to understand this phenomenon better. In this regard, rather than simply assessing the performance of poled and unpoled materials, it would be of interest to evaluate materials at a range of poling levels (and corresponding d33 levels); for example, Berlincourt [Citation361] produced a range of d33 values for the same material by varying the degree of poling from 0% to 100%.
The majority of the literature is focused upon biological analysis. While this provides insight into the effects of piezoelectric stimulation both in vitro and in vivo and develops an understanding of the electrochemical consequences of the stimulation of cells ((a)) it constricts the field into myopia, leaving the path to clinical application unclear. There is a fundamental need for a larger focus on clinical application-based material design and testing of materials for clinically relevant time periods ((b)). This is an issue which Jeong et al. [Citation323] clearly highlights in their work on KNN-based biomedical devices. This include a full dielectric and piezoelectric analysis of the scaffold under study, as highlighted in Piezoelectricity; these properties are fundamental for quantifying the surface charge or potential difference. This will allow researchers elucidate the degree of piezoelectric stimulation and allow a shift away for a case-by-case assessment and design of materials (see Piezoelectric Scaffolds). Finally, while materials, such as HA, are inherently biodegradable there is a lack of biodegradable composites in the literature. Clinically, the scaffold must act as a temporary matrix which will eventually be replaced by the tissue of the host body. All these factors fall into this notion of clinically relevant material design and needs to be discussed in the field is to see further success in the future.
Devices/implants
Biomedical devices and implants reported in the literature are often lead-based, although they have the distinct advantage of a high electrical output due to their large d33 coefficients, despite the challenge of possible Pb leakage and the related toxicity. Lead toxicity can affect almost all organs of the human body [Citation362] and therefore devices based on lead are being restricted.
Biocompatible systems, such as those based on PVDF, PVDF-TrFE and ZnO, overcome this issue but at the cost of lower piezoelectric coefficients and reduced electrical output. These thin films (PVDF and PVDF-TrFE) and NWs (ZnO) tend to provide lower power and therefore low sensitivity, though they are highly useful for systems which require ultra-low power stimulation, such as the cochlea. Another technical aspect to consider is impedance matching, whereby any transmittance of electrical energy from the piezoelectric energy harvesters requires all proceeding interconnected systems to match the devices impedance to maximise energy harvesting output. In the literature encompassing piezoelectric biomedical devices, the main parameters focused upon are voltage and current output, but these values are ultimately determined by the level of impedance matching when integrated into a larger system for sensing or energy harvesting. A simple approach to electrical impedance matching is to ensure the electrical load (RL) matches the impedance of the piezoelectric harvesting device so that (1/RL) = 2 π f Cp; where f is the frequency and Cp is the device capacitance. However, are a variety of methods to approach this issue which are outlined comprehensively by Rathod [Citation363]. In addition to electrical impedance matching, there is also a need for mechanical impedance matching to ensure transfer of mechanical strains from the body into the devices, and vice versa [Citation364]. Recent work on ultrasound powered implantable devices [Citation282] that uses ferroelectric ceramics for power generation provided an intriguing route to create autonomous systems.
Utilising a similar effect to piezoelectric materials, ferroelectrets are porous piezo-active polymer foams which gain their piezoelectric properties not from the inherent properties of the material but form the change in polarisation as a result of the deformation of their charged ellipsoidal pores [Citation365]. Ferroelectrets have shown high piezoelectric properties using many biocompatible materials such as PVDF (d33 ∼ 264 pC/N) [Citation366], PDMS (d33 ∼ 520 pC/N) [Citation367] and polypropylene (d33 ∼ 650 pC/N) [Citation368]. Furthermore, a range of ferroelectret nanogenerators have been observed in the literature, frequently for loudspeaker/microphone applications [Citation369–371], but also for sensing and biomedical applications [Citation372]. Despite their seemly high mechano-transductive properties, we can speculate that the infiltration of porosity with fluids in the body over time can affect their performance. However, this effect has not been studied directly in literature and is worthy of study. This can be mitigated by encapsulation of the ferroelectret material in a polymer but could negatively impact the mechano-transductive properties of the overall system. To investigate this in future, impedance spectroscopy would be useful to monitor the change in ferroelectret properties following soaking with fluid or implantation in vivo.
Triboelectric nanogenerators utilise a combination of triboelectric electrification and electrostatic induction between layers of electrostatically dissimilar materials as a means of generating energy [Citation373]. A range of triboelectric devices have been reported in the literature which utilise classically biocompatible materials and have achieved notable voltage outputs; these include PET-Kapton films (3.3 V) [Citation374], PTFE-eggshell membranes (79–160 V) [Citation375], degradable paper-based systems (20–200 V) [Citation376] and carboxymethyl cellulose aerogel-PDMS systems (150 V). However, for implantable biomedical applications, there are challenges, such as the need to overcome the need for a mechanically unfavourable air-gap in classical triboelectric systems [Citation377]. While these are not studied to the extent of implantable energy harvesting (IEH) materials, for a more comprehensive review of the variety of IEH materials, the reader is referred to the works of Dagdviren et al. [Citation378], Shi et al. [Citation379], Jiang et al. [Citation380] and electrically active materials for medical devices published by the RSC and edited by Tofail [Citation381], Hansen et al. [Citation382] and Li et al. [Citation383] for an insight into hybrid biomechanical and biochemical IEH devices.
Importantly there has been a large shift away from lead-based materials despite their impressive piezoelectric properties. Devices have been shown to produce a range of currents and voltage for a variety of biomedical applications, see (a). However, as with the scaffolds for tissue engineering, reports of devices/implants currently lack biocompatbility studies for clinically relavent time periods and comprehensive piezoelectric and dielectric properties to understand how their properties change overtime in vivo under strain. Furthermore, reports tend to observe the direct output of the devices which have not been impedance matched to provide specific power or act as part of a sensing array for a larger biomedical device, (b). While much progress has been made we believe a focus on clinical applications based material design will elevate the field closer to its final clinical goal.
Clinical perspective
The World Health Organisation (WHO) comprehensively details the life span and clinical application of biomedical devices [Citation384]. Two critical criteria which are highly emphasised are clinical effectiveness and device performance. Clinical effectiveness asks whether or not the system has fulfilled its intended purpose relative to the medical condition. In addition, device performance asks how well the device operates and performs in service. These criteria are difficult to implement to the scaffolds and devices mentioned in the literature as most encompassed the Phase 1 – Conception and development stages of clinical development, as outlined in . Therefore, a longer-term view of these scaffolds and devices is critical if they to achieve clinical use. Jeong et al. [Citation323] highlight the need for a longer-term approach with respect to Phase 6 – Use and the ultimate compatibility of these devices in the body, but these are not the only criteria to consider. Phase 2 – Manufacture, refers to larger scale manufacture outside the laboratory and Phase 3 – Disposal refers to the devices at end of life, which needs to be considered if we have to consider the ultimate clinical application of a devices. Ultimately such scaffolds and devices which achieve clinical application will be those with the appropriate combination of effectiveness, performance, risk, price, reproducibility and other important factors that need to be balanced to be considered viable for use on patients.
Figure 26. Major phases in the life span of a medical device, as outlined by the World Health Organisation (WHO) [Citation384].
![Figure 26. Major phases in the life span of a medical device, as outlined by the World Health Organisation (WHO) [Citation384].](/cms/asset/917eec31-8964-428a-822e-70bd5e1f6f93/yimr_a_1988194_f0026_oc.jpg)
The continued demand for smart piezoelectric scaffolds for tissue engineering applications and high-performance piezoelectric-based biomedical devices with high sensitivity or self-powered functionality will ensure there will be further developments in this fascinating research area [Citation385–390].
Acknowledgements
This research is funded by the RCH studentship through University of Bath Alumni. The authors would also like to acknowledge the support provided by the Centre for Sustainable and Circular Technologies (CSCT) at the University of Bath.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- “Home - GODT.” [cited 2021 Aug 30]. Available from: http://www.transplant-observatory.org/
- Azoth Analytics. Global organ transplantation market: world market review by product type (organ preservation products, transplant diagnostics, immunosuppressant drugs, others), by applications (kidney, liver, heart, others), by region, by country (2019 Edition): forecast, 2019.
- “WHO – Human organ transplantation.” WHO, 2013.
- Williams A. Central nervous system regeneration–where are we? QJM. 2014;107(5):335–339. DOI:10.1093/qjmed/hct260
- Seijffers R, Benowitz L.. CNS Regeneration Basic Science and Clinical Advances. Second Edition Academic Press; 2008. p. 1–39.
- Tandon B, Blaker JJ, Cartmell SH. Piezoelectric materials as stimulatory biomedical materials and scaffolds for bone repair. Acta Biomater. 2018;73:1–20. DOI:10.1016/J.ACTBIO.2018.04.026
- Zhang K, Wang S, Zhou C , et al Advanced smart biomaterials and constructs for hard tissue engineering and regeneration . DOI:10.1038/s41413-018-0032-9
- Zaszczynska A, Sajkiewicz P, Gradys A. Piezoelectric scaffolds as smart materials for neural tissue engineering. Polymers. 2020;12(1):161. DOI:10.3390/polym12010161
- Chorsi MT, Curry EJ, Chorsi HT, et al. Piezoelectric biomaterials for sensors and actuators. Adv Mater. 2019;31(1):1802084. DOI:10.1002/adma.201802084.
- Ribeiro C, Sencadas V, Correia DM, et al. Piezoelectric polymers as biomaterials for tissue engineering applications. Colloids Surf B Biointerfaces. 2015;136:46–55. DOI:10.1016/J.COLSURFB.2015.08.043
- Rajabi AH, Jaffe M, Arinzeh TL. Piezoelectric materials for tissue regeneration: a review. Acta Biomater. 2015;24:12–23. DOI:10.1016/J.ACTBIO.2015.07.010
- Moulson A.J, Herbert J.M. “Piezoelectric ceramics.” In Electroceramics. Second Edition. Chichester: John Wiley & Sons, Ltd; 2003. p. 339–410.
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926. DOI:10.1007/978-3-642-02824-3
- Howard D, Buttery LD, Shakesheff KM, et al. Tissue engineering: strategies, stem cells and scaffolds. J Anat. 2008;213:66–72. DOI:10.1111/j.1469-7580.2008.00878.x
- Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123(24):4195–4200. DOI:10.1242/JCS.023820
- Chan BP, Leong AKW. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J. 2008;17:467–479 DOI:10.1007/s00586-008-0745-3
- Liu Y, Lim J, Teoh S-H. Review: development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnol Adv. 2013;31(5):688–705. DOI:10.1016/j.biotechadv.2012.10.003
- Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomater Silver Jubil Compend. 2000;21:175–189. DOI:10.1016/B978-008045154-1.50021-6.
- Iqbal N, Samad Khan A, Asif A, et al. Recent concepts in biodegradable polymers for tissue engineering paradigms: a critical review. Int Mater Rev. 2018;64:91–126. DOI:10.1080/09506608.2018.1460943.
- Asghari F, Samiei M, Adibkia K, et al. Biodegradable and biocompatible polymers for tissue engineering application: a review. Artif Cells Nanomed Biotechnol. 2017;45(2):185–192. DOI:10.3109/21691401.2016.1146731
- Jafari M, Paknejad Z, Rad MR, et al. Polymeric scaffolds in tissue engineering: a literature review. J Biomed Mater Res Part B Appl Biomater. 2017;105(2):431–459. DOI:10.1002/jbm.b.33547
- Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24(24):4337–4351. DOI:10.1016/S0142-9612(03)00340-5
- Guo JL, Kim YS, Smith BT, et al. Modular, tissue-specific, and biodegradable hydrogel cross-linkers for tissue engineering. Sci Adv. 2019;5(6). DOI:10.1126/sciadv.aaw7396
- Kumar Meena L, Rather H, Kedaria D, et al. Polymeric microgels for bone tissue engineering applications – a review. Int J Polym Mater Polym Biomater. 2019: 1–17. DOI:10.1080/00914037.2019.1570512
- Venkatesan J, Bhatnagar I, Manivasagan P, et al. Alginate composites for bone tissue engineering: a review. Int J Biol Macromol. 2015;72:269–281. DOI:10.1016/j.ijbiomac.2014.07.008
- Fernandes HR, Gaddam A, Rebelo A, et al. Bioactive glasses and glass-ceramics for healthcare applications in bone regeneration and tissue engineering. Materials. 2018;11(12):2530. DOI:10.3390/ma11122530
- Hajiali F, Tajbakhsh S, Shojaei A. Fabrication and properties of polycaprolactone composites containing calcium phosphate-based ceramics and bioactive glasses in bone tissue engineering: a review. Polym Rev. 2018;58(1):164–207. DOI:10.1080/15583724.2017.1332640
- Afewerki S, Sheikhi A, Kannan S, et al. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: towards natural therapeutics. Bioeng Transl Med. 2019;4(1):96–115. DOI:10.1002/btm2.10124
- Mohebbi S, Nezhad MN, Zarrintaj P, et al. Chitosan in biomedical engineering: a critical review. Curr Stem Cell Res Ther. 2019;14(2):93–116. DOI:10.2174/1574888X13666180912142028
- Willerth SM, Sakiyama-Elbert SE. Approaches to neural tissue engineering using scaffolds for drug delivery. Adv Drug Deliv Rev. 2007;59(4–5):325–338. DOI:10.1016/J.ADDR.2007.03.014
- Sefat F, Mozafari M, Atala A. Introduction to tissue engineering scaffolds. In: Mozafari M, Sefat F, Atala A, editors. Handbook of tissue engineering scaffolds. Sawston, Cambridge: Woodhead Publishing; 2019, Vol. 1, p. 3–22. DOI:10.1016/B978-0-08-102563-5.00001-0.
- Esteve-Turrillas FA., Guardia M Handbook of smart materials in analytical chemistry. Hoboken, USA: John Wiley & Sons Ltd.; 2019. DOI:10.1002/9781119422587.
- Custódio CA, del Campo A, Reis RL, et al.. Smart polymers and their applications. Second Edition. Sawston, Cambridge: Woodhead Publishing; 2019. p. 411–438.
- Levin M, Bement W. Molecular bioelectricity: how endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol Biol Cell. 2014;25(24):3835–3850. DOI:10.1091/mbc.E13-12-0708
- Thrivikraman G, Boda SK, Basu B. Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: a tissue engineering perspective. Biomaterials. 2018;150:60–86. DOI:10.1016/j.biomaterials.2017.10.003
- Mrcho H, Sthatte M, Tsilvia D, et al. Transmembrane calcium influx induced by ac electric fields. FASEB J. 1999;13(6):677–683. DOI:10.1096/fasebj.13.6.677
- Ahadian S, Ostrovidov S, Hosseini V, et al. Electrical stimulation as a biomimicry tool for regulating muscle cell behavior. Organogenesis. 2013;9(2):87–92. DOI:10.4161/org.25121
- Donnelly K, Khodabukus A, Philp A, et al. A novel bioreactor for stimulating skeletal muscle in vitro. Tissue Eng Part C Methods. 2010;16(4):711–718. DOI:10.1089/ten.TEC.2009.0125
- Rao L, Qian Y, Khodabukus A, et al. Engineering human pluripotent stem cells into a functional skeletal muscle tissue. Nat Commun. 2018;9:126. DOI:10.1038/s41467-017-02636-4
- Zhang Q, Beirne S, Shu K, et al. Electrical stimulation with a conductive polymer promotes neurite outgrowth and synaptogenesis in primary cortical neurons in 3D open. Sci Rep. 2018;8:9855. DOI:10.1038/s41598-018-27784-5
- Zhang Q, Esrafilzadeh D, Crook JM, et al. Electrical stimulation using conductive polymer polypyrrole counters reduced neurite outgrowth of primary prefrontal cortical neurons from NRG1-KO and DISC1-LI Mice open. Sci Rep. 2017;7:42525. DOI:10.1038/srep42525
- Koppes AN, Keating KW, McGregor AL, et al. Robust neurite extension following exogenous electrical stimulation within single walled carbon nanotube-composite hydrogels. Acta Biomater. 2016;39:34–43, DOI:10.1016/J.ACTBIO.2016.05.014
- Tian J, Shi R, Liu Z, et al. Self-powered implantable electrical stimulator for osteoblasts’ proliferation and differentiation. Nano Energy. 2019;59:705–714. DOI:10.1016/J.NANOEN.2019.02.073
- Zhang J, Li M, Kang E-T, et al. Electrical stimulation of adipose-derived mesenchymal stem cells in conductive scaffolds and the roles of voltage-gated ion channels. Acta Biomater. 2016;32:46–56. DOI:10.1016/J.ACTBIO.2015.12.024
- Huang Y, Deng H, Fan Y, et al. Conductive nanostructured Si biomaterials enhance osteogeneration through electrical stimulation. Mater Sci Eng C. 2019;103:109748, DOI:10.1016/J.MSEC.2019.109748.
- Huang Z-B, Yin G-F, Liao X-M, et al. Conducting polypyrrole in tissue engineering applications. Front Mater Sci. 2014;8(1):39–45. DOI:10.1007/s11706-014-0238-8
- Qazi TH, Rai R, Boccaccini AR. Tissue engineering of electrically responsive tissues using polyaniline based polymers: a review. Biomaterials. 2014;35(33):9068–9086. DOI:10.1016/J.BIOMATERIALS.2014.07.020
- Pires F, Ferreira Q, Rodrigues CAV, et al. Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. Biochim Biophys Acta Gen Subj. 2015;1850(6):1158–1168. DOI:10.1016/J.BBAGEN.2015.01.020
- Vashist A, et al. Advances in carbon nanotubes-hydrogel hybrids in nanomedicine for therapeutics. Adv. Healthc. Mater. May 2018;7(9):1701213. DOI:10.1002/adhm.201701213
- Gajendiran M, et al. Conductive biomaterials for tissue engineering applications. J Ind Eng Chem. 2017;51:12–26. DOI:10.1016/j.jiec.2017.02.031
- Hickey RJ, Pelling AE. Cellulose biomaterials for tissue engineering. Front Bioeng Biotechnol. 2019;7. DOI:10.3389/fbioe.2019.00045
- Priya G, Madhan B, Narendrakumar U, et al. In vitro and in vivo evaluation of carboxymethyl cellulose scaffolds for bone tissue engineering applications. ACS Omega. 2021;6(2):1246–1253. DOI:10.1021/acsomega.0c04551
- Courtenay J, Sharma R, Scott J. Recent advances in modified cellulose for tissue culture applications. Molecules. 2018;23(3):654. DOI:10.3390/molecules23030654
- Lin Z, Tao Y, Huang Y, et al. Biomedical materials biomedical materials topical review applications of marine collagens in bone tissue engineering. Biomed Mater. 2021;16:42007, DOI:10.1088/1748-605X/abf0b6
- El Blidi O, El Omari N, Balahbib A, et al. Extraction methods, characterization and biomedical applications of collagen: a review. Biointerface Res Appl Chem. 2021;11(5):13587–13613. DOI:10.33263/BRIAC115.1358713613
- Szymkowiak S, Sandler N, Kaplan DL. Aligned silk sponge fabrication and perfusion culture for scalable proximal tubule tissue engineering. ACS Appl Mater Interfaces. 2021;13:10768–10777. DOI:10.1021/acsami.1c00548
- Zhang L, et al. Systematic review of silk scaffolds in musculoskeletal tissue engineering applications in the recent decade. ACS Biomater Sci Eng. 2021;7:840. DOI:10.1021/acsbiomaterials.0c01716
- Khademolqorani S, Tavanai H, Chronakis IS, et al. The determinant role of fabrication technique in final characteristics of scaffolds for tissue engineering applications: a focus on silk fibroin-based scaffolds. Mater Sci Eng C. 2021;122:111867. DOI:10.1016/j.msec.2021.111867
- Lu TY, Huang WC, Chen Y, et al. Effect of varied hair protein fractions on the gel properties of keratin/chitosan hydrogels for the use in tissue engineering. Colloids Surf B Biointerfaces. 2020;195:111258. DOI:10.1016/j.colsurfb.2020.111258
- Yang Y, Chen J, Migliaresi C, et al. Natural fibrous protein for advanced tissue engineering applications: focusing on silk fibroin and keratin. Adv Exp Med Biol. 2020;1249:39–49. DOI:10.1007/978-981-15-3258-0_3
- Chae I, Jeong CK, Ounaies Z, et al. Review on electromechanical coupling properties of biomaterials. ACS Appl Bio Mater. 2018;1(4):936–953. DOI:10.1021/acsabm.8b00309
- Tofail SAM, Bauer J. Electrically polarized biomaterials. Adv Mater. 2016;28(27):5470–5484. DOI:10.1002/ADMA.201505403
- Cao L, Qiu X, Jiao Q, et al. Polysaccharides and proteins-based nanogenerator for energy harvesting and sensing: a review. Int J Biol Macromol. 2021;173:225–243. DOI:10.1016/j.ijbiomac.2021.01.109
- Lee BY, Zhang J, Zueger C, et al. Virus-based piezoelectric energy generation. Nat Nanotechnol. 2012;7(6):351–356. DOI:10.1038/nnano.2012.69
- Nguyen V, Zhu R, Jenkins K, et al. Self-assembly of diphenylalanine peptide with controlled polarization for power generation. Nat Commun. 2016;7(1):1–6. DOI:10.1038/ncomms13566
- Song Y, Shi Z, Hu GH, et al. Recent advances in cellulose-based piezoelectric and triboelectric nanogenerators for energy harvesting: a review. J Mater Chem A. 2021;9(4):1910–1937. DOI:10.1039/d0ta08642h
- Sundelacruz S, Levin M, Kaplan DL. Role of membrane potential in the regulation of cell proliferation and differentiation. Stem Cell Rev Rep. 2009;5(3):231–246. DOI:10.1007/s12015-009-9080-2
- Cone CD. Unified theory on the basic mechanism of normal mitotic control and oncogenesis. J Theor Biol. 1971;30(1):151–181. DOI:10.1016/0022-5193(71)90042-7
- Branković Z, Branković G, Jovalekić Č, et al. Mechanochemical synthesis of PZT powders. Mater Sci Eng A. 2003;345(1–2):243–248. DOI:10.1016/S0921-5093(02)00475-6
- Buscaglia MT, Bassoli M, Buscaglia V, et al. Solid-State synthesis of nanocrystalline BaTiO3 : reaction kinetics and powder properties. J Am Ceram Soc. 2008;91(9):2862–2869. DOI:10.1111/j.1551-2916.2008.02576.x
- Tariverdian T, Behnamghader A, Brouki Milan P, et al. 3D-printed barium strontium titanate-based piezoelectric scaffolds for bone tissue engineering. Ceram Int. 2019;45(11):14029–14038. DOI:10.1016/J.CERAMINT.2019.04.102
- Villafuerte-Castrejón ME, Morán E, Reyes-Montero A, et al. Towards lead-free piezoceramics: facing a synthesis challenge. Materials. 2016;9(1):21. DOI:10.3390/ma9010021
- Selmi F, Guerin F, Yu XD, et al. Microwave calcination and sintering of barium strontium titanate. Mater Lett. 1992;12(6):424–428. DOI:10.1016/0167-577X(92)90206-Y
- Orlik K, Lorgouilloux Y, Marchet P, et al. Influence of microwave sintering on electrical properties of BCTZ lead free piezoelectric ceramics. J Eur Ceram Soc. 2019. DOI:10.1016/j.jeurceramsoc.2019.12.010.
- Mane SM, Tirmali PM, Kulkarni SB. Hybrid microwave sintering and shifting of Tc in lead-free ferroelectric composition x(Ba0.7Ca0.3TiO3)-(1-x)(BaZr0.2Ti0.8O3). Mater Chem Phys. 2018;213:482–491. DOI:10.1016/j.matchemphys.2018.04.059
- Taheri Mofassal A, Tajally M, Mirzaee O. Comparison between microwave and conventional calcination techniques in regard to reactivity and morphology of co-precipitated BaTiO3 powder, and the electrical and energy storage properties of the sintered samples. Ceram Int. 2017;43(11):8057–8064. DOI:10.1016/j.ceramint.2017.03.126
- Reza Bafandeh M, Gharahkhani R, Lee J-S. Dielectric and piezoelectric properties of sodium potassium niobate-based ceramics sintered in microwave furnace. Mater Chem Phys. 2015;156:254–260. DOI:10.1016/j.matchemphys.2015.03.018
- Manohar C. S., Kumar BS, Sadhu SPP et al., “Novel lead-free biocompatible piezoelectric hydroxyapatite (HA) – BCZT (Ba0.85Ca0.15Zr0.1Ti0.9O3) nanocrystal composites for bone regeneration. Nanotechnol Rev. 2019;8(1):61–78. DOI:10.1515/ntrev-2019-0006
- Amna T, Hassan MS, Sheikh FA, et al. Zinc oxide-doped poly(urethane) spider web nanofibrous scaffold via one-step electrospinning: a novel matrix for tissue engineering. Appl Microbiol Biotechnol. 2013;97(4):1725–1734. DOI:10.1007/s00253-012-4353-0
- Yáñez-Limón JM, Rivera-Ruedas G, Jesús FSD, et al. Synthesis of PZT ceramics by sol-gel method and mixed oxides with mechanical activation using different oxides as a source of Pb. Ferroelectr Mater Asp. 2011. DOI:10.5772/18125.
- Znaidi L. Sol-gel-deposited ZnO thin films: a review. Mater Sci Eng B Solid State Mater Adv Technol. 2010;174(1–3):18–30. DOI:10.1016/j.mseb.2010.07.001
- Chamankar N, Khajavi R, Yousefi AA, et al. Comparing the piezo, pyro and dielectric properties of PZT particles synthesized by sol–gel and electrospinning methods. J Mater Sci Mater Electron. 2019;30(9):8721–8735. DOI:10.1007/s10854-019-01197-0
- Kawamura G, Kazuhiro O, Tan WK, et al. Sol-gel template synthesis of BaTiO3 films with nano-periodic structures. Mater Lett. 2018;227:120–123. DOI:10.1016/j.matlet.2018.05.056
- Li L, Miao L, Pu X, et al. Recent progress in piezoelectric thin film fabrication: via the solvothermal process. J Mater Chem A. 2019;7(27):16046–16067. DOI:10.1039/c9ta04863d
- Chilibon I, Marat-Mendes JN. Ferroelectric ceramics by sol-gel methods and applications: a review. J Sol-Gel Sci Technol. 2012;64(3):571–611. DOI:10.1007/s10971-012-2891-7
- Sakka S, Petrykin V, Kakihana M. Processing, Characterization and Applications. In: Klein L, Aparicio M, Jitianu A, editors. Handbook of Sol-Gel Science and Technology. New York City, USA: Springer International Publishing; 2005.
- Lv J-H, Zhang M, Guo M, et al. Hydrothermal synthesis and characterization of KxNa(1−x)NbO3 powders. Int J Appl Ceram Technol. 2007;4(6):571–577. DOI:10.1111/j.1744-7402.2007.02165.x
- Deng Y, Liu L, Cheng Y, et al. Hydrothermal synthesis and characterization of nanocrystalline PZT powders. Mater Lett. 2003;57(11):1675–1678. DOI:10.1016/S0167-577X(02)01050-9
- Ma YJ, Cho JH, Lee YH, et al. Hydrothermal synthesis of (Bi1/2Na1/2)TiO3 piezoelectric ceramics. Mater Chem Phys. 2006;98(1):5–8. DOI:10.1016/j.matchemphys.2004.09.045
- Morita T. Piezoelectric materials synthesized by the hydrothermal method and their applications. Materials. 2010;3(12):5236–5245. DOI:10.3390/ma3125236
- Lin Y, Liu Y, Sodano HA. Hydrothermal synthesis of vertically aligned lead zirconate titanate nanowire arrays. Appl Phys Lett. 2009;95(12). DOI:10.1063/1.3237170
- Ou C, Sanchez-Jimenwz PE, Datta A, et al. Template-assisted hydrothermal growth of aligned zinc oxide nanowires for piezoelectric energy harvesting applications. ACS Appl Mater Interfaces. 2016;8(22):13678–13683. DOI:10.1021/acsami.6b04041
- Xu Y, Yu Q, Li JF. A facile method to fabricate vertically aligned (K,Na)NbO3 lead-free piezoelectric nanorods. J Mater Chem. 2012;22(43):23221–23226. DOI:10.1039/c2jm35090d
- Laudise RA. Hydrothermal synthesis of crystals. Chem Eng News. 1987;65(39):30–43. DOI:10.1021/cen-v065n039.p030
- Li C, Liu S, Li G, et al. Hydrothermal synthesis of large-sized hydroxyapatite whiskers regulated by glutamic acid in solutions with low supersaturation of precipitation. Adv Powder Technol. 2011;22(4):537–543. DOI:10.1016/j.apt.2010.07.013
- Suaste-Gomez E. Piezoelectric ceramics. Rijeka, Croatia: Sciyo; 2010. DOI:10.5772/241.
- Ehterami A, Kazemi M, Nazari B, et al. Fabrication and characterization of highly porous barium titanate based scaffold coated by Gel/HA nanocomposite with high piezoelectric coefficient for bone tissue engineering applications. J Mech Behav Biomed Mater. 2018;79:195–202. DOI:10.1016/j.jmbbm.2017.12.034
- Creedon MJ, Schulze WA. Axially distorted 3-3 piezoelectric composites for hydrophone applications. Ferroelectrics. 1994;153(1):333–339. DOI:10.1080/00150199408016589
- Cunningham E, Dunne N, Walker G, et al. Hydroxyapatite bone substitutes developed via replication of natural marine sponges. J Mater Sci Mater Med. 2010;21(8):2255–2261. DOI:10.1007/s10856-009-3961-4
- Miao X, Hu Y, Liu J, et al. Porous calcium phosphate ceramics prepared by coating polyurethane foams with calcium phosphate cements. Mater Lett. 2004;58(3–4):397–402. DOI:10.1016/S0167-577X(03)00510-X
- Pinheiro ED, Deivarajan T. A concise review encircling lead free porous piezoelectric ceramics. Acta Phys Pol A. 2019;136. DOI:10.12693/APhysPolA.136.555
- Li D, Xia Y. Electrospinning of nanofibers: reinventing the wheel? Adv Mater. 2004;16(14):1151–1170. DOI:10.1002/adma.200400719
- Subbiah T, Bhat GS, Tock RW, et al. Electrospinning of nanofibers. J Appl Polym Sci. 2005;96(2):557–569. DOI:10.1002/app.21481
- Gomes J, Nunes JS, Sencadas V, et al. Influence of the β-phase content and degree of crystallinity on the piezo-and ferroelectric properties of poly(vinylidene fluoride). Smart Mater Struct. 2010;19(6):065010. DOI:10.1088/0964-1726/19/6/065010
- Guo H-F, Li Z, Dong S, et al. Piezoelectric PU/PVDF electrospun scaffolds for wound healing applications. Colloids Surf B Biointerfaces. 2012;96:29–36. DOI:10.1016/J.COLSURFB.2012.03.014
- Greeshma T, Balaji R, Jayakumar S. PVDF phase formation and its influence on electrical and structural properties of PZT-PVDF composites. Ferroelectr Lett Sect. 2013;40(1–3):41–55. DOI:10.1080/07315171.2013.814460
- Menzies KL, Jones L. The impact of contact angle on the biocompatibility of biomaterials. Optom Vis Sci. 2010;87(6):387–399, DOI:10.1097/OPX.0b013e3181da863e
- Antonis N. SEM and TEM: what’s the difference? Lambda News, 2018. [cited 2021 Aug 30]. Available from: https://www.lambdaphoto.co.uk/news/2018/02/21/sem-and-tem-whats-the-difference/
- Marino A, Arai S, Hou Y, et al. Piezoelectric nanoparticle-assisted wireless neuronal stimulation. ACS Nano. 2015;9(7):7678–7689. DOI:10.1021/acsnano.5b03162
- Kumar A, Dixit CK. Methods for characterization of nanoparticles. In: Nimesh S, Chandra R, Gupta N, editors. Advances in nanomedicine for the delivery of therapeutic nucleic acids. Sawston, Cambridge: Woodhead Publishing; 2017, p. 43–58. DOI:10.1016/B978-0-08-100557-6.00003-1.
- Siqveland LM, Skjæveland SM. Derivations of the young-laplace equation. Capillarity. 2021;4(2):23–30. DOI:10.46690/CAPI.2021.02.01
- Peinador RI, Calvo JI, Ben Aim R. Comparison of capillary flow porometry (CFP) and liquid extrusion porometry (LEP) techniques for the characterization of porous and face mask membranes. Appl Sci. 2020;10(16):5703. DOI:10.3390/APP10165703
- Akhtar R, Sherratt MJ, Cruickshank JK, et al. Characterizing the elastic properties of tissues. Mater Today. 2011;14(3):96–105. DOI:10.1016/S1369-7021(11)70059-1
- Alexander H, Cook T. Variations with age in the mechanical properties of human skin in vivo. J Tissue Viability. 2006;16(3):6–11. DOI:10.1016/S0965-206X(06)63002-7
- Choudhury N, Bouchot O, Rouleau L, et al. Local mechanical and structural properties of healthy and diseased human ascending aorta tissue. Cardiovasc Pathol. 2009;18(2):83–91. DOI:10.1016/j.carpath.2008.01.001
- Eros S, Reitz JR. Elastic constants by the ultrasonic pulse echo method. J Appl Phys. 1958;29(4):683–686. DOI:10.1063/1.1723250
- Börger A, Supancic P, Danzer R. The ball on three balls test for strength testing of brittle discs: stress distribution in the disc. J Eur Ceram Soc. 2002;22(9–10):1425–1436. DOI:10.1016/S0955-2219(01)00458-7
- Yu S-W, Kuo S-T, Tuan W-H, et al. Cytotoxicity and degradation behavior of potassium sodium niobate piezoelectric ceramics. Ceram Int. 2012;38(4):2845–2850. DOI:10.1016/J.CERAMINT.2011.11.056
- Ciofani G, Danti S, D'Alessandro D, et al. Enhancement of neurite outgrowth in neuronal-like cells following boron nitride nanotube-mediated stimulation. ACS Nano. 2010;4(10):6267–6277. DOI:10.1021/nn101985a
- Haugland RP, MacCoubrey IC, Moore PL. Dual-fluorescence cell viability assay using ethidium homodimer and calcein AM, US5314805A, 1994.
- King MA. Detection of dead cells and measurement of cell killing by flow cytometry. J Immunol Methods. 2000;243(1–2):155–166. DOI:10.1016/S0022-1759(00)00232-5
- Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4(1):26–49. DOI:10.1002/smll.200700595
- Zhang Y, Chen X, Gueydan C, et al. Plasma membrane changes during programmed cell deaths. Cell Res. 2018;28(1):9–21. DOI:10.1038/cr.2017.133
- Buttke TM, McCubrey JA, Owen TC. Use of an aqueous soluble tetrazolium/formazan assay to measure viability and proliferation of lymphokine-dependent cell lines. J Immunol Methods. 1993;157(1–2):233–240. DOI:10.1016/0022-1759(93)90092-L
- Präbst K, Engelhardt H, Ringgeler S, et al. Basic colorimetric proliferation assays: MTT, WST, and resazurin. Methods Mol Biol. 2017;1601:1–17. DOI:10.1007/978-1-4939-6960-9_1
- Sultan Aslantürk Ö. In vitro cytotoxicity and cell viability assays: principles, advantages, and disadvantages provisional chapter in vitro cytotoxicity and cell viability assays: principles, advantages, and disadvantages. 2016. DOI:10.5772/intechopen.71923
- Zhang G, Gurtu V, Kain SR, et al. Early detection of apoptosis using a fluorescent conjugate of annexin V. Biotechniques. 1997;23(3):525–531. DOI:10.2144/97233pf01
- Patel R, Rinker L, Peng J, et al. Reactive oxygen species: the good and the bad. In: Filip C, Albu E, editors. Reactive oxygen species (ROS) in living cells. London, UK: IntechOpen; 2017. p. 69–8910.5772/INTECHOPEN.71547.
- Christov A, Hamdheydari L, Grammas P. Detection of reactive oxygen species by flow cytometry. In: Hensley K, Floyd RA, editors. Methods in biological oxidative stress. Totowa, USA: Humana Press; 2003, p. 175–184. DOI:10.1385/1-59259-424-7:175.
- Jonkman JEN, Cathcart JA, Xu F, et al. An introduction to the wound healing assay using live-cell microscopy. Cell Adh Migr. 2014;8:440–451. DOI:10.4161/cam.36224
- Freeman WM, Walker SJ, Vrana KE. Quantitative RT-PCR: pitfalls and potential. Aug. 2018;26(1):112–125. DOI:10.2144/99261rv01.
- Wells L, Edwards KA, Bernstein SI. Myosin heavy chain isoforms regulate muscle function but not myofibril assembly. EMBO J. 1996;15(17):4454–4459. DOI:10.1002/J.1460-2075.1996.TB00822.X
- Abmayr SM, Pavlath GK. Myoblast fusion: lessons from flies and mice. Development. 2012;139(4):641–656. DOI:10.1242/DEV.068353
- Ikeda K, Ito A, Sato M, et al. Improved contractile force generation of tissue-engineered skeletal muscle constructs by IGF-I and Bcl-2 gene transfer with electrical pulse stimulation. Regen Ther. 2016;3:38–44. DOI:10.1016/J.RETH.2015.12.004
- Florencio-Silva R, Rodrigues Da G, Sasso S, et al. Biology of bone tissue: structure, function, and factors that influence bone cells. BioMed Res Int. 2015. DOI:10.1155/2015/421746
- Virtanen P, Isotupa K. Staining properties of alizarin red S for growing bone in vitro. Cells Tissues Organs. 1980;108(2):202–207. DOI:10.1159/000145301
- Golub EE, Boesze-Battaglia K. The role of alkaline phosphatase in mineralization. [cited 2019 Aug 20]. Available from: https://pdfs.semanticscholar.org/90d3/c7d6cebfaa5c8cbaa9cdc68b412316199acc.pdf
- Wen J, Liu M. Piezoelectric ceramic (PZT) modulates axonal guidance growth of rat cortical neurons via RhoA, Rac1, and Cdc42 pathways. J Mol Neurosci. 2014;52(3):323–330. DOI:10.1007/s12031-013-0149-7
- Anti-microtubule associated protein 2 (MAP2) antibody. [cited 2019 Apr 28]. Available from: https://www.phosphosolutions.com/wp-content/uploads/2015/12/1100-MAP2-datasheet-4.pdf
- Finci L, Zhang Y, Meijers R, et al. Signaling mechanism of the netrin-1 receptor DCC in axon guidance. Prog Biophys Mol Biol. 2015;118(3):153–160. DOI:10.1016/j.pbiomolbio.2015.04.001
- Guo J, Walss-Bass C, Ludueña RF. The beta isotypes of tubulin in neuronal differentiation. Cytoskeleton. 2010;67(7):431–441. DOI:10.1002/cm.20455
- Tischler AS, Ruzicka LA, Perlman RL. Mimicry and inhibition of nerve growth factor effects: interactions of staurosporine, forskolin, and K252a in PC12 cells and normal rat chromaffin cells in vitro. J Neurochem. 1990;55(4):1159–1165. DOI:10.1111/j.1471-4159.1990.tb03120.x
- Lansman JB. Blockade of current through single calcium channels by trivalent lanthanide cations effea of ionic raztius on the rates of ion entry and exit. DOI:10.1085/jgp.95.4.679
- Gee KR, Brown KA, Chen WNU, et al. Chemical and physiological characterization of fluo-4 Ca2+-indicator dyes. Cell Calcium. 2000;27(2):97–106. DOI:10.1054/CECA.1999.0095
- Meier SD, Kovalchuk Y, Rose CR. Properties of the new fluorescent Na+ indicator CoroNa green: comparison with SBFI and confocal Na+ imaging. J Neurosci Methods. 2006;155(2):251–259. DOI:10.1016/J.JNEUMETH.2006.01.009
- Hamill OP, McBride DW. The pharmacology of mechanogated membrane ion channels. Pharmacol. Rev. 1996;48(2):231–252.
- Molleman A. Patch clamping : an introductory guide to patch clamp electrophysiology. Hoboken, USA: John Wiley; 2003.
- Ribeiro C, et al. In vivo demonstration of the suitability of piezoelectric stimuli for bone reparation. Mater Lett. 2017;209:118–121. DOI:10.1016/j.matlet.2017.07.099
- Rosai J. Why microscopy will remain a cornerstone of surgical pathology. Lab Invest. 2007;87(5):403–408. DOI:10.1038/labinvest.3700551
- Liu J, Gu H, Liu Q, et al. An intelligent material for tissue reconstruction: the piezoelectric property of polycaprolactone/barium titanate composites. Mater Lett. 2019;236:686–689. DOI:10.1016/j.matlet.2018.11.036
- Genchi GG, Ceseracciu L, Marino A, et al. P(VDF-TrFE)/BaTiO3 nanoparticle composite films mediate piezoelectric stimulation and promote differentiation of SH-SY5Y neuroblastoma cells. Adv Healthc Mater. 2016;5(14):1808–1820. DOI:10.1002/adhm.201600245
- Poon KK, Wurm MC, Evans DM, et al. Biocompatibility of (Ba,Ca)(Zr,Ti)O3 piezoelectric ceramics for bone replacement materials. J Biomed Mater Res Part B Appl Biomater. 2019. DOI:10.1002/jbm.b.34477.
- Ribeiro C, Moreira S, Correia V, et al. Enhanced proliferation of pre-osteoblastic cells by dynamic piezoelectric stimulation. RSC Adv. 2012;2(30):11504–11509. DOI:10.1039/C2RA21841K
- Martins PM, et al. Effect of poling state and morphology of piezoelectric poly(vinylidene fluoride) membranes for skeletal muscle tissue engineering. RSC Adv. 2013;3(39):17938, DOI:10.1039/c3ra43499k
- Ribeiro S, Gomes AC, Etxebarria I, et al. Electroactive biomaterial surface engineering effects on muscle cells differentiation. Mater Sci Eng C. 2018;92:868–874. DOI:10.1016/j.msec.2018.07.044
- Ribeiro C, Pärssinen J, Sencadas V, et al. Dynamic piezoelectric stimulation enhances osteogenic differentiation of human adipose stem cells. J Biomed Mater Res Part A. 2015;103(6):2172–2175. DOI:10.1002/jbm.a.35368
- Hoop M, Chen X-Z, Ferrari A, et al. Ultrasound-mediated piezoelectric differentiation of neuron-like PC12 cells on PVDF membranes. Sci Rep. 2017;7(1):4028. DOI:10.1038/s41598-017-03992-3
- Royo-Gascon N, Wininger M, Scheinbeim JI, et al. Piezoelectric substrates promote neurite growth in rat spinal cord neurons. Ann Biomed Eng. 2013;41(1):112–122. DOI:10.1007/s10439-012-0628-y
- Ricotti L, Fujie T, Vazão H, et al. Boron nitride nanotube-mediated stimulation of cell co-culture on micro-engineered hydrogels. PLoS One. 2013;8(8):e71707. DOI:10.1371/journal.pone.0071707
- Seil JT, Webster TJ. Decreased astroglial cell adhesion and proliferation on zinc oxide nanoparticle polyurethane composites. Int J Nanomed. 2008;3(4):523–531. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19337420
- Costa J, Peixoto T, Ferreira A, et al. Development and characterization of ZnO piezoelectric thin films on polymeric substrates for tissue repair. J Biomed Mater ResPart A. 2019;107(10):2150–2159. DOI:10.1002/jbm.a.36725
- Carville NC, Collins L, Manzo M, et al. Biocompatibility of ferroelectric lithium niobate and the influence of polarization charge on osteoblast proliferation and function. J Biomed Mater Res Part A. 2015;103(8):2540–2548. DOI:10.1002/jbm.a.35390
- Kanemaru K, Okubo Y, Hirose K, et al. Regulation of neurite growth by spontaneous Ca2 oscillations in astrocytes. J Neurosci. 2007;27(33):8957–8966. DOI:10.1523/JNEUROSCI.2276-07.2007
- Lankford KL, Letourneau PC. Evidence that calcium may control neurite outgrowth by regulating the stability of actin filaments. J Cell Biol. 1989;109(3):1229–1243. DOI:10.1083/jcb.109.3.1229
- Rønn LCB, Dissing S, Holm A, et al. Increased intracellular calcium is required for neurite outgrowth induced by a synthetic peptide ligand of NCAM. FEBS Lett. 2002;518(1–3):60–66. DOI:10.1016/S0014-5793(02)02644-3
- Lee KH, Warchol ME. Promotion of neurite outgrowth and axon guidance in spiral ganglion cells by netrin-1. Arch Otolaryngol Neck Surg. 2008;134(2):146. DOI:10.1001/archoto.2007.6
- Mei T, Dai Q, Zheng W, et al. Strain properties and piezoelectric constant of lead-free barium titanate ceramics. Mater Res Express. 2019;6(10):106301. DOI:10.1088/2053-1591/ab34b1
- Genchi GG, Marino A, Rocca A, et al. Barium titanate nanoparticles: promising multitasking vectors in nanomedicine. Nanotechnology. 2016;27(23):232001. DOI:10.1088/0957-4484/27/23/232001
- Ciofani G, Ricotti L, Canale C, et al. Effects of barium titanate nanoparticles on proliferation and differentiation of rat mesenchymal stem cells. Colloids Surf B Biointerfaces. 2013;102:312–320. DOI:10.1016/j.colsurfb.2012.08.001
- Yhee JY, Son S, Kim SH, et al. Self-assembled glycol chitosan nanoparticles for disease-specific theranostics. J Control Release. 2014;193:202–213. DOI:10.1016/j.jconrel.2014.05.009
- Lee SJ, Min HS, Ku SH, et al. Tumor-targeting glycol chitosan nanoparticles as a platform delivery carrier in cancer diagnosis and therapy. Nanomedicine. 2014;9(11):1697–1713. DOI:10.2217/nnm.14.99
- Yin W, Li W, Rubenstein DA, et al. Biocompatible and target specific hydrophobically modified glycol chitosan nanoparticles. Biointerphases. 2016;11(4):04B301. DOI:10.1116/1.4948265
- Sun IC, et al. Biocompatible glycol chitosan-coated gold nanoparticles for tumor-targeting CT imaging. Pharm. Res. Aug. 2014;31(6):1418–1425. DOI:10.1007/s11095-013-1142-0.
- Yun Nam H, Na JH, Jeong SY, et al. Cellular uptake mechanism and intracellular fate of hydrophobically modified glycol chitosan nanoparticles. J Control Release. 2009;135:259–267. DOI:10.1016/j.jconrel.2009.01.018
- Alberts B, Johnson A, Lewis J, et al. Molecular biology of the cell. New York: Garland Science.; 2014.
- McBeath R, Pirone DM, Nelson CM, et al. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6(4):483–495. DOI:10.1016/S1534-5807(04)00075-9
- ter Haar G. Therapeutic applications of ultrasound. Prog Biophys Mol Biol. 2007;93(1–3):111–129. DOI:10.1016/J.PBIOMOLBIO.2006.07.005
- Arai S, Lee S-C, Zhai D, et al. A molecular fluorescent probe for targeted visualization of temperature at the endoplasmic reticulum. Sci Rep. May 2015;4(1):6701. DOI:10.1038/srep06701
- Deng Y, Liu X, Xu A, et al. Effect of surface roughness on osteogenesis in vitro and osseointegration in vivo of carbon fiber-reinforced polyetheretherketone-nanohydroxyapatite composite. Int J Nanomed. 2015;10:1425–1447. DOI:10.2147/IJN.S75557
- Ball JP, Mound BA, Nino JC, et al. Biocompatible evaluation of barium titanate foamed ceramic structures for orthopedic applications. J Biomed Mater ResPart A. 2014;102(7):2089–2095. DOI:10.1002/jbm.a.34879
- Vijatović MM, Bobić JD, Stojanović BD. History and challenges of barium titanate: part I. Sci Sinter. 2008;40:155–165. DOI:10.2298/SOS0802155V
- Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008;9(6):446–454. DOI:10.1038/nrm2406
- Zhang Y, Chen L, Zeng J, et al. Aligned porous barium titanate/hydroxyapatite composites with high piezoelectric coefficients for bone tissue engineering. Mater Sci Eng C. 2014;39:143–149. DOI:10.1016/J.MSEC.2014.02.022
- Zhou Z, Li W, He T, et al. Polarization of an electroactive functional film on titanium for inducing osteogenic differentiation. Sci Rep. 2016;6(1):35512. DOI:10.1038/srep35512
- Cappariello A, Maurizi A, Veeriah V, et al. The great beauty of the osteoclast. Arch Biochem Biophys. 2014;558:70–78. DOI:10.1016/J.ABB.2014.06.017
- Jeong J, Kim JH, Shim JH, et al. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater Res. 2019;23:4. DOI:10.1186/s40824-018-0149-3
- Galow A-M, Rebl A, Koczan D, et al. Increased osteoblast viability at alkaline pH in vitro provides a new perspective on bone regeneration. Biochem Biophys Rep. 2017;10:17–25. DOI:10.1016/J.BBREP.2017.02.001
- Wang P, Li Y, Lu Y. Enhanced piezoelectric properties of (Ba0.85Ca0.15)(Ti0.9Zr0.1)O3 lead-free ceramics by optimizing calcination and sintering temperature. J Eur Ceram Soc. 2011;31(11):2005–2012. DOI:10.1016/j.jeurceramsoc.2011.04.023
- Poon KK, Wurm MC, Evans DM, et al. Biocompatibility of (Ba,Ca)(Zr,Ti)O3 piezoelectric ceramics for bone replacement materials. J Biomed MaterRes Part B Appl Biomater. 2019. DOI:10.1002/jbm.b.34477
- Lerman MJ, Lembong J, Muramoto S, et al. The evolution of polystyrene as a cell culture material. Tissue Eng Part B Rev. 2018;24. DOI:10.1089/ten.teb.2018.0056
- Baxter FR, Turner IG, Bowen CR, et al. An in vitro study of electrically active hydroxyapatite-barium titanate ceramics using Saos-2 cells. J Mater Sci MaterMed. 2009;20(8):1697–1708. DOI:10.1007/s10856-009-3734-0
- Kawai H. The piezoelectricity of poly (vinylidene fluoride). Jpn J Appl Phys. 1969;8(7):975–976. DOI:10.1143/JJAP.8.975
- Foster FS, Harasiewicz KA, Sherar MD. A history of medical biological imaging with polyvinylidene fluoride (PVDF) transducers. IEEE Trans Ultrason Ferroelectr Freq Control. 2000;47(6):1363–1371. DOI:10.1109/58.883525
- Adnane M, Jiang Z. A wearable sensor design for cardiorespiratory signals acquisition based on PVDF sensors. Proceedings - 2018 IEEE International Conference on bioinformatics and biomedicine, BIBM 2018, Jan. 2019, p. 1099–1102. DOI:10.1109/BIBM.2018.8621519
- Sharma T, Aroom K, Naik S, et al. Flexible thin-film PVDF-TrFE based pressure sensor for smart catheter applications. Ann Biomed Eng. 2013;41(4):744–751. DOI:10.1007/s10439-012-0708-z
- Bifulco P, et al. Monitoring of respiration, seismocardiogram and heart sounds by a PVDF piezo film sensor. IMEKO International Measurement Federation Secretariat. 2014, p. 786–789.
- Qasaimeh MA, Sokhanvar S, Dargahi J, et al. PVDF-based microfabricated tactile sensor for minimally invasive surgery. J Microelectromech Syst. 2009;18(1):195–207. DOI:10.1109/JMEMS.2008.2008559
- Bae J-H, Chang S-H. PVDF-based ferroelectric polymers and dielectric elastomers for sensor and actuator applications: a review. Funct Compos Struct. 2019;1(1):012003. DOI:10.1088/2631-6331/ab0f48
- Ribeiro C, Correia DM, Ribeiro S, et al. Piezoelectric poly(vinylidene fluoride) microstructure and poling state in active tissue engineering. Eng Life Sci. 2015;15(4):351–356. DOI:10.1002/elsc.201400144
- Tschoeke B, Flanagan TC, Cornelissen A, et al. Development of a composite degradable/nondegradable tissue-engineered vascular graft. Artif Organs. 2008;32(10):800–809. DOI:10.1111/j.1525-1594.2008.00601.x
- Fernandez-Yague MA, Vallejo-Giraldo C, Aceret GO, et al. Biological activity on piezoelectric PVDF. In: Tofail SAM, Bauer J, editors. Electrically active materials for medical devices. London,UK: Imperial College Press; 2016. p. 167–176.
- Inderherbergh J. Polyvinylidene fluoride (PVDF) appearance, general properties and processing. Ferroelectrics. 1991;115(4):295–302. DOI:10.1080/00150193.1991.11876614
- Online R, Correia DM, Ribeiro C, et al. Strategies for the development of three dimensional scaffolds from piezoelectric poly(vinylidene fluoride). Mater Design. 2016;92:674–681. Available from: http://ro.uow.edu.au/eispapers/5124
- Chen L, Mccrate JM, Lee JC-M, et al. The role of surface charge on the uptake and biocompatibility of hydroxyapatite nanoparticles with osteoblast cells. Nanotechnology 2011;22:105708. DOI:10.1088/0957-4484/22/10/105708
- Itoh S, Nakamura S, Kobayashi T, et al. Effect of electrical polarization of hydroxyapatite ceramics on New bone formation. Calcif Tissue Int. 2006;78:133–142. DOI:10.1007/s00223-005-0213-6
- Ayatollahi M, Geramizadeh B, Zakerinia M, et al. Human bone marrow-derived mesenchymal stem cell: A source for cell-based therapy. Int JOrgan TransplantMed. 2012;3(1):32.
- Mizuno H, Tobita M, Cagri Uysal A. Concise review: adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012;30(5):804–810. DOI:10.1002/stem.1076
- Ribeiro C, et al. Fibronectin adsorption and cell response on electroactive poly(vinylidene fluoride) films. Biomed. Mater. 2012;7(3):035004. DOI:10.1088/1748-6041/7/3/035004.
- Ribeiro C, et al. “Enhanced proliferation of pre-osteoblastic cells by dynamic piezoelectric stimulation,” DOI:10.1039/c2ra21841k.
- Li M, Zhang P, Zhang D. PVDF piezoelectric neural conduit incorporated pre-differentiated adipose-derived stem cells may accelerate the repair of peripheral nerve injury. Med Hypotheses. 2018;114:55–57. DOI:10.1016/j.mehy.2018.02.027.
- Niu Y, Chen X, Yao D, et al. Enhancing neural differentiation of induced pluripotent stem cells by conductive graphene/silk fibroin films. J Biomed Mater Res Part A. 2018;106(11):2973–2983. DOI:10.1002/jbm.a.36486
- Guo W, Qiu J, Liu J, et al. Graphene microfiber as a scaffold for regulation of neural stem cells differentiation. Sci Rep. 2017;7(1):5678. DOI:10.1038/s41598-017-06051-z
- Zhu R, Sun Z, Li C, et al. Electrical stimulation affects neural stem cell fate and function in vitro. Exp Neurol. 2019;319:112963. DOI:10.1016/J.EXPNEUROL.2019.112963
- Heo DN, Acquah N, Kim J, et al. Directly induced neural differentiation of human adipose-derived stem cells using three-dimensional culture system of conductive microwell with electrical stimulation. Tissue Eng Part A. 2018;24(7–8):537–545. DOI:10.1089/ten.tea.2017.0150
- Zack-Williams SDL, Griffin M, Butler P, et al. Electrical stimulation differentiates adipose derived stem cells (ADSC) to a neurological phenotype. Plast Reconstr Surg. 2015;136:77. DOI:10.1097/01.prs.0000472377.27490.6c
- Zhang J, Neoh KG, Kang E. Electrical stimulation of adipose-derived mesenchymal stem cells and endothelial cells co-cultured in a conductive scaffold for potential orthopaedic applications. J Tissue Eng Regen Med. 2018;12(4):878–889. DOI:10.1002/term.2441
- Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87(Pt 4):387.
- Ciofani G, et al. Investigation of interactions between poly-L-lysine-coated boron nitride nanotubes and C2C12 cells: up-take, cytocompatibility, and differentiation. Int J Nanomed. 2010;5(1):285–298. DOI:10.2147/IJN.S9879
- Ciofani G, et al. Pilot in vivo toxicological investigation of boron nitride nanotubes. Int J Nanomed. 2012;7:19. DOI:10.2147/IJN.S28144
- Salvetti A, Rossi L, Iacopetti P, et al. In vivo biocompatibility of boron nitride nanotubes: effects on stem cell biology and tissue regeneration in planarians. Nanomedicine. 2015;10(12):1911–1922. DOI:10.2217/nnm.15.46
- Ciofani G, et al. “Enhancement of Neurite Outgrowth in Neuronal-Like Cells following Boron Nitride Nanotube-Mediated Stimulation,” 2010, DOI:10.1021/nn101985a.
- Lavenius E, Gestblom C, Johansson I, et al. Transfection of TRK-A into human neuroblastoma cells restores their ability to differentiate in response to nerve growth factor. Cell Growth Differ. 1995;6(6):727–736.
- Eggert A, Ikegaki N, Liu X, et al. Molecular dissection of TrkA signal transduction pathways mediating differentiation in human neuroblastoma cells. Oncogene. 2000;19(16):2043–2051. DOI:10.1038/sj.onc.1203518
- Mirzaei H, Darroudi M. Zinc oxide nanoparticles: biological synthesis and biomedical applications. Ceram. Int. Jan. 2017;43(1):907–914. DOI:10.1016/J.CERAMINT.2016.10.051.
- Sirelkhatim A, Mahmud S, Seeni A, et al. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015;7(3):219–242. DOI:10.1007/s40820-015-0040-x
- Hameed ASH, Karthikeyan C, Ahamed AP, et al. In vitro antibacterial activity of ZnO and Nd doped ZnO nanoparticles against ESBL producing Escherichia coli and Klebsiella pneumoniae. Sci Rep. 2016;6(1):24312. DOI:10.1038/srep24312
- Rajendra R, Balakumar C, Ahammed H, et al. Use of zinc oxide nano particles for production of antimicrobial textiles. Int J Eng Sci Technol. 2010;2(1):202–208. DOI:10.4314/ijest.v2i1.59113
- Pasquet J, Chevalier Y, Pelletier J, et al. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surf A Physicochem Eng Asp. 2014;457:263–274. DOI:10.1016/j.colsurfa.2014.05.057
- He L, Liu Y, Mustapha A, et al. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol Res. 2011;166(3):207–215. DOI:10.1016/J.MICRES.2010.03.003
- Jamdagni P, Khatri P, Rana JS. Green synthesis of zinc oxide nanoparticles using flower extract of nyctanthes arbor-tristis and their antifungal activity. J King Saud Univ Sci. 2018;30(2):168–175. DOI:10.1016/J.JKSUS.2016.10.002
- Zhang Z-Y, Xiong H-M. Photoluminescent ZnO nanoparticles and their biological applications. Materials. 2015;8(6):3101–3127. DOI:10.3390/ma8063101
- Hassan HFH, Mansour AM, Abo-Youssef AMH, et al. Zinc oxide nanoparticles as a novel anticancer approach; in vitro and in vivo evidence. Clin Exp Pharmacol Physiol. 2017;44(2):235–243. DOI:10.1111/1440-1681.12681
- Rasmussen JW, Martinez E, Louka P, et al. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin Drug Deliv. 2010;7(9):1063–1077. DOI:10.1517/17425247.2010.502560
- Kou LZ, Guo WL, Li C. Piezoelectricity of ZNO and its nanostructures. 2008 Symposium on Piezoelectricity, Acoustic Waves, and Device Applications, SPAWDA 2008, p. 354–359, DOI:10.1109/SPAWDA.2008.4775808
- Kao Y-Y, Chen Y-C, Cheng T-J, et al. Zinc oxide nanoparticles interfere with zinc ion homeostasis to cause cytotoxicity. Toxicol Sci. 2012;125(2):462–472. DOI:10.1093/toxsci/kfr319
- Sharma V, Shukla RK, Saxena N, et al. DNA damaging potential of zinc oxide nanoparticles in human epidermal cells. Toxicol Lett. 2009;185(3):211–218. DOI:10.1016/J.TOXLET.2009.01.008
- Heng BC, Zhao X, Tan EC, et al. Evaluation of the cytotoxic and inflammatory potential of differentially shaped zinc oxide nanoparticles. Arch Toxicol. 2011;85(12):1517–1528. DOI:10.1007/s00204-011-0722-1
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–156. DOI:10.1038/nrn1326
- Silver J, Schwab ME, Popovich PG. Central nervous system regenerative failure: role of oligodendrocytes, astrocytes, and microglia. Cold Spring Harb Perspect Biol. 2014;7(3):a020602. DOI:10.1101/cshperspect.a020602
- Seog HJ, et al. Recent progress in potassium sodium niobate lead-free thin films. J Korean Phys Soc. 2018;72(12):1467–1483. DOI:10.3938/jkps.72.1467
- The European Parliament and the Council of the European Union. Directive 2002/95/EC of the European Parliament and of the Council of 27 January 2003 on the restriction of the use of certain hazardous substances in electrical and electronic equipment. [cited 2019 Sep 29]. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32002L0095&from=EN
- M. A. Schwartz, G. Both, and C. Lechene, Effect of cell spreading on cytoplasmic pH in normal and transformed fibroblasts. 1989. [cited 2019 Aug 28]. Available from: https://www.pnas.org/content/pnas/86/12/4525.full.pdf
- ISO Standard, . 2009. Biological evaluation of medical devices — Part 5: Tests for in vitro cytotoxicity. Geneva, Switzerland; ISO10993-5:2009 www.iso.org.
- Cong P, Ko WH, Young DJ. Wireless batteryless implantable blood pressure monitoring microsystem for small laboratory animals. IEEE Sens J. 2010;10(2):243–254. DOI:10.1109/JSEN.2009.2030982
- Yu L, Kim B, Meng E. Chronically implanted pressure sensors: challenges and state of the field. Sensors. 2014;14(11):20620–20644. DOI:10.3390/s141120620
- Cong P, Young DJ, Hoit B, et al. Novel long-term implantable blood pressure monitoring system with reduced baseline drift. Conf Proc IEEE Eng Med Biol Soc. 2006;1:1854–1857. DOI:10.1109/IEMBS.2006.260692
- Birnie D, Williams K, Guo A, et al. Reasons for escalating pacemaker implants. Am J Cardiol. 2006;98(1):93–97. DOI:10.1016/J.AMJCARD.2006.01.069
- Hill PE. Complications of permanent transvenous cardiac pacing: a 14-year review of all transvenous pacemakers inserted at one community hospital. Pacing Clin Electrophysiol. 1987;10(3):564–570. DOI:10.1111/j.1540-8159.1987.tb04521.x
- Mattson AR, Eggen MD, Iaizzo PA. The cardiac pacemaker: a crossroads of engineering and medicine. Eng Med. 2019:153–178. DOI:10.1016/B978-0-12-813068-1.00006-3
- Ezekowitz JA, Armstrong PW, McAlister FA. Implantable cardioverter defibrillators in primary and secondary prevention. Ann Intern Med. 2003;138(6):445. DOI:10.7326/0003-4819-138-6-200303180-00007
- Theuns DAMJ, Smith T, Hunink MGM, et al. Effectiveness of prophylactic implantation of cardioverter-defibrillators without cardiac resynchronization therapy in patients with ischaemic or non-ischaemic heart disease: a systematic review and meta-analysis. Europace. 2010;12(11):1564–1570. DOI:10.1093/europace/euq329
- Aziz S, Leon AR, El-Chami MF. The subcutaneous defibrillator. J Am Coll Cardiol. 2014;63(15):1473–1479. DOI:10.1016/j.jacc.2014.01.018
- Steffen MM, Osborn JS, Cutler MJ. Cardiac implantable electronic device therapy: permanent pacemakers, implantable cardioverter defibrillators, and cardiac resynchronization devices. Med Clin North Am 2019;103(5):931–943. DOI:10.1016/j.mcna.2019.04.005
- Mittal S, Rogers J, Sarkar S, et al. Real-world incidence of pacemaker and defibrillator implantation following diagnostic monitoring with an insertable cardiac monitor. Am J Cardiol. 2019;123(12):1967–1971. DOI:10.1016/J.AMJCARD.2019.03.014
- Elliott M, Momin S, Fiddes B, et al. Pacemaker and defibrillator implantation and programming in patients with deep brain stimulation. Arrhythmia Electrophysiol Rev. 2019;8(2):138–142. DOI:10.15420/aer.2018.63.2
- Shen HH. Core concept: can deep brain stimulation find success beyond Parkinson’s disease? Proc Natl Acad Sci USA. 2019;116(11):4764–4766. DOI:10.1073/pnas.1900442116
- The Vagus Nerve Stimulation Study Group. A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. Neurology. 1995;45(2):224–230. DOI:10.1212/wnl.45.2.224
- Ben-Menachem E, Revesz D, Simon BJ, et al. Surgically implanted and non-invasive vagus nerve stimulation: a review of efficacy, safety and tolerability. Eur J Neurol. 2015;22(9):1260–1268. DOI:10.1111/ene.12629
- Arndt S, Laszig R, Aschendorff A, et al. Cochlear implant treatment of patients with single-sided deafness or asymmetric hearing loss. HNO. 2017;65(S2):98–108. DOI:10.1007/s00106-016-0297-5
- Friedmann DR, Roland JT, Waltzman SB. Cochlear implants as treatment of single-sided deafness in children. In: Young NM, Kirk KL, editors. Pediatric cochlear implantation. New York (NY): Springer; 2016. p. 247–253.
- Aschendorff A, Arndt S, Laszig R, et al. Treatment and auditory rehabilitation of intralabyrinthine schwannoma by means of cochlear implants. HNO. 2017;65(S1):46–51. DOI:10.1007/s00106-016-0217-8
- Wilson BS, Dorman MF. A brief history of the cochlear implant and related treatments. Neuromodulation. 2018:1197–1207. DOI:10.1016/B978-0-12-805353-9.00099-1
- Alteheld N, Roessler G, Walter P. Towards the bionic eye — the retina implant: surgical, opthalmological and histopathological perspectives. Acta Neurochir Suppl. 2007;97(PART 2):487–493. DOI:10.1007/978-3-211-33081-4_56
- Bore M, Choudhari N, Chaurasia S. Management of complications of cosmetic iris implants in a phakic eye: a case report and literature review. Int Ophthalmol. 2019;39(5):1141–1146. DOI:10.1007/s10792-018-0893-3
- Koydemir HC, Ozcan A. Wearable and implantable sensors for biomedical applications. Annu Rev Anal Chem. 2018;11(1):127–146. DOI:10.1146/annurev-anchem-061417-125956
- Hwang GT, Byun M, Jeong CK, et al. Flexible piezoelectric thin-film energy harvesters and nanosensors for biomedical applications. Adv Healthc Mater. 2015;4(5):646–658. DOI:10.1002/adhm.201400642
- Bock DC, Marschilok AC, Takeuchi KJ, et al. Batteries used to power implantable biomedical devices. Electrochim Acta. 2012;84. DOI:10.1016/j.electacta.2012.03.057
- Mallela VS, Ilankumaran V, Rao NS. Trends in cardiac pacemaker batteries. Indian Pacing Electrophysiol J. 2004;4(4):201–212.
- Horlbeck FW, Mellert F, Kreuz J, et al. Real-world data on the lifespan of implantable cardioverter-defibrillators depending on manufacturers and the amount of ventricular pacing. J Cardiovasc Electrophysiol. 2012;23(12):1336–1342. DOI:10.1111/j.1540-8167.2012.02408.x
- Hodgins D, et al. Healthy aims: developing New medical implants and diagnostic equipment. IEEE Pervasive Comput. 2008;7(1):14–21. DOI:10.1109/MPRV.2008.8
- Chen B, Li H, Tian W, et al. PZT based piezoelectric sensor for structural monitoring. J Electron Mater. 2019;48(5):2916–2923. DOI:10.1007/s11664-019-07034-8
- Lu B, Chen Y, Ou D, et al. Ultra-flexible piezoelectric devices integrated with heart to harvest the biomechanical energy. Sci Rep. 2015;5(1):16065. DOI:10.1038/srep16065
- Hammoud AN, Baumann ED, Overton E, et al., High temperature dielectric properties of apical, kapton, peek, teflon af, and upilex polymers. AAnnual Report: Conference on Electrical Insulation and Dielectric Phenomena, CEIDP; 1992, Vol. 1992, p. 549–554, DOI:10.1109/CEIDP.1992.283158
- Lindner E, Cosofret VV, Ufer S, et al. Flexible (Kapton-based) microsensor arrays of high stability for cardiovascular applications. J Chem Soc Faraday Trans. 1993;89(2):361. DOI:10.1039/ft9938900361
- Dupont. DuPont TM Kapton ® Summary of Properties. 2017 http://www.dupont.com/content/dam/dupont/products-and-services/membranes-and-films/polyimde-films/documents/DEC-Kapton-summary-of-properties.pdf
- Southcott M, MacVittie K, Halámek J, et al. A pacemaker powered by an implantable biofuel cell operating under conditions mimicking the human blood circulatory system – battery not included. Phys Chem Chem Phys. 2013;15(17):6278. DOI:10.1039/c3cp50929j
- Park DY, Joe DJ, Kim DH, et al. Self-powered real-time arterial pulse monitoring using ultrathin epidermal piezoelectric sensors. Adv Mater. 2017;29(37):1702308. DOI:10.1002/adma.201702308
- Lee HS, Chung J, Hwang G-T, et al. Flexible inorganic piezoelectric acoustic nanosensors for biomimetic artificial hair cells. Adv Funct Mater. 2014;24(44):6914–6921. DOI:10.1002/adfm.201402270
- Dagdeviren C, et al. Conformal piezoelectric systems for clinical and experimental characterization of soft tissue biomechanics. Nat Mater. 2015;14(7):728–736. DOI:10.1038/nmat4289.
- Han JH, Shi Y, Joe P, et al. Basilar membrane-inspired self-powered acoustic sensor enabled by highly sensitive multi tunable frequency band. Nano Energy. 2018;53:198–205. DOI:10.1016/J.NANOEN.2018.08.053
- Mielczarek M, Norena A, Schlee W, et al. Excitation of the auditory system as a result of non-invasive extra-cochlear stimulation in normal subjects and tinnitus patients. Front. Neurosci. 2018;12. DOI:10.3389/fnins.2018.00146
- Sonmezoglu S, Fineman JR, Maltepe E, et al. Monitoring deep-tissue oxygenation with a millimeter-scale ultrasonic implant. Nat. Biotechnol. 2021. DOI:10.1038/s41587-021-00866-y
- Hwang G-T, Park H, Lee JH, et al. Self-powered cardiac pacemaker enabled by flexible single crystalline PMN-PT piezoelectric energy harvester. Adv Mater. 2014;26(28):4880–4887. DOI:10.1002/adma.201400562
- Southcott M, MacVittie K, Halámek J. et al. A pacemaker powered by an implantable biofuel cell operating under conditions mimicking the human blood circulatory system–battery not included. [cited 2019 Oct 11]. Available from: https://pubs.rsc.org/en/content/articlehtml/2013/cp/c3cp50929j
- Hill WE, Murray A, Bourke JP, et al. Minimum energy for cardiac pacing. Clin Phys Physiol Meas. 1988;9(1):41–46. DOI:10.1088/0143-0815/9/1/003
- Kim DH, Shin HJ, Lee H, et al. In vivo self-powered wireless transmission using biocompatible flexible energy harvesters. Adv Funct Mater. 2017;27(25):1700341. DOI:10.1002/adfm.201700341
- Douglas WR. Of pigs and men and research. Space Life Sci. 1972;3(3):226–234. DOI:10.1007/BF00928167
- Crick SJ, Sheppard MN, Ho SY, et al. Anatomy of the pig heart: comparisons with normal human cardiac structure. J Anat. 1998;193(1):105–119. DOI:10.1046/J.1469-7580.1998.19310105.X
- Ye ZG, editor. Handbook of advanced dielectric, piezoelectric and ferroelectric materials: synthesis, properties and applications, 1st ed. Sawston, Cambridge: Woodhead Publishing, 2008.
- Zhang S, Luo J, Hackenberger W, et al. Electromechanical characterization of Pb(In0.5Nb0.5)O3–Pb(Mg1/3Nb2/3)O3–PbTiO3 crystals as a function of crystallographic orientation and temperature. J Appl Phys. May 2009;105(10):104506. DOI:10.1063/1.3131622.
- Hwang G-T, Kim Y, Lee J-H, et al. Self-powered deep brain stimulation via a flexible PIMNT energy harvester. Energy Environ Sci. 2015;8(9):2677–2684. DOI:10.1039/C5EE01593F
- Tennant KA, Adkins DL, Donlan NA, et al. The organization of the forelimb representation of the C57BL/6 mouse motor cortex as defined by intracortical microstimulation and cytoarchitecture. Cereb Cortex. 2011;21(4):865–876. DOI:10.1093/cercor/bhq159
- Cornejo-Garrido H, Kibanova D, Nieto-Camacho A, et al. Oxidative stress, cytoxicity, and cell mortality induced by nano-sized lead in aqueous suspensions. Chemosphere. 2011;84(10):1329–1335. DOI:10.1016/j.chemosphere.2011.05.018
- Silbergeld EK. Facilitative mechanisms of lead as a carcinogen. Mutat Res. 2003;533(1–2):121–133. DOI:10.1016/j.mrfmmm.2003.07.010
- Ahamed M, Siddiqui MKJ. Low level lead exposure and oxidative stress: current opinions. Clin Chim Acta. 2007;383(1–2):57–64. DOI:10.1016/j.cca.2007.04.024
- Saito Y, Takao H, Tani T, et al. Lead-free piezoceramics. Nature. 2004;432(7013):84–87. DOI:10.1038/nature03028
- Zhang Y, Kim H, Wang Q, et al. Progress in lead-free piezoelectric nanofiller materials and related composite nanogenerator devices. Nanoscale Adv. 2020;2(8):3131–3149. DOI:10.1039/c9na00809h
- Wu J. Advances in lead-free piezoelectric materials. Singapore: Springer; 2018.
- Li Z, Yang R, Yu M, et al. Cellular level biocompatibility and biosafety of ZnO nanowires. J Phys Chem C. 2008;112(51):20114–20117. DOI:10.1021/jp808878p
- Jiang J, Pi J, Cai J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg Chem Appl. 2018;2018:1–18, DOI:10.1155/2018/1062562
- Espitia PJP, Otoni CG, Soares NFF. Zinc oxide nanoparticles for Food packaging applications. Antimicrob Food Packag. 2016: 425–431. DOI:10.1016/B978-0-12-800723-5.00034-6
- Xia T, Kovochich M, Liong M, et al. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano. 2008;2(10):2121–2134. DOI:10.1021/nn800511k
- Ghosh M, Rao MG. Growth mechanism of ZnO nanostructures for ultra-high piezoelectric d33 coefficient. Mater Express. 2013;3(4):319–327. DOI:10.1166/mex.2013.1134
- Fortunato M, Chandraiahgari CR, Bellis GD, et al. Piezoelectric thin films of ZnO-nanorods/ nanowalls grown by chemical bath deposition article. IEEE Trans Nanotechnol. 2018;17(2):311–319. DOI:10.1109/TNANO.2018.2800406
- Zhou B, Wang J, Pan Y, et al. Fabrication and physical properties of high-quality zinc oxide thin films; 2008, 69840S. DOI:10.1117/12.792356
- Bukowski TJ, McCarthy K, McCarthy F, et al. Piezoelectric properties of sol-gel derived ZnO thin films. Integr Ferroelectr. 1997;17(1–4):339–347. DOI:10.1080/10584589708013008
- Mai W, Liang Z, Zhang L, et al. Strain sensing mechanism of the fabricated ZnO nanowire-polymer composite strain sensors. Chem Phys Lett. 2012;538:99–101. DOI:10.1016/J.CPLETT.2012.04.041
- Zhang W, Zhu R, Nguyen V, et al. Highly sensitive and flexible strain sensors based on vertical zinc oxide nanowire arrays. Sensors Actuators A Phys. 2014;205:164–169. DOI:10.1016/J.SNA.2013.11.004
- Gullapalli H, Vemuru VSM, Kumar A, et al. Flexible piezoelectric ZnO-paper nanocomposite strain sensor. Small. 2010;6(15):1641–1646. DOI:10.1002/smll.201000254
- Zhu R, Yang R. Ultra-Sensitive strain/stress sensing. Cham: Springer; 2018.
- Li Z, Zhu G, Yang R, et al. Muscle-driven in vivo nanogenerator. Adv Mater. 2010;22(23):2534–2537. DOI:10.1002/adma.200904355
- Yang R, Qin Y, Dai L, et al. Power generation with laterally packaged piezoelectric fine wires. Nat Nanotechnol. 2009;4(1):34–39. DOI:10.1038/nnano.2008.314
- Cheng X, Xue X, Ma Y, et al. Implantable and self-powered blood pressure monitoring based on a piezoelectric thinfilm: simulated, in vitro and in vivo studies. Nano Energy. 2016;22:453–460. DOI:10.1016/J.NANOEN.2016.02.037
- Zhang H, Zhang X-S, Cheng X, et al. A flexible and implantable piezoelectric generator harvesting energy from the pulsation of ascending aorta: in vitro and in vivo studies. Nano Energy. 2015;12:296–304. DOI:10.1016/J.NANOEN.2014.12.038
- Joffres M. Distribution of blood pressure and hypertension in Canada and the United States. Am J Hypertens. 2001;14(11):1099–1105. DOI:10.1016/S0895-7061(01)02211-7
- Sharma T, Je S-S, Gill B, et al. Patterning piezoelectric thin film PVDF–TrFE based pressure sensor for catheter application. Sensors Actuators A Phys. Apr. 2012;177:87–92. DOI:10.1016/J.SNA.2011.08.019
- Sharma T, Aroom K, Naik S, et al. Flexible thin-film PVDF-TrFE based pressure sensor for smart catheter applications. Ann Biomed Eng. 2013;41(4):744–751. DOI:10.1007/s10439-012-0708-z
- Inaoka T, Shintaku H, Nakagawa T, et al. Piezoelectric materials mimic the function of the cochlear sensory epithelium. Proc Natl Acad Sci USA. 2011;108(45):18390–18395. DOI:10.1073/pnas.1110036108
- Sarpeshkar R, Lyon RF, Mead C. A low-power wide-dynamic-range analog VLSI cochlea. Analog Integr. Circuits Signal Process. 1998;16(3):245–274. DOI:10.1023/A:1008218308069
- Graz I, Krause M, Bauer-Gogonea S, et al. Flexible active-matrix cells with selectively poled bifunctional polymer-ceramic nanocomposite for pressure and temperature sensing skin. J Appl Phys. 2009;106(3):034503. DOI:10.1063/1.3191677.
- Kim D-I, Trung TQ, Hwang B-U, et al. A sensor array using multi-functional field-effect transistors with ultrahigh sensitivity and precision for bio-monitoring. Sci Rep. 2015;5(1):12705. DOI:10.1038/srep12705
- Kim B-Y, Lee W-H, Hwang H-G, et al. Resistive switching memory integrated with nanogenerator for self-powered bioimplantable devices. Adv Funct Mater. 2016;26(29):5211–5221. DOI:10.1002/adfm.201505569
- Jeong CK, Han JH, Palneedi H, et al. Comprehensive biocompatibility of nontoxic and high-output flexible energy harvester using lead-free piezoceramic thin film. APL Mater. 2017;5(7):074102. DOI:10.1063/1.4976803
- Dsouki NA, Pereira de Lima M, Corazzini R, et al. Cytotoxic, hematologic and histologic effects of niobium pentoxide in Swiss mice. J Mater Sci Mater Med. 2014;25(5):1301–1305. DOI:10.1007/s10856-014-5153-0
- De Sairre MI, Bronze-Uhle ÉS, Donate PM. Niobium(V) oxide: a new and efficient catalyst for the transesterification of β-keto esters. Tetrahedron Lett. 2005;46(15):2705–2708. DOI:10.1016/j.tetlet.2005.01.158
- Fairhall LT. Lead studies. J Biol Chem. 1924;60(3):481–484. DOI:10.1016/s0021-9258(18)85180-8
- Yuan M, Cheng Li, Xu Q, et al. Biocompatible nanogenerators through high piezoelectric coefficient 0.5Ba(Zr0.2 Ti0.8)O3 -0.5(Ba0.7 Ca0.3)TiO3 nanowires for in-vivo applications. Adv Mater. 2014;26(44):7432–7437. DOI:10.1002/adma.201402868
- Liu W, Ren X. Large piezoelectric effect in Pb-free ceramics. Phys Rev Lett. 2009;103(25). DOI:10.1103/PhysRevLett.103.257602
- Won SS, et al. Lead-free Bi0.5 (Na0.78 K0.22)TiO3 nanoparticle filler–elastomeric composite films for paper-based flexible power generators. Adv Electron Mater. 2020;6(2):1900950. DOI:10.1002/aelm.201900950
- Park K, et al. Piezoelectric BaTiO3 thin film nanogenerator on plastic substrates. Nano Lett. 2010;10(12):4939–4943. DOI:10.1021/nl102959k
- Kim D, Han SA, Kim JH, et al. Biomolecular piezoelectric materials: from amino acids to living tissues. Adv Mater. 2020;32(14):1906989. DOI:10.1002/ADMA.201906989
- Kim K, Ha M, Choi B, et al. Biodegradable, electro-active chitin nanofiber films for flexible piezoelectric transducers. Nano Energy. 2018;48:275–283. DOI:10.1016/J.NANOEN.2018.03.056
- Lee BY, Zhang J, Zueger C, et al. Virus-based piezoelectric energy generation. Nat Nanotechnol. 2012;7(6):351–356. DOI:10.1038/nnano.2012.69
- Nenghui Z, Jinying S, Jingjing X. Piezoelectric properties of single-strand DNA molecular brush biolayers. Acta Mech Solida Sin. 2007;20(3):206–210. DOI:10.1007/S10338-007-0724-Y
- Li J, Long Y, Yang F, et al. Degradable piezoelectric biomaterials for wearable and implantable bioelectronics. Curr Opin Solid State Mater Sci. 2020;24(1). DOI:10.1016/J.COSSMS.2020.100806
- Iitaka Y. The crystal structure of γ-glycine. Acta Crystallogr. 1961;14(1):1–10. DOI:10.1107/S0365110X61000012
- Yang F, Long Y, Zhang Z, et al. Wafer-scale heterostructured piezoelectric bio-organic thin films. Science. 2021;373(6552):337–342. DOI:10.1126/SCIENCE.ABF2155
- Guerin S, et al. Racemic amino acid piezoelectric transducer. Phys. Rev. Lett. Jan. 2019;122(4):047701), DOI:10.1103/PhysRevLett.122.047701.
- Karan SK, et al. Nature driven spider silk as high energy conversion efficient bio-piezoelectric nanogenerator. Nano Energy. 2018;49:655–666. DOI:10.1016/J.NANOEN.2018.05.014
- Lee J-H, Lee JH, Xiao J, et al. Vertical self-assembly of polarized phage nanostructure for energy harvesting. Nano Lett. 2019;19(4):2661–2667. DOI:10.1021/ACS.NANOLETT.9B00569
- Jayasuriya AC, Ghosh S, Scheinbeim JI, et al. A study of piezoelectric and mechanical anisotropies of the human cornea. Biosens Bioelectron. 2003;18(4):381–387. DOI:10.1016/S0956-5663(02)00144-6
- Dong X-x, Ospeck M, Iwasa KH. Piezoelectric reciprocal relationship of the membrane motor in the cochlear outer hair cell. Biophys J. 2002;82(3):1254–1259. DOI:10.1016/S0006-3495(02)75481-7
- Guerin S, Stapleton A, Chovan D, et al. Control of piezoelectricity in amino acids by supramolecular packing. Nat Mater. 2017;17(2):180–186. DOI:10.1038/nmat5045
- Guerin S, Syed TAM, Thompson D. Deconstructing collagen piezoelectricity using alanine-hydroxyproline-glycine building blocks. Nanoscale. 2018;10(20):9653–9663. DOI:10.1039/C8NR01634H
- Xu Q, Gao x, Zhao S, et al. Construction of bio-piezoelectric platforms: from structures and synthesis to applications. Adv Mater. 2021;33(27):2008452. DOI:10.1002/ADMA.202008452
- Guerin S, Tofail SAM, Thompson D. Organic piezoelectric materials: milestones and potential. NPG Asia Mater. 2019;11(1):1–5. DOI:10.1038/s41427-019-0110-5
- Tofail S. editor, Biological Interactions with surface charge in biomaterials. Cambridge: Royal Society of Chemistry, 2011.
- Tofail SAM, Bauer J, Tofail SAM, et al. Electrically Polarized Biomaterials, 2016. DOI:10.1002/adma.201505403
- Valentini RF, Vargo TG, Gardella JA, et al. Electrically charged polymeric substrates enhance nerve fibre outgrowth in vitro. Biomaterials. 1992;13(3):183–190. DOI:10.1016/0142-9612(92)90069-Z
- Chernozem RV, Guselnikova O, Surmeneva MA, et al. Diazonium chemistry surface treatment of piezoelectric polyhydroxybutyrate scaffolds for enhanced osteoblastic cell growth. Appl Mater Today. 2020;20:100758. DOI:10.1016/j.apmt.2020.100758
- Young TH, Chang HH, Lin DJ, et al. Surface modification of microporous PVDF membranes for neuron culture. J Memb Sci. 2010;350(1–2):32–41. DOI:10.1016/j.memsci.2009.12.009
- Kang G, Cao Y. Application and modification of poly(vinylidene fluoride) (PVDF) membranes – a review. J Memb Sci. 2014;463:145–165. DOI:10.1016/J.MEMSCI.2014.03.055
- Liu F, Hashim NA, Liu Y, et al. Progress in the production and modification of PVDF membranes. J Memb Sci. 2011;375(1–2):1–27. DOI:10.1016/J.MEMSCI.2011.03.014
- Sheikh Z, Abdallah MN, Hanafi AA, et al. Mechanisms of in vivo degradation and resorption of calcium phosphate based biomaterials. Materials. 2015;8(11):7913–7925. DOI:10.3390/ma8115430
- Konan S, Haddad FS. A clinical review of bioabsorbable interference screws and their adverse effects in anterior cruciate ligament reconstruction surgery. Knee. 2009;16(1):6–13. DOI:10.1016/j.knee.2008.06.001
- Haffiz M, Radzi M, Leong KS. Investigation of the piezoelectric charge coefficient d 33 of thick-film piezoelectric ceramics by varying poling and repoling conditions. AIP Conf Proc. 2015;1660:70083. DOI:10.1063/1.4915801
- Bijalwan V, Sokolov I, Tofel P. Poling procedures and piezoelectric response of (Ba 0.85 Ca 0.15 Zr 0.1T 0.9)O 3 ceramics. J. Asian Ceram. Soc. 2021;9(1):229–236. DOI:10.1080/21870764.2020.1860438
- Du HL, Tang FS, Li ZM, et al. Effect of poling condition on piezoelectric properties of K0.5Na0.5)NbO3 ceramics. Trans Nonferrous Met Soc China. 2006;16(suppl 1):462–465. DOI:10.1016/s1003-6326(06)60234-3
- Fukada E, Yasuda I. On the piezoelectric effect of bone. J Phys Soc Jpn. 1957;12(10):1158–1162. DOI:10.1143/JPSJ.12.1158
- Kao FC, Chiu PY, Tsai TT, et al. The application of nanogenerators and piezoelectricity in osteogenesis. Sci Technol Adv Mater. 2019;20(1):1103–1117. DOI:10.1080/14686996.2019.1693880
- Berlincourt D. Variation of electroelastic constants of polycrystalline lead titanate zirconate with thoroughness of poling. J Acoust Soc Am. 1964;36(3):515–520. DOI:10.1121/1.1918990
- Wani AL, Ara A, Usmani JA. Lead toxicity: a review. Interdiscip Toxicol. 2015;8(2):55–64. DOI:10.1515/intox-2015-0009
- Rathod VT. A review of electric impedance matching techniques for piezoelectric sensors, actuators and transducers. Electronics. 2019;8(2):169. DOI:10.3390/electronics8020169
- Uchino K. Piezoelectric energy harvesting systems—essentials to successful developments. Energy Technol. 2018;6(5):829–848. DOI:10.1002/ente.201700785
- Zhang M, Zuo X, Yang TQ, et al. Research progress of piezoelectrets based micro-energy harvesting. Wuli Xuebao/Acta Phys Sin. 2020;69(24):247701 DOI:10.7498/aps.69.20200815
- Zhang Y, Bowen CR, Deville S. Ice-templated poly(vinylidene fluoride) ferroelectrets. Soft Matter. 2019;15(5):825–832. DOI:10.1039/C8SM02160K
- Shi J, Luo Z, Dibin Z, et al. Optimization a structure of MEMS based PDMS ferroelectret for human body energy harvesting and sensing. Smart Mater Struct. 2019;28(7):075010. DOI:10.1088/1361-665X/ab1ce2
- Zhang X, Wu L, Sessler GM. Energy harvesting from vibration with cross-linked polypropylene piezoelectrets. AIP Adv. 2015;5(7):077185. DOI:10.1063/1.4928039
- Dsouza H, Van Schyndel A, Pastrana J, et al. Ferroelectret nanogenerators for loudspeaker applications: a comprehensive study. J Sound Vib. 2020;468:115091. DOI:10.1016/j.jsv.2019.115091
- Cao Y, Li W, Figueroa J, et al. Impact-activated programming of electro-mechanical resonators through ferroelectret nanogenerator (FENG) and vanadium dioxide. Nano Energy. 2018;43:278–284. DOI:10.1016/j.nanoen.2017.10.066
- Li W, et al. In situ click chemistry generation of cyclooxygenase-2 inhibitors. Nat Commun. 2017;8(1):1–9. DOI:10.1038/ncomms15310
- Li W, Torres D, Wang T, et al. Flexible and biocompatible polypropylene ferroelectret nanogenerator (FENG): On the path toward wearable devices powered by human motion. Nano Energy. 2016;30:649–657. DOI:10.1016/J.NANOEN.2016.10.007
- Fan FR, Tian ZQ, Lin Wang Z. Flexible triboelectric generator. Nano Energy. 2012;1(2):328–334. DOI:10.1016/J.NANOEN.2012.01.004
- Fan F-R, Tian Z-Q, Lin Wang Z. Flexible triboelectric generator!, 2012. DOI:10.1016/j.nanoen.2012.01.004
- Yan S, Zhang Z, Xu S, et al. Eggshell membrane and expanded polytetrafluoroethylene piezoelectric-enhanced triboelectric bio-nanogenerators for energy harvesting. Int J Energy Res. 2021. DOI:10.1002/er.6589
- Han J, Xu N, Liang Y, et al. Paper-based triboelectric nanogenerators and their applications: a review. Beilstein J Nanotechnol. 2021;12:151–171. DOI:10.3762/BJNANO.12.12
- Tang Y, Zheng Q, Chen B, et al. A new class of flexible nanogenerators consisting of porous aerogel films driven by mechanoradicals. Nano Energy. 2017;38:401–411. DOI:10.1016/j.nanoen.2017.06.022
- Dagdeviren C, Li Z, Wang ZL. Energy harvesting from the animal-human body for self-powered electronics. Annu Rev Biomed Eng. 2017;19:85–108. DOI:10.1146/annurev-bioeng-071516-044517
- Shi B, Liu Z, Zheng Q, et al. Body-Integrated self-powered system for wearable and implantable applications. ACS Nano. 2019;13(5):6017–6024. DOI:10.1021/acsnano.9b02233
- Jiang D, Shi B, Ouyang H, et al. Emerging implantable energy harvesters and self-powered implantable medical electronics. ACS Nano. 2020;14(6):6436–6448. DOI:10.1021/acsnano.9b08268
- Tofail SAM, Bauer J. Electrically active materials for medical devices. London, UK: Imperial College Press; 2016.
- Hansen BJ, Liu Y, Yang R, et al. Hybrid Nanogenerator for Concurrently Harvesting Biomechanical and Biochemical Energy; 2010. DOI:10.1021/nn100845b
- Li H, Zhang X, Zhao L, et al. A hybrid biofuel and triboelectric nanogenerator for bioenergy harvesting. Nano-Micro Lett. 2020;12:50. DOI:10.1007/s40820-020-0376-8
- MEDICAL DEVICE REGULATIONS Global overview and guiding principles. 2003. [cited 2021 Apr 27]. Available from: www.who.int/bct
- Serrano MC, Pagani R, Vallet-Regí M, et al. In vitro biocompatibility assessment of poly(ϵ-caprolactone) films using L929 mouse fibroblasts. Biomaterials. 2004;25(25):5603–5611. DOI:10.1016/J.BIOMATERIALS.2004.01.037
- Wang L, Abedalwafa M, Wang F, et al. 23 Biodegradable poly-epsilon-caprolactone (PCL) for tissue engineering applications: a review; 2013. [cited 2019 Jun 28]. Available from: https://pdfs.semanticscholar.org/f393/80a9d2ecac8005a7ab2aee0f3eb1fc6cbe01.pdf
- Kattimani VS, Kondaka S, Lingamaneni KP. Hydroxyapatite–-past, present, and future in bone regeneration. Bone Tissue Regen Insights. 2016;7:BTRI.S36138. DOI:10.4137/BTRI.S36138
- Hench LL, Thompson I. Twenty-first century challenges for biomaterials. J R Soc Interface. 2010;7(suppl_4):S379–S391. DOI:10.1098/rsif.2010.0151.focus
- Dubey AK, Basu B, Balani K, et al. Dielectric and pyroelectric properties of HAp-BaTiO3 composites. Ferroelectrics. 2011;423(1):63–76. DOI:10.1080/00150193.2011.618382
- Inthong S, Tunkasiri T, Rujijanagul G, et al. Dielectric, mechanical, and microstructural characterization of HA–BST composites. Ceram Int. 2015;41:S481–S486. DOI:10.1016/J.CERAMINT.2015.03.205