Abstracts
Background: Implantation failure of in vitro fertilization (IVF) cycles is recognized as one of key problems in contemporary reproductive medicine. Implantation itself is a multifactorial process and one can hardly expect to find a single criterion for the endometrium receptivity. Endometrium biopsy still remains the most applicable technique to diagnose abnormalities causing decrease or complete loss of endometrial receptivity.
Materials and methods: We have studied 95 endometrial biopsy samples from 45 patient with I/II stage endometriosis and 40 controls from October 2014 to December 2015. Immunohistochemical analysis of key biological molecules participating in implant window formation (LIF, ER, PR, integrin, TGF-β1 and VEGF) was done to assess their predicting value for endometrial receptivity troubles.
Results: The discriminant analysis demonstrated that highest information capacity was characteristic for LIF expression percent area, integrin αVβ3 both percent area and optic density in endometrial stroma and glands and finally TGFβ1 and VEGF-А percent area expression in endometrial stroma. The model test done on a checking group showed 89.1% correct discrimination. Cross-checking in a teaching group showed a bit lower but still high correct answer percentage (88.8%). A decision-making classification tree was worked out.
Conclusion: The produced model is sufficient for predicting IVF treatment failure and allows producing reasonable treatment tactics as well as encourages IVF treatment effectiveness improvement in patients with endometriosis.
Introduction
Infertile marriage is a serious medical, social and economic problem. According to WHO, it has a 10–15% frequency with no observed tendency decrease. The insufficiency of natural fertility recovering techniques stipulates development of new assisted reproductive technologies (ART), such as in vitro fertilization (IVF) with 28.5–32.5% effectiveness [Citation1]. IVF effectiveness optimization is straightforwardly dependent on solving the key reproductive medicine problem: endometrial receptivity period identification for determining endometrium functional ready state for blastocyst implanting [Citation2]. The endometrial receptivity is a complex of structural and functional endometrium properties with distinct temporal constants, determining the endometrium capability of assuring embryo implantation. Endometrium receptivity is expressed by biologically active molecules growing quantity, such as cytokines, growth factors, adhesion molecules, etc. Traditionally the endometrium receptivity is estimated by biopsy effective enough to assess the lutein phase sufficiency [Citation3,Citation4]. However, this technique does not permit getting full information on the endometrium receptivity. Researchers accept that histological visualization does not allow functional subthreshold damages of endometrium receptivity.
The immunohistochemical (IHC) technique aimed at detecting endometrial receptivity assuring signaling molecules is effective to estimate the endometrium functional ready state for implantation.
Endometrium activity functional disturbance is also observed in morphologically normal state and is seen in balance changes of regulatory molecules responsible for endometrial receptivity. Receptivity disturbance detection allows assigning complex therapy for women with unharmed ovulatory cycle, effective to encourage trophic and metabolic processes in endometrium.
Objectives
Verification of the most specific and significant IHC endometrial receptivity markers for working out an IVF outcome predicting algorithm in case of external genital I–II stage endometriosis.
Material and techniques
The investigation was carried out at Russian Medical Academy Institute named after D.O. Ott and embraced 95 endometrium biopsy cases of women who underwent IVF treatment with further embryo transfer into uterine cavity. The cases were divided into three groups where the first one included 25 women with I–II stage endometriosis (according to ASRM classification) with positive result treatment (sonography confirmed pregnancy); the second group consisted of 30 women with I–II degree EGE without pregnancy after IVF. The third, which was the control group, embraced 40 women who underwent men infertility caused treatment with positive program outcome. The investigation included 24–35 years aged women with infertility factor and with follicle-stimulating hormone level on third-to-fifth day of menstrual cycle (d.m.c.) under 11ME/l. The ultrasound examination on third-to-fifth day m.c.d. showed 7–12 antral follicles in the maximal ovarian section. All women underwent either standard IVF or IVF/ICSI protocol accompanied by gonadotrophin-releasing hormone antagonists. The cycle lutein phase was supported by micronized progesterone. Only high-quality embryos assessed by morphological criteria were used for transfer on the fourth-to-fifth cultivation day. The average recombinant gonadotrophin dose, the amount of obtained oocytes and transferred embryos were the same for all clinical groups. Pregnancy was verified by detecting the chorionic gonadotropin hormone in blood on the 14th day after embryo transfer into uterine cavity together with ultrasonography on the 21th day. All the significant exponents were standardized on the stage of study groups formation.
Endometrium biopsy was made within the assumed implantation window on the seventh-to-ninth day after ovulation in the cycle preceding the IVF stage using suction curette Pipelle de Cornier (Jiangsu Suyun Medical Materials Co., Ltd., Jiangsu, China). The ovulation was confirmed through ultrasonography along with determining the luteinizing hormone peak in urine. The obtained endometrium samples were standardly treated to get paraffin blocks. Histology analysis was made in accordance with criteria suggested by Mazur and Kurman [Citation5]. Endometrium samples containing medium stage secretion were selected with no signs of inflammations or fibrosis. In dubious cases, van Gison additional staining technique was applied. To disclose chronic inflammations CD20, CD138 leucocyte subpopulations were detected by histochemical analysis.
IHC investigation was made on deparaffinized and dehydrated 4–6 mcm thick sections applying avidin–biotin immunoperoxidase technique. Following expression levels were measured: leukemia inhibiting factor (LIF)–anti-LIF, Abcam (ab135629), 1:100; vascular endothelial growth factor type A (VEGF-А)–anti-VЕGF-А, Abcam (ab28775), 1:50; transforming growth factor β-1 (TGF-β1)–anti-TGF-β1, Novocastra,1:40; alpha type estrogen receptor (ER)–anti-ER, DAKO (1D5), 1:35; A type progesterone receptor–anti-PR, DAKO (636), 1:35; alphaV beta3 integrin (integrin αVβ3)–anti-integrin αVβ3, Abcam (ab7166), 1:250.
IHC reaction quantitative estimation required making pictures by Nikon DXM 1200 camera along with “AST-1” (version 2.12) program. The image was fixed at 40× enlargement. Morphometric picture analysis was applied to assess IHC staining accompanied by computer microscope image analyzing system Morphology 5.2 (Videotest, St. Petersburg, Russia). In each case, the entire material was explored excluding fields of vision with defected staining or artifacts.
Nuclear-localized antigen-related reaction (ER, PR) was estimated by histochemical score (HS) with maximum of 300 points. The intensity of cytoplasm and cell membrane-localized antigen-related reaction (LIF, VEGF-A, integrin αVβ3, TGF-b1) was estimated by two options: relative expression square and optic density. The relative expression percent area was calculated as percentage ratio of immune positive cell square to the general cell area within the field of vision. The expression optic density was measured in conventional units. The IHC reaction estimation was made on stromal and glandular endometrium histologic structures.
The obtained data was statistically processed by Microsoft Excel standard set together with Statistica for Windows program packs version 6.0, StatSoft Inc., Tulsa, OK and SPSS-19, applying both parametric and non-parametric statistic methods.
The descriptive statistics included mean value (M) and standard error of mean determination. The intergroup sign value difference was estimated applying Student’s t-criterion along with Mann–Whitney range U-criterion. Only differences with р < 0.05 (95% significance level) and р < 0.01 (99% significance level) were considered statistically relevant. Discriminant analysis was performed to reveal the highest predictive capacity features. To work out an IVF result-predicting algorithm in endometriosis patients, the classification tree technique was used.
Results
The endometrial receptivity within ART cycles is achieved by estradiol and progesterone affect through interaction with corresponding steroid receptors. The results on ER and PR expression in the studied groups are shown in .
Table 1. Estrogen and progesterone expression endometrial stroma and glands in the studied groups.
The obtained ER expression data for stromal and glandular components show value growth in the group with negative result (р < 0.005); in the meantime, lower ER levels in the positive result group also differ from that of the control group.
PR expression was high both in the control and positive result group and remained within normal “implantation window”; while in the negative result group, PR level was considerably lower both in stromal and glandular components.
LIF expression could be seen mainly in glandular and luminal epithelium while stromal expression was detected in women with histologically and immunohistochemically confirmed chronic endometrium inflammation, these women thus were excluded from the study.
Within blastocyst apposition and adhesion phases, LIF is intermediary for maternal decidual leukocytes and the incorporated trophoblast interaction.
We have noticed maximal LIF expression values in the control group. In the group of women with endometriosis, the values were lower compared to the control group and stayed within 14–10% in the positive outcome group while in the negative outcome group we see a downfall both of the percent area (<10%) and the optic density [<0.200 c.u. (concentration units)].
Integrin αVβ3 expression in the group of women with endometriosis showed minimal values both of percent area expression and optic density with no detected difference in the control group (р > 0.05).
The obtained data show direct influence of integrin αVβ3 expression values on IVF positive result. In the group of women with verified pregnancy, the percent area values varied within 8–14%; while in that of negative outcome they were <6.8%. The optic density was also at the highest points in the control group and at the lowest in the endometriosis and negative result groups.
VEGF-А expression could be seen only in the endometrial stroma with no signs of it in the external and glandular epithelium. The results of VEGF-А expression in women’s endometrium are given in . VEGF-А expression in endometrial stroma in women with endometriosis did not differ much with the control group by percent area while the optic density in the positive outcome group was higher than that in the negative outcome group. VEGF-А expression percent area values in women with endometriosis were twice higher than that of control group (р < 0.05).
Table 2. The results of PAE and OD of LIF, αVβ3, VEGF-А and TGFβ1 in endometrial stroma (str) and glands (gl) in study groups.
TGFβ1 expression in the glandular component is higher than in stroma. TGFβ1 endometrium expression data is drawn in . The group comparison shows definitely higher percent area values in the positive outcome group, while higher optic density values in the negative outcome group. The latter had lower percent area values compared to the control group
Another study task was to estimate the information capacity of IHC markers for further IVF outcome prediction in the group of women with endometriosis. For this purpose, we applied the discriminant analysis which proved that the highest information capacity was characteristic for LIF expression percent area, integrin αVβ3 both percent area and optic density in endometrial stroma and glands and finally TGFβ1 and VEGF-А growth factors’ percent area expression in endometrial stroma.
Step-by-step variable inclusion procedure based on Wilks statistics allowed calculating discriminant functions:
P = 0.04 A + 126.0 B + 3.7 C − 23.5
O = 0.09 A + 83.9 B + 1.9 C – 13.4
where, P is IVF positive outcome discriminant function, O stands for IVF negative outcome discriminant function, A for PR expression in glandular endometrium component, B for LIF expression optic density in glandular endometrium component and C for TGFβ1 expression percent area in stromal endometrium component.
All the variables used in the discriminant functions definitely differ from zero. The model test done on a checking group showed 89.1% correct discrimination. Cross-checking in a teaching group showed a bit lower but still high correct answer percentage (88.8%).
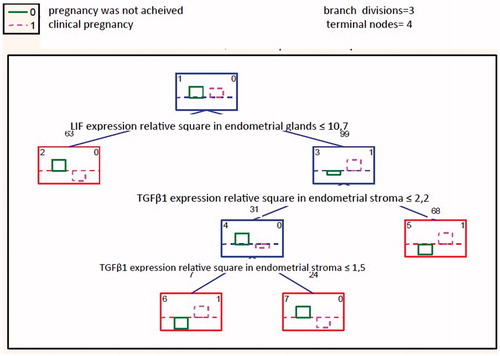
The IVF outcome predicting algorithm based on IHC markers expression was worked out using classification tree model—a special technique allowing object inclusion into a certain class depending on respective one or several variables. This model allows classifying objects by decisive rules. The best tree choice was made relying on the lowest rate of incorrectly classified observations. The chosen prognosis had the lowest incorrect classification percentage (). The classification tree analysis proved the TGFβ1 expression percent area in endometrial stroma (range 100), LIF expression percent area and LIF expression optic density in endometrial glands (range 71) to be informationally most relevant indexes.
Notes: the rectangles denote decision joints where the left numeric is the rectangle number and the right one denotes the class to which it is related. The characters above the rectangles mark the quantity of observations done for the joint, while the comments below denote the condition of branch division
The dividing condition was the following: when a variable value corresponds or is equal to the condition noted below the decision joint, the left branch is chosen, in the contrary situation—the right one. Thus we go along the branches till the final joint pointing out the expected IVF treatment outcome.
Discussion
Lack of implantation within IVF cycles appears to be one of the main contemporary reproduction problems. The implantation itself is a multifactorial process and one can hardly imagine just one criteria allowing endometrial receptivity proper determination. However, clinical significance of reliable endometrium receptivity diagnostics can be hardly overestimated. Endometrium biopsy still remains the most spread technique of diagnosing abnormalities leading to serious decrease or even complete loss of endometrial implant capability.
A reliable method for detecting endometrial receptivity may allow avoiding emotional stress, restraining from lutein phase and prospect less embryo transfers in order to preserve them for postponed transfers.
Cardinal regulators of the implantation process include growth factors, cytokines and their receptors [Citation6]. Integrin αVβ3 participates in the initial blastocyst attachment and also regulates intercellular interactions. Its expression has been observed both in stromal and glandular endometrium components. The VEGF is an important angiogenesis regulator, a mitogen for vessel endothelial cells. TGFβ1 is a growth factor and its expression is detected in all endometrium structures: stroma, glandular component and luminal epithelium. It was recently mentioned, that endometrial receptivity is almost on the same level from cycle to cycle. This mechanism is supported by constant genomic profile [Citation7]. Our previous study demonstrated that LIF and VEGF are of great importance for IVF treatment outcome prediction in general infertile population [Citation8]. Thus applying IHC test of endometrium obtained in “implantation window” period before the ART treatment looks enough substantiated.
The result of our investigation is the following: the most specific and significant endometrium receptivity markers for IVF treatment outcome prediction are TGFβ1 and LIF. This newly obtained data allowed working out an IVF treatment outcome prediction algorithm for women with I–II stage endometriosis.
The model built makes it possible to predict negative outcome group and allows taking scientifically substantiate decisions aimed at raising the taken treatment measures effectiveness.
Basing on the created model we have suggested the following negative outcome prediction algorithm in the endometriosis patients:
When planning, IVF treatment for endometriosis patients endometrium biopsy along with histology should be necessarily done.
In case of undetected circumstances evidently impeding endometrium receptivity (hyperplastic processes or inflammations), a complex endometrium functional status estimation applying IHC analysis should be done.
Conclusion
We become more and more definite about the fact that there is no ideal biomarker for endometrium receptivity assessment. In patients with I–II stage endometriosis the discriminant analysis demonstrated that the following markers had the highest information capacity: LIF expression percent area, integrin αVβ3 both percent area and optic density in endometrial stroma and glands and finally TGFβ1 and VEGF-А percent area expression in endometrial stroma. The produced IVF treatment outcome-predicting model is sufficient for determining treatment failure and allows producing reasonable treatment tactics as well as encourages improvement of IVF treatment effectiveness in patients with endometriosis.
It is necessary to carry out large clinical trials on big women populations both with normal and disturbed fertility helpful to determine the normal rage of different molecule expression, standardize techniques and allow their further application for predictive diagnostics and receptivity correction.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Kupka MS, Ferraretti AP, de Mouzon J, et al. Assisted reproductive technology in Europe, 2010: results generated from European registers by ESHRE. Hum Reprod 2014;29:2099–113
- Edwards RG. Human implantation: the last barrier in assisted reproduction technologies? Reprod Biomed Online 2006;13:887–904
- Devroey C, Bourgain NS, Macklon, et al. Reproductive biology and IVF: ovarian stimulation and endometrial receptivity. Trends Endocrinol Metab 2004;15:84–90
- Simon A, Laufer N. Repeated implantation failure: clinical approach. Fertil Steril 2012;97:1039–43
- Mazur M, Kurman RJ. Diagnosis of endometrial biopsies and curettings: a practical approach. New York: Springer; 2005
- Boomsma CM, Kavelaars A, Eijkemans MJ, et al. Endometrial secretion analysis identifies a cytokine profile predictive of pregnancy in IVF. Hum Reprod 2009;24:1427–35
- Ruiz-Alonso M, Blesa D, Díaz-Gimeno P, et al. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril 2013;100:818–24
- Sharfi Y, Dzhemlikhanova LK, Niauri DA, et al. Endometrial receptivity evaluation in IVF cycles. Gynecol Endocrinol 2015;31:74–8