Abstract
The purpose of this study was to evaluate the effect of two doses of D-chiro-inositol (DCI) in combination with Myo-inositol (MYO) in women with PCOS undergoing ICSI. This was a multicenter controlled, randomized, double-blind parallel group study with two MYO-DCI formulations for 12 weeks. The study group (SG) was administered 550 mg of MYO + 150 mg of DCI twice daily; the control group (CG) was administered 550 mg of MYO + 13.8 mg of DCI twice daily. The participants comprised 60 women with PCOS undergoing ICSI. At baseline, no differences were found between the two groups regarding age, BMI, HOMA-IR or testosterone levels. The pregnancy and live birth rates were significantly higher in the SG than in the CG (65.5 vs. 25.9 and 55.2 vs. 14.8, respectively) [risk ratio (RR) = 0.4; 95%CI (0.2, 0.79); p = .003 and RR = 0.27; 95%CI (0.10, 0.70); p = .002 respectively]. The risk of ovarian hyperstimulation syndrome (OHSS) was lower in the SG (3.44 vs. 18.5%, p = .07). The combination of MYO-DCI at high doses of DCI improves the pregnancy rates and reduces the risk of OHSS in women with PCOS undergoing ICSI.
摘要
本研究的目的是评价两种剂量的D-手性肌醇(DCI)联合肌醇(MYO)治疗多囊卵巢综合征ICSI的疗效。这是一项多中心对照、随机、双盲平行组研究, 使用两种不同剂量的MYO-DCI制剂, 为期12周。研究小组(SG)服用550毫克的MYO + 150毫克的DCI每天两次;对照组(CG)服用550毫克的MYO+每天13.8毫克的DCI两次。参与者包括60名接受ICSI的PCOS女性。两组在年龄、BMI、 HOMA-IR及睾酮方面的基线水平无差异, SG组的妊娠率和活产率明显高于CG组(分别为65.5 vs 25.9和55.2 vs 14.8)【风险比(RR)0.4;95%可信区间(0.2, 0.79);p= .003和RR 0.27;95%可信区间(0.10, 0.70);p= .002】。卵巢过度刺激综合征(OHSS)的风险在SG中较低(3.44 vs 18.5%, p= .07)。高剂量DCI联合使用MYO-DCI可提高妊娠率, 并降低合并多囊卵巢综合征(PCOS)的ICSI女性发生OHSS的风险。
The Chinese abstracts are translated by Prof. Dr. Xiangyan Ruan and her team: Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing 100026, China.
Introduction
Polycystic ovary syndrome (PCOS) is a complex disease that involves reproductive and metabolic problems [Citation1]. For women with PCOS undergoing fertility treatments, reproductive improvements have been reported using drugs such as metformin or inositol in different forms, combinations or doses [Citation2,Citation3].
Inositol has two major stereoisomers: myo-inositol (MYO) and D-chiro-inositol (DCI). MYO supplements have been observed to improve the metabolic profile and hyperandrogenism of women with PCOS [Citation4,Citation5], in addition to increasing the clinical pregnancy rate in infertile women undergoing ovulation induction for ICSI or IVF-ET, probably improving the quality of embryos and reducing the number of unsuitable oocytes [Citation6]. However, a recent systematic review showed that MYO supplementation is not sufficient to improve oocyte maturation, embryo quality or the pregnancy rate [Citation7].
Regarding DCI, the existence of tissue-specific ratios, namely, in the ovary, has prompted researchers to recently develop a treatment based on both molecules in the proportion of 40 (MI) to 1 (DCI) [Citation8]. This ratio has been effective in improving endocrine and metabolic parameters in obese PCOS women [Citation9,Citation10]. In a mouse model, a higher DCI dose was ineffective or even had a negative effect on clinical-pathological outcomes [Citation11]. However, based on available data, the specific MI:DCI ratio to be administered to PCOS patients could not be established [Citation12]. Therefore, the role of DCI supplementation also remains controversial or unknown, and future studies of the appropriate dose and duration are vital to clarify the role of DCI in the management of PCOS.
The aim of this study was to evaluate the effect of two DCI doses in combination with MYO in women with PCOS undergoing ICSI.
Materials and methods
Study design
This study was a double-blind, multicenter randomized clinical trial (RCT) with quadruple masking (Participant, Care Provider, Investigator and Outcomes Assessor). This study was conducted at five sites in Spain from February 2016 to April 2017 and was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines; it is registered at clinicaltrials.gov with the identifier NCT03201601.
For a superiority trial to test that the pregnancy rate is higher under experimental treatment, with double the pregnancy rate than under normal treatment, assuming a 30% pregnancy rate under control treatment with a statistical power of 80% and a significance level of 5%, the sample per group needed was 30 patients per group.
Randomization system
The volunteers were randomly assigned to one of two groups according to a randomization scheme generated by a computer program (SIGESMU®). To maintain blinding, the investigator received a treatment allocation number for each subject from the IRT system.
Subjects
Inclusion criteria: women aged between 18 and 40 years with PCOS according to the Rotterdam criteria [Citation13] with a body mass index (BMI) <30 who were undergoing ICSI and signed the informed consent document. All participants were required to have a normal uterine cavity. Before randomization, the patients were offered the possibility of doing inseminations or ICSI.
Exclusion criteria: Contraindications for ICSI, adrenal hyperplasia, hyperprolactinemia, thyroid disease, severe endometriosis, poor responder and severe male factor.
Methods
Sixty subjects were randomized to receive oral soft gelatin capsules of 550 mg of MYO + 150 mg of DCI twice daily (3.6: 1) [Study group (SG)] or 550 mg of MYO + 13.8 mg of DCI twice daily (40:1) [Control group (CG)] over 12 weeks until the day of ovarian puncture. Intake of other vitamins or antioxidants was not permitted during the study except for folic acid (400 mcg/day), which was provided to all the patients.
Initial ovulation stimulation was performed homogeneously in all participating centers using a GnRH antagonist cycle and an initial dose of 150 units of FSH for 5 days. After the initial stimulation, each patient was treated individually according to her response. Triggering was performed with 0.25 mg of hCG in all cases except when the risk of ovarian hyperstimulation syndrome (OHSS) was suspected. Follicular puncture was scheduled approximately 36–37 h after triggering. Embryo transfer (ET) was carried out individually according to the characteristics of each volunteer and her response, although in no case was more than three embryos transferred. In all ET cases, treatment with micronized natural progesterone for luteal phase support was prescribed.
Outcomes
The primary outcome was the pregnancy rate, and the secondary outcomes were oocyte maturation, embryo quality, testosterone levels and insulin sensitivity.
Pregnancy was defined as a positive test at 2 weeks from ET. Oocyte maturation was defined by the percentage of metaphase II (MII) oocytes. Embryos were assessed according to ESHRE criteria [Citation14]. Participants who conceived were followed at the clinical site for ultrasound evidence of a viable intrauterine pregnancy and were referred for obstetrical care.
Ethical approval
The study was approved at each clinical site. Patients signed an informed consent document informing them about the procedure and possible risks of the study. ICSI was performed in all cases; in the circumstances of normal sperm, the patients were informed of possible higher fetal anomalies in ICSI vs IVF.
Statistical methodology
Bivariate statistical tests for the personal variables were performed to determine the homogeneity of the women’s characteristics between the treatment groups. Frequencies, percentages and chi-square tests were performed on qualitative variables. For quantitative variables, the mean, standard deviation (SD) and 95% confidence intervals were obtained, and the asymptotic t-test or bootstrap technique for the t-test was performed to compare groups. Additionally, statistical multivariate modeling was applied to check differences between groups regarding the evolution of parameters, which used multivariate linear mixed regression models and intra-subject random effect and was fitted with the patients’ characteristics.
The main outcome success of pregnancy was also described with the odds of pregnancy per group and corresponding OR and confidence interval.
Intention to treat analysis is the comparison of the treatment groups including all patients as originally allocated after randomization. We mainly present the per-protocol analysis for primary and secondary outcomes. However, to compare results, avoid possible bias in the primary outcome and increase the credibility of the results, an intention to treat analysis was also presented using R project 3.3.
Results
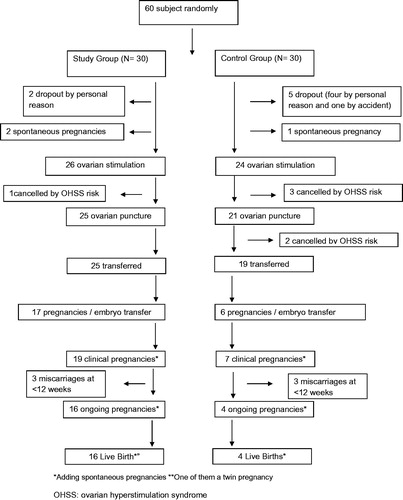
At baseline, no differences were found between the two groups (see ). Four patients were removed from the study due to the risk of OHSS (one in the SG and three in the CG). Another two patients in the CG also did not undergo ET due to the risk of OHSS. For these patients, triggering was performed with leuprolide 0.2 mg and all embryos were frozen. When ET was not achieved due to the OSSH risk, HOMA-IR and testosterone tests were performed at the time of ovarian puncture. Globally, the percentage of exclusions due to the risk of OSSH was lower in the SG (3.84% vs. 20.83%, p = .065) (see ).
Table 1. Patients’ characteristics at baseline.
Regarding compliance, all the patients returned the container to verify that they had complied with the intake of the medication. No participant showed side effects with any of the two combinations studied.
At the end of the ICSI cycle, the duration of ovarian stimulation (SG = 10.48 ± 1.39 vs. CG = 10.38 ± 0.86) and units of FSH used per cycle (SG = 1219.23 ± 102 vs. CG = 1137.5 ± 68) were similar in both groups. Likewise, total testosterone, glucose and insulin levels, HOMA-IR, number of MII oocytes and percentage of good-quality embryos were also similar in both groups ().
Table 2. Summary results for reproductive and analytic outcomes.
Forty-four patients underwent ET (25 in the SG and 19 in the CG). No differences in the average number of embryos transferred or percentage of women with more than one embryo transferred were found. However, the pregnancy rate was significantly higher in the SG than in the CG (65.5% vs. 25.9%, p = .003). Additionally, pregnancies after ET were higher in the SG (68% vs. 31.6%, p = .017). The global data of each pregnancy rate are shown in .
Table 3. Pregnancy outcomes.
Concerning the testosterone levels, a significant difference was found from the baseline to end of the study. However, the difference was similar in both the groups (Dif = −0.13; 95% (−0.17, −0.08); p values < .001). Similarly, a decrease from the baseline to the end of the study in HOMA-IR was observed in both the groups (Dif = −0.70; 95%CI (−1.23, −0.16); p = .011).
Discussion
The primary findings of this study show that high doses of DCI combined with MYO increase the percentage of pregnancy rates in women with PCOS undergoing ICSI. To our knowledge, this is the first RCT investigating the effect of different doses of DCI concerning reproductive outcomes.
Studies carried out over the 30-year history of ART coincide in highlighting the importance of the quality of embryos/oocytes as the main predictors of positive results, and it seems that both MYO and DCI can improve the outcomes by diverse mechanisms: improving insulin sensibility, increasing ovulation or reducing oxidative stress of follicular fluid [Citation10,Citation15–22]. However, data obtained from a meta-analysis indicate a lack of evidence to justify that MYO supplementation is enough to improve the oocyte or embryo quality and pregnancy rates [Citation7].
The main strength of this study is that the pregnancy rates are high with 150 mg of DCI twice daily and could involve DCI in early embryonic implantation and development. The main weakness of this study is the sample size, although the calculation of the sample size indicated a priori that 60 subjects were sufficient to draw conclusions.
There are precedents that indicate that inositol improves the metabolic parameters of women with PCOS [Citation23–26]. In our study, significant improvement was found from the baseline to the end of the study both in the testosterone level and HOMA-IR, although the difference was similar in both the groups.
Regarding the effect of DCI on oocyte and embryo quality, studies that have analyzed its use have reported contradictory results. A study comparing both inositol forms observed that MYO supplementation rather than DCI could improve oocyte and embryo quality [Citation21]. In other, an increased DCI dosage progressively worsened oocyte maturation [Citation27]. However, Piomboni et al. observed a higher number of MII oocytes with DCI [Citation16], and Colazingari et al. showed that only the MYO-DCI-treated group (physiological ratio: 1.1 g + 27.6 mg) increased the embryo quality [Citation28]. Interestingly, in the current study and in all the studies reviewed, no side effects were reported at any dose of MYO or DCI, resulting in a high degree of patient compliance.
Studies performed with the combination of MYO:DCI at a ratio 40:1 showed better results than the administration of DCI or MYO alone. The ratio 40:1 was based on the physiological blood ratio of the two molecules, although each tissue has its own ratio [Citation11]. Thus, some authors pointed out that the ratio was not as important as the absolute concentration of either MYO or DCI. These authors suggest that the amount of DCI in studies using the 40:1 ratio was very low (13.8–27.6 mg/day) and might have been insufficient to achieve adequate levels of DCI as predicted by the studies showing a beneficial effect [Citation12]. We agree with these authors that more studies should be performed to evaluate different ratios or dosages to determine the most efficient dose for each PCOS condition [Citation7]. Therefore, we performed a study comparing the 40:1 ratio with other proposed MYO:DCI combinations containing higher dose of DCI, more in line with the dose used in the studies showing beneficial effects in PCOS. To determine the dose, we considered the results published by Isabella and Raffone in 2012 showing that a high dose of DCI might affect oocyte quality [Citation27]. Although these results were contradictory to those posteriorly published by Piomboni et al. in 2014 [Citation16], we decided to use 300 mg, which was also in line with the estimated daily conversion of MYO to DCI in a healthy person.
In our study, the improvement in the pregnancy rates observed in the SG was not accompanied by improvements in intermediate parameters, such as oocyte or embryo quality. It is true that the percentage of pregnancies in the control group was too low, without the need for justification. Taken together, these findings suggest that DCI might affect other variables of oocyte or embryo quality that go unnoticed in the classifications that are made in a generalized manner. In this sense, it would be valuable to know how supplementation with MYO or DCI affects other markers of oocyte quality.
Another hypothesis that justifies the percentage of pregnancies is higher with higher doses of DCI suggests that DCI could influence embryo implantation. There are no clinical studies that indicate whether inositol influences embryo implantation; thus, study to test this hypothesis is warranted.
Another relevant finding of this study is the difference in cancelations due to OHSS, a result that has not been previously published. However, OHSS was not previously defined as an objective of this study. Additionally, the criteria to define OHSS risk were not defined at the beginning of the study; therefore, they were left to the criteria of each participating center. Although it has been suggested that metformin can reduce OHSS [Citation29,Citation30], this was not corroborated in a Cochrane review [Citation31]. Concerning inositol, there are only two studies, including one in an animal model in which vascular permeability as well as VEGF and COX-2 expression were reduced in animals treated with MYO [Citation32]. An RCT in women with PCOS undergoing intrauterine insemination showed that supplementation with MYO prevented OHSS [Citation18].
In our study, the CG OHSS rates were similar to those described for women with PCOS [Citation33,Citation34]. This finding reinforces the conclusions of a previous meta-analysis that showed MYO alone did not seem to influence the results of the stimulation cycle, but DCI supplementation could prevent it [Citation3]. The result did not reach statistical significance (p = .07), probably due to the small sample size, which was not calculated a priori to assess this parameter. However, these data are relevant from a clinical point of view and should be contrasted in future studies.
In conclusion, the combination of MYO-DCI at high doses of DCI improves the pregnancy rate with respect to its physiological concentration. This same combination reduces the risk of OHSS. These results highlight the importance of DCI supplementation in women with PCOS undergoing ICSI.
TRIAL REGISTRATION NUMBER
NCT01850030 (clinicaltrials.gov).
Acknowledgements
The statistical analyses were performed by Llenalia García Fernandez, Seplin Soluciones Estadísticas S.L.
Springer Nature Author Services provided English language editing services.
The study has been carried out in the framework of the CIEN METASIN Strategic Project entitled “Research, Development and Innovation in new multifunctional foods for Metabolic Syndrome” (IDI-20150571), funded by the Centre for Industrial Technological Development (CDTI)
We acknowledge Ma José Bravo and Ma Isabel Galan in the recruitment of patients and oocytes/embryos.
Disclosure statement
Díaz-Ropero MP, Maldonado-Lobón JA, Olivares M, Fonollá J are workers of Biosearch Life, a company that produces DCI from carob fruit. The remaining authors declare that they have no competing interests.
Additional information
Funding
References
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841–2855.
- Naderpoor N, Shorakae S, de Courten B, et al. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015; 21:560–574.
- Genazzani AD. Inositol as putative integrative treatment for PCOS. Reprod Biomed Online. 2016; 33:770–780.
- Zheng X, Lin D, Zhang Y, et al. Inositol supplement improves clinical pregnancy rate in infertile women undergoing ovulation induction for ICSI or IVF-ET. Medicine (Baltimore). 2017; 96:e8842.
- Unfer V, Nestler JE, Kamenov ZA, et al. Effects of inositol(s) in women with PCOS: a systematic review of randomized controlled trials. Int J Endocrinol. 2016;2016:1849162.
- Unfer V, Facchinetti F, Orrù B, et al. Myo-inositol effects in women with PCOS: a meta-analysis of randomized controlled trials. Endocr Connect. 2017;6:647–658.
- Mendoza N, Pérez L, Simoncini T, et al. Inositol supplementation in women with polycystic ovary syndrome undergoing intracytoplasmic sperm injection: a systematic review and meta-analysis of randomized controlled trials. Reprod Biomed Online. 2017;35:529–535.
- Facchinetti F, Bizzarri M, Benvenga S, et al. Results from the International Consensus Conference on Myo-inositol and d-chiro-inositol in Obstetrics and Gynecology: the link between metabolic syndrome and PCOS. Eur J Obstet Gynecol Reprod Biol. 2015;195:72–76.
- Benelli E, Del Ghianda S, Di Cosmo C, et al. A combined therapy with myo-inositol and D-chiro-inositol improves endocrine parameters and insulin resistance in PCOS young overweight women. Int J Endocrinol. 2016;2016:1.
- Minozzi M, Nordio M, Pajalich R. The combined therapy myo-inositol plus D-Chiro-inositol, in a physiological ratio, reduces the cardiovascular risk by improving the lipid profile in PCOS patients. Eur Rev Med Pharmacol Sci. 2013;17:537–540.
- Bevilacqua A, Dragotto J, Giuliani A, et al. Myo-inositol and D-chiro-inositol (40:1) reverse histological and functional features of polycystic ovary syndrome in a mouse model. J Cell Physiol. 2018. doi:10.1002/jcp.27623.
- Sortino MA, Salomone S, Carruba MO, et al. Polycystic ovary syndrome: insights into the therapeutic approach with inositols. Front Pharmacol. 2017;8:341
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25.
- Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–1283.
- Chiu TT, Rogers MS, Law EL, et al. Follicular fluid and serum concentrations of myo-inositol in patients undergoing IVF: relationship with oocyte maturation. Hum Reprod. 2002;17:1591–1596.
- Piomboni P, Focarelli R, Capaldo A, et al. Protein modification as oxidative stress marker in follicular fluid from women with polycystic ovary syndrome: the effect of inositol and metformin. J Assist Reprod Genet. 2014;31:1269–1276.
- Papaleo E, Unfer V, Baillargeon JP, et al. Myo-inositol may improve oocyte quality in intracytoplasmic sperm injection cycles. A prospective, controlled, randomized trial. Fertil Steril. 2009;91:1750–1754.
- Emekçi Özay Ö, Özay AC, Çağlıyan E, et al. Myo-inositol administration positively effects ovulation induction and intrauterine insemination in patients with polycystic ovary syndrome: a prospective, controlled, randomized trial. Gynecol Endocrinol. 2017;33:524–528.
- Croze ML, Soulage CO. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie. 2013;95:1811–1827.
- Sun TH, Heimark DB, Nguygen T, et al. Both myo-inositol to chiro-inositol epimerase activities and chiro-inositol to myo-inositol ratios are decreased in tissues of GK type 2 diabetic rats compared to Wistar controls. Biochem Biophys Res Commun. 2002;293:1092–1098.
- Unfer V, Carlomagno G, Rizzo P, et al. Myo-inositol rather than D-chiro-inositol is able to improve oocyte quality in intracytoplasmic sperm injection cycles. A prospective, controlled, randomized trial. Eur Rev Med Pharmacol Sci. 2011;15:452–457.
- De Leo V, La Marca A, Cappelli V, et al. [Evaluation of the treatment with D-chiro-inositol on levels of oxidative stress in PCOS patients]. Minerva Ginecol. 2012;64:531–538.
- Heimark D, McAllister J, Larner J. Decreased myo-inositol to chiro-inositol (M/C) ratios and increased M/C epimerase activity in PCOS theca cells demonstrate increased insulin sensitivity compared to controls. Endocr J. 2014;61:111–117.
- Pizzo A, Laganà AS, Barbaro L. Comparison between effects of myo-inositol and D-chiro-inositol on ovarian function and metabolic factors in women with PCOS. Gynecol Endocrinol. 2014;30:205–208.
- Formuso C, Stracquadanio M, Ciotta L. Myo-inositol vs. D-chiro inositol in PCOS treatment. Minerva Ginecol. 2015;67:321–325.
- Rolland AL1, Peigné M, Plouvier P, et al. Could myo-inositol soft gel capsules outperform clomiphene in inducing ovulation? Results of a pilot study. Eur Rev Med Pharmacol Sci. 2017;21:10–14.
- Isabella R, Raffone E. CONCERN: does ovary need D-chiro-inositol? J Ovarian Res. 2012;5:14.
- Colazingari S, Treglia M, Najjar R, et al. The combined therapy myo-inositol plus D-chiro-inositol, rather than D-chiro-inositol, is able to improve IVF outcomes: results from a randomized controlled trial. Arch Gynecol Obstet. 2013;288:1405–1411.
- Balen AH, Morley LC, Misso M, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update. 2016;22:687–708.
- Guo JL, Zhang DD, Zhao Y, et al. Pharmacologic interventions in preventing ovarian hyperstimulation syndrome: a systematic review and network meta-analysis. Sci Rep. 2016;11:19093.
- Bordewijk EM, Nahuis M, Costello MF, et al. Metformin during ovulation induction with gonadotrophins followed by timed intercourse or intrauterine insemination for subfertility associated with polycystic ovary syndrome. Cochrane Database Syst Rev. 2017;1:CD009090.
- Turan GA, Eskicioglu F, Sivrikoz ON, et al. Myo-inositol is a promising treatment for the prevention of ovarian hyperstimulation syndrome (OHSS): an animal study. Arch Gynecol Obstet. 2015;292:1163–1171.
- Jacob SL, Brewer C, Tang T, et al. A short course of metformin does not reduce OHSS in a GnRH antagonist cycle for women with PCOS undergoing IVF: a randomised placebo-controlled trial. Hum Reprod. 2016;31:2756–2764.
- Mohammadi Yeganeh L, Moini A, Shiva M, et al. Methylprednisolone for prevention of ovarian hyperstimulation syndrome in patients with polycystic ovarian syndrome undergoing in-vitro fertilisation: a randomised controlled trial. J Obstet Gynaecol. 2018;38:241–246.