Abstract
The trial objective was to determine the peripheral blood NK cells cytotoxic activity effect on trophoblast cells at recurrent pregnancy loss (RPL). The investigation involved non-pregnant women with PRL in proliferating and secretory menstrual cycle phases (PMCPh and SMCPh, respectively); women of 6–7 weeks pregnancy with RPL in past medical history; healthy fertile non-pregnant women in PMCPh and SMCPh, women of 6–7 weeks physiological pregnancy, nulliparity healthy women with regular menstrual function in PMCPh and SMCPh. NK cells cytotoxic activity was determined using peripheral blood mononuclear cells. The target cells were JEG-3 line trophoblasts. It has been established that NK cells cytotoxic activity effect on trophoblasts is lower in SMCPh than in PMCPh in non-pregnant fertile women. The NK cells cytotoxic activity was higher in SMCPh than in PMCPh in non-pregnant women with PRL and also higher than the same value in SMCPh in non-pregnant fertile women. The increased NK cells cytotoxic activity values in SMCPh in women with RPL may be the reason for miscarriage.
Introduction
The recurrent pregnancy loss (RPL) frequency is still persistently high. The pathology is caused by genetic disorders, hormone production imbalance, uterine structure disorders, infections, and immune conflicts [Citation1,Citation2]. The immune interaction disorders in mother–fetus system are one of the main pathogenic factors [Citation1]. In the meantime, the existing immune laboratory testes (lupus anticoagulant test, anticardiolipin, phosphatidylserine antibody, Factor V Leiden mutation, glucose test, C and S protein functional activity, prolactin level, hyperandrogenemia, etc.) allow to determine changes surpassing normal values only in 40% women with RPL [Citation3]. In 60% women with RPL, the disease cause remains unascertained [Citation3]. Among a variety of immune parameters, the NK cells role in RPL pathogenesis, especially their cytotoxic function is often stressed [Citation4].
NK cells are lymphocytes capable of virus-infected and tumor cells contact cytolysis. Endometrial NK cells are a specific NK population and their representation in uterus varies depending оn menstrual cycle (MC) phase and pregnancy [Citation5–7]. NK cells have a regulatory function during a physiological pregnancy which means creating optimal conditions for blastocyst invasion, the invasion process control depending on gestation terms as well as taking part in uterus spiral artery remodeling and uterus–placenta normal blood flow establishment [Citation7–10]. Trials in rats showed that NK cells decrease in uterus causes disorders of spiral artery restructuring [Citation11].
In case of miscarriage, the NK cells activity may vary [Citation11]. Attempts are made to work out miscarriage risk diagnostics and assessment methods based on NK cells functional activity variation measuring [Citation12]. There are two approaches to obtain biological material for NK cells investigation in miscarriage cases. The less invasive one is peripheral blood sampling with further NK cells isolation. The other source for NK cells is endometrium biopsy material [Citation12]. Generally, the functional status of peripheral blood NK cells reflects the same of uterus NK cells [Citation12,Citation13]. The peripheral blood sampling variant is more preferable because it is more standard and less invasive compared to biopsy method. Apart from that, blood sampling is possible both within and out of pregnancy while in case of biopsy we have a local tissue sampling which is possible only out of pregnancy, offers only sub-microgram of the material and is bound to cell loss during the isolation procedure [Citation14].
At the present time, chronic myeloid leukemia K562 line cells are used as targeting cells to determine NK cells cytotoxic activity. Different ways are applied to label the targeting cells: fluorescent dyeing [Citation15,Citation16], transfection with plasmid containing green fluorescent protein gene [Citation17], and some others. All the mentioned methods simulate the cytotoxic NK cells activity leaving out the peculiarities of NK cells and targeting cells interaction during pregnancy and its pathologies. Invasive extravillous trophoblast cells are the most obvious targeting cells for NK cells in decidua. Due to that, the trial objective was to determine the peripheral blood NK cells cytotoxic activity effect on trophoblast cells in case of recurrent miscarriage.
Materials and methods
Materials
The trial embraced non-pregnant women with PRL in MC proliferating (PPh) (n = 19) and secretory phases (SPh) (n = 23); women of 6–7 weeks pregnancy with RPL in past medical history (n = 23). The comparison groups included healthy non-pregnant fertile women, who had normal delivery ended previous pregnancy (henceforward non-pregnant fertile women) in PMCPh (n = 20) and SMCPh (n = 20), as well as women with physiological 6–7 weeks pregnancy (n = 25). The women involved were of normal karyotype, without antiphospholipid syndrome, hereditary high risk thrombophilia, hypertensive disease, diabetes, or adiposity. The control group included reproductive age healthy nulliparity women with regular menstrual function and no aggravated obstetric and gynecological anamnesis in PMCPh (n = 25) and SMCPh (n = 25). Another control group consisted of healthy men (n = 15). All the people involved in the trial were Caucasian race coming from The North-West region of Russia. The trial was carried out in the scientific-ambulatory and pregnancy pathology Departments of ‘Research Institute of Obstetrics, Gynecology, and Reproductology named after D.O. Ott’ together with Saint-Petersburg Miscarriage prevention, diagnostics and treatment Scientific and Practice Center. The study was prospective and cohort.
Methods
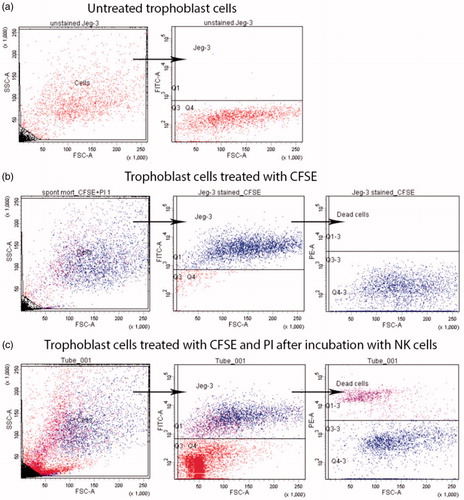
To determine the peripheral blood NK cells cytotoxic activity effect on trophoblast cells, mononuclear cells were applied which were isolated from patients’ peripheral blood by standard density gradient centrifugation Histopaque®-1077 (Sigma Aldrich, St. Louis, MO) [Citation18]. After isolation, the peripheral blood mononuclear cells containing NK cells were incubated in a complete culture medium containing DMEM, 10% fetal calf serum (FCS). 100 U/ml of penicillin and 100 mkg/ml of streptomycin, 2 mM of l-glutamine and 10 mM of sodium pyruvate (Sigma, St. Louis, MO). Then, the peripheral blood mononuclear cells were cultivated for four days both in presence or absence of recombinant IL-2 (‘Roncoleukine’ NPK ‘BIOTECH’, Saint Petersburg, Russia). The applied trophoblast cells were of JEG-3 line (ATCC, Manassas, VA), simulating morphological, phenotypic and functional characteristics of first trimester pregnancy trophoblasts [Citation19]. Twenty-four hours before the experiment (on the third incubation day), the trophoblast cells were prepared, the monolayer disintegrated through exposition in trypsin–Versene solution (1:1), then half of the obtained cell suspension was placed in a new flask for adhesive culture in a complete culture medium, containing 1% of HEPES solution and 10% FCS (Sigma, St. Louis, MO). On the next day, the trophoblast cells were disintegrated, treated by carboxyfluorescein succinimidyl ester (CFSE) 5-6 solution then incubated for 4 h with peripheral blood mononuclear cells in effector–target 10:1 rate. A part of trophoblast cells was incubated in a similar cultural medium without mononuclear cell addition to determine the base trophoblast cell death and then the number of viable and inviable trophoblast cells was measured by propidium iodide solution staining in a final 0.01 mg/ml concentration (Sigma Aldrich, St. Louis, MO). Part of trophoblast cells were not treated by CFSE and propidium iodide solutions but were used as negative control for cell autofluorescence estimation. For each patient, NK cells cytotoxic activity was measured both in presence or absence of IL-2. During each NK cells cytotoxic activity estimation, the base trophoblast cell death was also measured in absence of mononuclear cells ().
Figure 1. JEG-3 line trophoblast cells gating strategy after incubation with peripheral blood NK cells. (a) Trophoblast cells in FSC-SSC and FSC-FITC coordinates (negative control); (b) CFSE and propidium iodide solution treated trophoblast cells after incubation in complete culture medium in FSC-SSC and FSC-FITC coordinates (base death); (c) CFSE and propidium iodide solution treated trophoblast cells after incubation in presence of peripheral blood mononuclear cells containing NK cells in FSC-SSC and FSC-FITC coordinates (NK cells cytotoxic activity caused death).
Statistics
The data obtained were statistically processed by Statistica 10 program. For data comparison, the Mann–Whitney U-criteria along with Wilcoxon’s criteria were applied. Differences at p < .05, p < .01, p < .001 were estimated statistically relevant.
Results
It was established that the NK cells cytotoxic effect on trophoblast cells was higher in presence of IL-2 compared to the same indicator after incubation without IL-2 in all tested patient groups ().
Figure 2. Cytotoxic activity effect on trophoblasts of NK cells incubated both in presence and in absence of IL-2. Data are presented in box diagrams, where the midline corresponds to the median and the upper and lower box borders – to 75% and 25% quartiles, respectively. The group differences within incubation conditions: ***p < .001.
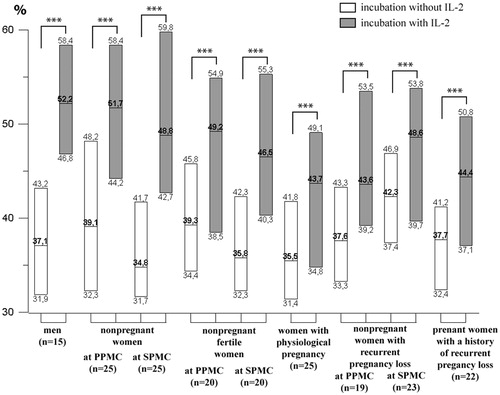
The groups of healthy non-pregnant women without pregnancies in past history in PMCPh and SMCPh did not have differences from the healthy men group in NK cells cytotoxic effect on trophoblast cells both in IL-2 presence and absence.
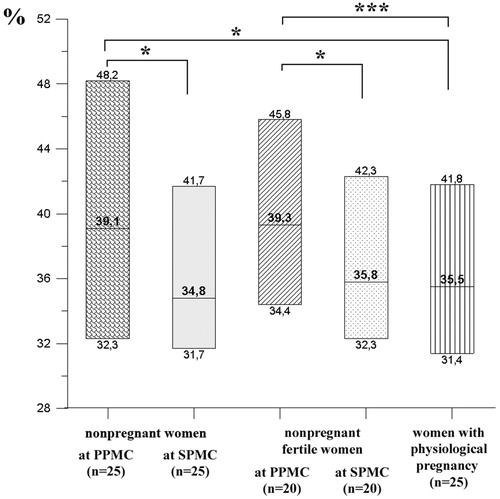
The NK cells cytotoxic activity without IL-2 in healthy non-pregnant women in PMCPh with no pregnancies in past history was higher than in those with 6–7 weeks physiological pregnancy (). In the meantime, the NK cells cytotoxic activity in healthy non-pregnant women in SMCPh was lower compared to the same group in PMCPh and did not differ from the same value in women with physiological pregnancy (). The comparison of healthy non-pregnant fertile women group in both phases to the group of physiologically pregnant women showed similar tendencies. The peripheral blood NK cells cytotoxic activity in case of incubation without IL-2 was higher in healthy non-pregnant fertile women in PMCPh compared to the values in physiologically pregnant women group (). The NK cells cytotoxic activity in case of incubation without IL-2 was lower in fertile non-pregnant women in SMCPh compared to the same in PMCPh (). No difference was indicated between the group of non-pregnant fertile women in SMCPh and of those physiologically pregnant ().
Figure 3. Cytotoxic activity effect on trophoblasts of NK cells incubated without IL-2 in non-pregnant and pregnant women. Data presented in box diagrams, where the midline corresponds to the median and the upper and lower box borders – to 75% and 25% quartiles, respectively. Group differences: *p < .05, **p < .01, ***p < .001.
In presence of IL-2, the NK cells cytotoxic activity in non-pregnant women without previous pregnancies in PMCPh (51.7% (44.2%; 58.4%)) and SMCPh (48.8% (42.7%; 59.8%)) was higher than in those with 6–7 weeks term physiological pregnancy (43.7% (34.8%; 49.0%); p ˂ .001).
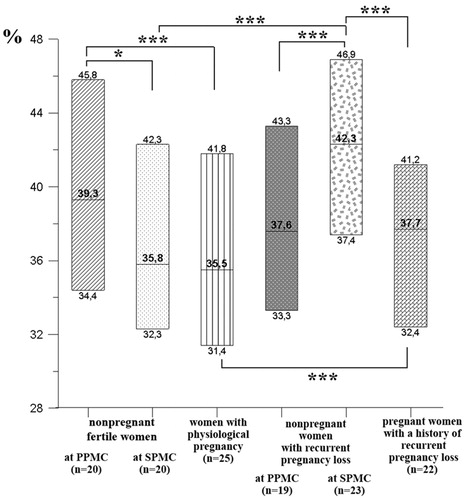
The NK cells cytotoxic activity effect on trophoblast in women with RPL was higher in SMCPh than in PMCPh in case of incubation without IL-2 (). Apart from that, the cytotoxic activity of NK cells obtained from non-pregnant women with PRL was higher in SMCPh compared to the same value in healthy fertile women also in SMCPh (). The NK cells cytotoxic activity effect on trophoblasts in pregnant women with RPL in case of incubation without IL-2 was lower compared to that in non-pregnant women with RPL in SMCPh (). Along with that, the NK cells cytotoxic activity effect on trophoblasts in case of incubation in presence of IL-2 was also lower in pregnant women with RPL (44.4% (37.0%; 50.8%)) compared to non-pregnant women with RPL in SMCPh (48.6% (39.7%; 53.8%); p ˂ .05).
Figure 4. Cytotoxic activity effect on trophoblasts of NK cells incubated without IL-2 in non-pregnant and pregnant women with RPL. Data are presented in box diagrams, where the midline corresponds to the median and the upper and lower box borders – to 75% and 25% quartiles, respectively. Group differences: *p < .05, ***p < .001.
Discussion
Chronic myeloid leukemia K562 line cells are used as targeting cells to determine the NK cells cytotoxic activity in the most widely applied methods [Citation15–17,Citation20,Citation21]. Some attempts were previously made to estimate the NK cells cytotoxic activity effect on trophoblasts by using BeWo linear cells together with placenta isolated trophoblast cells. Those methods showed similar activity of NK cells compared to cytotoxiс effect on K562 cells [Citation21]. However, the placenta isolated trophoblast cells contained macrophage and B-lymphocyte admixtures [Citation21] which could affect the trial results. Along with that placenta isolated trophoblast cells usage is not reasonable because the surface receptor expression of population difference by trophoblast cells may also affect the obtained results [Citation22]. Immune histochemical studies showed BeWo line cells inclination to syncytium fanning which is not observed in case of JEG-3 line cells [Citation23]. It is likely to get distorted results due to BeWo line cells damage in process of preparing them as target cells for NK cells cytotoxicity testing. For this reason, we preferred JEG-3 line trophoblast cells as target cells for the present trial.
We have established that the NK cells cytotoxic activity effect on trophoblast cells was higher in presence of IL-2 in all tested patient groups. Thus, the peripheral blood NK cells ability to increase the cytotoxic activity in presence of an activator (IL-2) within our simulating system affirms the possibility of applying the suggested experimental scheme to estimate cells functional status.
We have also established that NK cells cytotoxic activity was lower in non-pregnant women, both without previous pregnancies and the fertile ones in SMCPh, and did not differ from NK cells cytotoxic activity in women with physiological pregnancy. Taking into consideration the uterine NK cells cytopoiesis possibility on the base of migration from peripheral blood, the performed difference may be the effect of endometrium preparation for blastocyte implanting, which normally takes place in 6–7 days after ovulation and ovum fertilization [Citation10]. During this period, NK cells number increase in uterus is observed [Citation24,Citation25] along with variance both of NK cells and macrophage secretion and adhesive molecule expression by endometrium cells [Citation24]. It is likely that hormonal context variance taking place during MC affects not only cell population representation in uterus [Citation10,Citation25], but also peripheral blood NK cells functional characteristics.
It has been also established that NK cells cytotoxic activity effect on trophoblast cells in non-pregnant women with recurrent miscarriage is higher in SMCPh compared to PMCPh and also compared to the same value in non-pregnant fertile women in SMCPh. Thus, contrary to the case of non-pregnant fertile women where NK cells cytotoxic activity decrease in SMCPh is observed, we see cytotoxic activity increase in SMCPh in women with RPL.
Pregnant women with RPL in past history show decreased NK cells cytotoxic effect on trophoblast compared to the same value in non-pregnant women with RPL in SMCPh. This difference may be seen as pregnancy consequence together with macrophage and regulatory T-cell decidua affect outcome [Citation26,Citation27], stimulating implantation and placentation, and also that of yellow body and chorion secreted progesterone [Citation4,Citation10].
Following the results obtained we may suppose that NK cells cytotoxic activity effect on trophoblast varies during the MC. It is higher in the proliferative phase and decreases in the secretion phase. This variation may be determinative for blastocyst implantation and pregnancy success. NK cells cytotoxic activity increased values in women with RPL in SMCPh may stipulate the miscarriage. It should be noted that according to the research literature the NK cells content as well as NK cells cytotoxic activity investigation has not been seen yet as top priority for RPL patients’ treatment [Citation12,Citation28,Citation29]. Basing on data obtained, we would recommend to introduce immune disorders diagnostics including NK cells cytotoxic activity effect on trophoblast cells monitoring into the treatment practice of patients with RPL.
Acknowledgments
The authors express thanks to L.P. Viazmina for technical assistance.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Kwak-Kim J, Bao S, Lee SK, et al. Immunological modes of pregnancy loss: inflammation, immune effectors, and stress. Am J Reprod Immunol. 2014;72:129–140.
- El Hachem H, Crepaux V, May-Panloup P, et al. Recurrent pregnancy loss: current perspectives. IJWH. 2017;9:331–345.
- Jaslow CR, Carney JL, Kutteh WH. Diagnostic factors identified in 1020 women with two versus three or more recurrent pregnancy losses. Fertil Steril. 2010;93:1234–1243.
- Pandey MK, Rani R, Agrawal S. An update in recurrent spontaneous abortion. Arch Gynecol Obstet. 2005;272:95–108.
- Tabiasco J, Rabot M, Aguerre-Girr M, et al. Human decidual NK cells: unique phenotype and functional properties – a review. Placenta. 2006;27:34–39.
- Keskin DB, Allan DS, Rybalov B, et al. TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16– NK cells with similarities to decidual NK cells. Proc Natl Acad Sci USA. 2007;104:3378–3383.
- Zhang J, Dunk C, Croy AB, et al. To serve and to protect: the role of decidual innate immune cells on human pregnancy. Cell Tissue Res. 2016;363:249–265.
- Wallace AE, Fraser R, Cartwright JE. Extravillous trophoblast and decidual natural killer cells: a remodelling partnership. Hum Reprod Update. 2012;18:458–471.
- Sharkey AM, Xiong S, Kennedy PR, et al. Tissue-specific education of decidual NK cells. J Immunol. 2015;195:3026–3032.
- Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345:1400–1408.
- Chakraborty D, Rumi MA, Konno T, et al. Natural killer cells direct hemochorial placentation by regulating hypoxia-inducible factor dependent trophoblast lineage decisions. Proc Natl Acad Sci USA. 2011;108:16295–16300.
- Templer S, Sacks G. A blessing and a curse: is high NK cell activity good for health and bad for reproduction? Hum Fertil. 2016;19:166–172.
- Park DW, Lee HJ, Park CW, et al. Peripheral blood NK cells reflect changes in decidual NK cells in women with recurrent miscarriages. Am J Reprod Immunol. 2010;63:173–180.
- Tang AW, Alfirevic Z, Quenby S. Natural killer cells and pregnancy outcomes in women with recurrent miscarriage and infertility: a systematic review. Hum Reprod. 2011;26:1971–1980.
- Thum MY, Bhaskaran S, Abdalla HI, et al. Prednisolone suppresses NK cell cytotoxicity in vitro in women with a history of infertility and elevated NK cell cytotoxicity. Am J Reprod Immunol. 2008;59:259–265.
- Roussev RG, Dons’koi BV, Stamatkin C, et al. Preimplantation factor inhibits circulating natural killer cell cytotoxicity and reduces CD69 expression: implications for recurrent pregnancy loss therapy. Reprod Biomed Online. 2013;26:79–87.
- Kantakamalakul W, Jaroenpool J, Pattanapanyasat K. A novel enhanced green fluorescent protein (EGFP)-K562 flow cytometric method for measuring natural killer (NK) cell cytotoxic activity. J Immunol Methods. 2003;272:189–197.
- Boyum A. Separation of leukocytes from blood and bone marrow. Introduction. Scand J Clin Lab Investig Suppl. 1968;97:7.
- Kohler PO, Bridson WE. Isolation of hormone-producing clonal lines of human choriocarcinoma. J Clin Endocrinol Metabol. 1971;32:683–687.
- Karami N, Boroujerdnia MG, Nikbakht R, et al. Enhancement of peripheral blood CD56(dim) cell and NK cell cytotoxicity in women with recurrent spontaneous abortion or in vitro fertilization failure. J Reprod Immunol. 2012;95:87–92.
- Ferry BL, Sargent IL, Starkey PM, et al. Cytotoxic activity against trophoblast and choriocarcinoma cells of large granular lymphocytes from human early pregnancy decidua. Cell Immunol. 1991;132:140–149.
- Al-Nasiry S, Spitz B, Hanssens M, et al. Differential effects of inducers of syncytialization and apoptosis on BeWo and JEG-3 choriocarcinoma cells. Hum Reprod. 2006;21:193–201.
- Zidi I, Rizzo R, Bouaziz A, et al. sHLA-G1 and HLA-G5 levels are decreased in Tunisian women with multiple abortion. Hum Immunol. 2016;77:342–345.
- van Mourik MS, Macklon NS, Heijnen CJ. Embryonic implantation: cytokines, adhesion molecules, and immune cells in establishing an implantation environment. J Leuk Biol. 2008;85:4–19.
- Lee SK, Kim CJ, Kim DJ, et al. Immune cells in the female reproductive tract. Immune Netw. 2015;15:16–26.
- Sokolov DI, Selkov SA. Decidual macrophages: the role in immunologic dialogue of mother and the fetus. Immunologia (Russia)/Immunol. 2014;35:113–117.
- Sokolov DI, Stepanova OI, Selkov SA. The role of the different subpopulations of CD4+ T-lymphocytes during pregnancy. Med Immunol. 2016;18:521–536.
- Kwak-Kim J, Han AR, Gilman-Sachs A, et al. Current trends of reproductive immunology practices in in vitro fertilization (IVF) – a first world survey using IVF-Worldwide.com. Am J Reprod Immunol. 2013;69:12–20.
- Moffett A, Shreeve N. First do no harm: uterine natural killer (NK) cells in assisted reproduction. Hum Reprod. 2015;30:1519–1525.
