Abstract
Aim: The aim of this study is to summarize the outcomes of transfers of mosaic embryos, which were classified according to guidelines and in strong collaboration of reproductologists, clinical geneticists and patients approved as suitable for transfer.
Material and Methods: Retrospective data were collected from 70 patients from a private IVF center to whom embryos with mosaic changes in chromosomal material were transferred from 2015 to 2019.
Results and Conclusion: Implantation outcomes and continuing pregnancies showed slight differences, when compared to fully normal embryos. Artifacts have to be differentiated from undeniable aberrations, and correct interpretation of results must be done with following patient counselling and prenatal testing if necessary.
Keywords:
Introduction
Preimplantation genetic testing for aneuploidy (PGT-A) is developed as one of the opportunities to achieve better results in IVF pregnancies. The great challenge with this method is interpretation of results of embryos when mosaic aneuploidies are observed. Full aneuploidies in the embryo are mainly meiotic and are the result of improper chromosome segregation in the development of gametes [Citation1]. As second event mitotic errors are possible in embryo growth and results in chromosomally mosaic organism [Citation2]. Chromosomal mosaicism is the appearance of different chromosomally distinct cell lines in an embryo or developed individual. The mosaicism might have adverse effects on the phenotype if present in ICM (inner cell mass) of the embryo. Aberrant cells tend to become apoptotic or move to trophectoderm, which later forms the placenta, and in such manner, the embryo performs self-correction. Various karyotypes of placental cells might cause adverse results of pregnancy, for example, fetal growth restriction or placental insufficiency [Citation3]. Mosaicism can be divided into general and confined form. General mosaicism appears as a presence of mosaic cells in both, placenta and fetus [Citation3]. Confined mosaicism of cells is defined as aberrant cells in outer tissues of embryo, while the fetal cells are fully diploid [Citation4]. Different testing approaches, like FISH (fluorescent in situ hybridization), aCGH (array comparative genomic hybridization), or NGS (next generation sequencing) have approved the heterogeneity of chromosomal material [Citation5]. It is complicated to distinguish artifacts from true aberrations in segmental chromosome rearrangements due to various methodological limitation, technical biases, and quality of TE biopsy. Several guidelines have been developed to summarize available information for different possible chromosomal testing results [Citation6,Citation7]. Using these guidelines, when making decisions of ambiguous PGT-A results, allows to choose embryos which have high possibility to develop into a healthy child and avoid undesirable events in case of pregnancy. The aim of this study is to summarize the results of transfers of mosaic embryos, which are classified according to guidelines and in strong collaboration of reproductologists, clinical geneticists, and patients approved as suitable for transfer.
Methods
Patients
Results were collected from 70 patients from a private IVF center, to whom embryos with mosaic changes in chromosomal material were transferred from 2015 to 2019. As a control group patients with 168 transfers of euploid embryos were selected. All patients have signed informed consents and approved the use of their anonymized data. Data collection was done following the principles of the Declaration of Helsinki.
Patients were classified in groups according to age: ‘below advanced maternal age’ (<35 years old) and ‘advanced maternal age’ (>35 years old). Patients from the subject group were divided into subgroups according to the method used for PGT-A – aCGH and NGS.
To exclude chromosomal aberrations in patients, parental karyotyping was performed using classical G band cytogenetic approach according to ISCN (The International System for Human Cytogenetic Nomenclature) criteria.
In addition, endometrial receptivity test (ERA, Igenomix) was done for female patients to exclude endometrial receptivity shift.
IVF and embryo biopsy procedure
Patients underwent controlled ovarian stimulation with agonists and antagonists using standard protocols. The dosages were calculated after the evaluation of ovarian reserves and AMH (anti-Mullerian hormone) values. When follicles reached a size of 18–20 mm in diameter, the trigger was injected. Oocytes were retrieved 36 h after injection, and ICSI (intracytoplasmic sperm injection), in some cases PICSI (Physiological intracytoplasmic sperm injection), for cells, reached MII state, was performed. The sperm from male patients was selected using discontinuous density gradient according to WHO laboratory manual for the examination and processing of human semen. Cultivation of embryos was for 5–6 days in Embryoscope. When the embryo reached the blastocyst stage, the embryologist performed hatching with laser Saturn 5 (Cooper Surgical) and obtained trophectodermal cells through the opening in zona pellucida. Classification of embryo quality was made as follows: ‘low quality,’ ‘moderate,’ and ‘high quality’ according to quality of inner cell mass and trophectodermal cells using Gardner and Schoolcraft criteria. Kitazato vitrification approach was used for embryo vitrification.
Whole genome amplification (WGA) and chromosomal analysis
PGT-A was done either by aCGH with Illumina 24Sure array protocol from 2015 to 2017 or by NGS with Illumina VeriSeq PGS protocol from 2018 to 2019. An analysis was performed with Bluefuse software. Interpretation of results was made by at least two molecular geneticists and at least one clinical geneticist. Chromosomal aberrations were classified according to guidelines: euploid, low-level mosaic (20–50%), high-level mosaic (50–80%), aneuploid. A decision regarding transfer was made according to chromosomes involved and type of aberration (whole chromosome versus partial).
Counseling
Transfer of embryo with mosaic aneuploidies was offered to the patient only in case if there were no euploid embryos for transfer. All patients in the subject group visited reproductologist and clinical geneticist before the planned transfer. Embryos with mosaic aberrations were transferred after informed consent. Transfer day was chosen according to ERA test results to obtain more successful results.
Statistics
Due to the small number of mosaic embryo cases (n = 70), statistical analysis was not performed.
Results
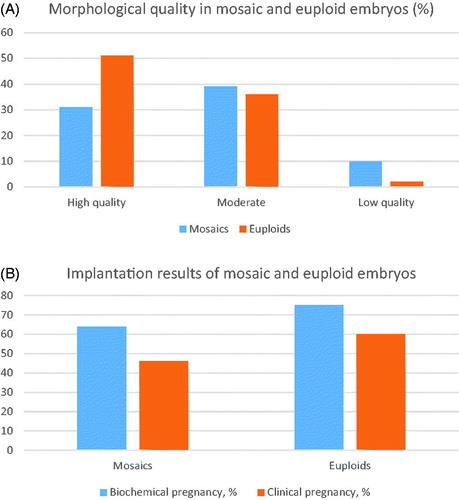
In the subject group, 61% of women (n = 43) were in advanced maternal age (>35 years old), and in 12 cases oocytes were retrieved from donors (17%). In the control group (n = 168), 37% (n = 63) patients reached advanced maternal age (>35 years) during therapy time. In the control group, only 7 oocytes were obtained from donor follicles (4%). Patients from the subject group were divided into subgroups according to the method used for PGT-A. In aCGH ‘below advanced maternal age’ subject group (<35 years old, n = 4, 18%), 49 oocytes and 18 embryos were retrieved. In aCGH ‘advanced maternal age’ subject group (>35 years old, n = 18, 82%), 198 oocytes and 73 embryos were obtained. In NGS subject ‘below advanced maternal age’ subject group (n = 15, 32%), 174 oocytes and 61 embryos were obtained, in ‘advanced maternal age’ subject group (>35 years old, n = 33), 316 oocytes and 118 embryos were obtained. From all 70 cases in subject group, morphology of embryo was defined as ‘high quality’ in 31% (n = 22) cases, 39% (n = 27) mosaic embryos were evaluated as moderate, in 10% (n = 7) embryo morphology was described as ‘very low quality.’ Amongst control group, there were only 2% (n = 3) with morphology defined as ‘low quality,’ 44% (n = 74) was in moderate morphology group and 54% (n = 91) embryo quality was high (). From subject group, total count of transfers with mosaic embryo was in aCGH group – 22, and in NGS group – 48.
Figure 1. (A) Comparison of mosaic and euploid embryo morhology. (B) Comparison of outcomes of mosaic and euploid embryo transfers.
Thirty-three of detected mosaics included whole chromosome aneuploidies, 22 of mosaic results were segmental aberrations, in 15 cases chromosomal changes included more than four regions and were described as complex chaotic chromosomal changes. Most of the detected aberrations in the subject group were considered as low-level mosaics. In some cases, repeated testing was performed to exclude false-positive result due to technical bias, mosaic level fluctuated in 10% range. In the aCGH group, 50% (n = 11) embryos were diagnosed with whole chromosome aneuploidies; 36% (n = 8) were recognized with segmental aberrations; results of 14% (n = 3) were described as complex chaotic chromosomal changes. In the mosaic embryo group, analyzed with NGS, 47% (n = 22) were characterized as whole chromosome mosaics; in 30% (n = 14) mosaic aberrations included partial regions of chromosomes; in 25% (n = 12) changes of chromosomal material were described as complex chaotic chromosomal changes.
Implantation outcomes and continuing pregnancies showed slight differences when compared to fully normal embryos. Biochemical pregnancy rates were 64% in the subject group and 75% in the control group. The clinical pregnancy rate was 46% and 60% accordingly (). The first screening and anomaly scan with USG was without abnormalities in all cases, where pregnancy continued.
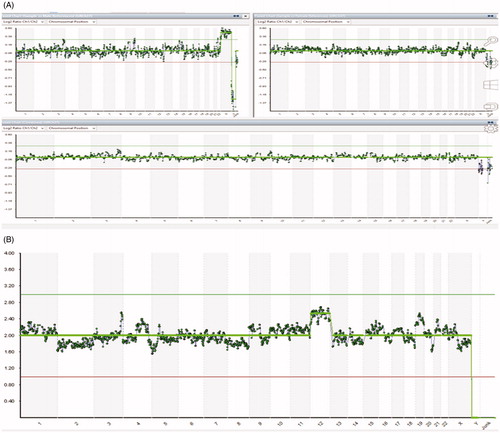
Figure 2. (A) aCGH result of the product of conception, case 2. (B) NGS result of the corresponding embryo, case 2.
Comparing results of transfer with mosaic embryos in the subject group between methods used for PGT-A, the percentage of biochemical pregnancies in the aCGH group was 59% (n = 13), and in the NGS group, it was 63% (n = 30). The clinical pregnancy rates achieved 45% in the aCGH group (n = 10) and 48% in the NGS group (n = 23). The livebirth rate in the aCGH mosaic group was 31% (n = 7). The livebirth rate in the NGS group at this moment is 37% (n = 19), and 4 more ongoing pregnancies at the moment of article writing.
Artifacts
Due to technical biases interpretation of PGT-A analysis is sometimes difficult. Mosaic chromosomal profiles and given embryo status can be impacted by faulty amplifications of certain chromosomal regions. In the subject group, 21% (n = 15; 3 in aCGH group, 12 in NGS group) embryos had low-level complex chaotic chromosomal changes, that included mosaic deletions of subtelomeric regions. Repeating mosaic partial aberrations of specific chromosomes are observed in different embryos not only in the subject group. In the aCGH group, it was 3 cases with segmental changes in autosome 18. In the NGS group, there were 13 segmental mosaic cases in different chromosomes. 50% (n = 6) of them included mosaic aberrations in acrocentric chromosomes. Frequent unreliable cases of mosaic trisomy of chromosome 19 are detected, but were not included in the subject group.
Case descriptions
Six cases, where patient consents were obtained for detailed description, are summarized in . In five cases, livebirth without congenital anomalies was achieved, in one case there is ongoing pregnancy. In all six cases, pregnancy was achieved after transfer with mosaic embryo. Each of embryo had different mosaic chromosomal aberrations.
Table 1. Summary of six cases, in which transfer with mosaic embryo was performed.
In case 2, two IVF cycles were performed. In the first IVF cycle, the patient chose to perform embryo transfer without PGT-A, although biopsies of embryos were done. At week 5 she miscarried. The product of conception was sent to aCGH analysis, the result showed no chromosomal aneuploidies – aCGH(1-22,X)x2 (). Chromosomal analysis of the embryo biopsy, used for the transfer, was executed afterward to exclude possible mosaicism. NGS analysis showed mosaic trisomy of autosome 12, seq[GRCh37](12)x2 ∼ 3 (). This shows the possible impact of mosaic aneuploidies on pregnancy continuity. Successful pregnancy in case 2 was achieved with mosaic embryo after PGT-A analysis was done.
Discussion
Mosaicism of genetic material is described as two or more different cell lineages in one organism. This phenomena in PGT-A genetic analysis by aCGH and NGS sometimes is difficult to recognize or to distinguish from artifacts because of tissue specificity, technical conditions, or phenotypic variations. In our subject group, biochemical and clinical pregnancy/livebirth rates differed only slightly between aCGH and NGS groups. Our results are similar to those obtained by Yang et al. [Citation8], where diversities in pregnancies and livebirths did not exceed 10%. The effect of each mosaic is unique in each case, and it is problematic to make adequate interpretations and statistically significant results in the shortage of well-defined genotypes and phenotypes, and limited subject groups [Citation9]. Embryos, where chromosomal aberration is derived from meiosis mainly develop as 100% aneuploids. In such cases, the prediction of pregnancy outcome can be made convincingly [Citation10]. The source for trisomic mosaics is either of a somatic or meiotic origin. Somatic aneuploidies are more often, but are excluded by placental tissue and with marginal clinical result [Citation11]. The consequences of embryo development involve many variables – chromosome number, proportion of abnormal cells, and the location of aberrant cell lineages [Citation12]. Mosaicism of trisomies is present in 1–2% of chorion villus samples (CVS). In cases of confined chorionic mosaicism (CCM), pregnancy outcomes are mostly normal, and adverse results arise from aberrant cells in the fetus or uniparental disomies [Citation13].
We compared embryo implantation and ongoing pregnancy rates between mosaic and euploid embryos. Our results illustrated the assumption that conceptions with mosaic embryos can still lead to successful livebirths, and pregnancy rates are lower, but still relevant. Almost none of the mosaic aberrations in transferred embryos from our group exceeded 50% borderline. Lin et al. [Citation14] demonstrated that mosaics, higher than 50%, still result in live birth cases, however, miscarriage levels are 25% higher, when compared to low-level mosaics. The reported case of Kahraman et al. [Citation15], where healthy offspring with 2% cells with monosomy of autosome 2 was born, does not exclude the possibility, that there could be more similar cases after the transfer of mosaic embryos. The fact, that children are born healthy, only proves that IVF clinics should do more careful follow-up not only during the pregnancy, but at the neonatal stage as well. In Case 2, from our results, mosaic trisomy of autosome 12 was detected in embryo TE cells, however, the same aberration was not identified in chorionic material of POC. All the possibilities of errors were excluded. Therefore we can discuss the theory about embryo self-correction [Citation16]. Confined placental mosaicism (CPM) could occur as well in this case [Citation4]. Very likely abnormal cells from trophectodermal cells can be eliminated, while abnormal cells in the inner layers stay and impair the continuing development. There is no evidence about placental cells passing the basal lamina, therefore the movement could have happened in very early stages of development. Discrepancies between chorion villi and embryo chromosomal material are described before. This is caused by isolated non-disjunction in trophectodermal cells. Fetal blood is more strictly linked to chorion than to embryo itself [Citation17]. Despite, when fetal blood was compared to chorion, 50% results were discordant [Citation18].
Segmental mosaicism is another issue in preimplantation diagnostics. Zore et al. [Citation19] proved 66% lower birth rates and 22% elevated miscarriage levels in segmental aberrations group. Sub-chromosomal aberrations are of mitotic origin in 70% cases. Therefore, positive predictive value for full-chromosome aneuploidies in ICM is higher (97.18% vs 70.8%) [Citation20]. Diagnostics of segmental chromosomal rearrangements are more problematic when compared with whole chromosome mosaics and can be impacted by different reasons, such as confined mosaicism, WGA artifacts, reciprocal errors, S-phase artifacts, or algorithm imperfections [Citation21,Citation22]. Victor et al. [Citation23] showed that the type of mosaicism is the playing factor for implantation – embryos with segmental aneuploidies implanted better, when compared with whole chromosome mosaics.
The quality of the embryo and the following aspect of biopsy leaves an impact on PGT-A and fertilization outcome. Fiorentino et al. [Citation24] demonstrated the importance of aneuploidy type, poorer results of IVF cycles were in cases with complex or segmental mosaic aberrations, when compared with whole chromosome aneuploidies. Our clinic experience shows, that the problematic biopsies arise from the lower grade embryos. If blastocyst is not separated from zona pellucida, the quality of inner cell mass and trophectoderm is faulty, cell division is evaluated as low, and the cells obtained for biopsy are few and might be damaged. So, the embryo quality impacts biopsy and following amplification artifacts in PGT-A result. Such results arise additional risk to reject chromosomally normal embryos. False-positive mosaics sometimes might be recognized by odd patterns of probe aberrations – only several probes duplicated/deleted, making skewed, vague borders, wavy profiles, or frequent aberrations with usually rare frequency in certain regions. In Case 2, six embryos were obtained in the second IVF cycle, and four of them exhibited mosaic deletions of 5p region. After FISH investigation of corresponding regions in both partners, no similar findings were detected, embryo without mosaic deletion in 5p region was selected for transfer, to avoid possible impact on the child, if such event is present in ICM. Here we can consider whether frequent segmental mosaics are real, or just artifacts because of the PGT-A method’s imperfections or quality of biopsy. Liedo et al. [Citation25] did comparison of pregnancy outcomes after transfers of euploid and mosaic embryos and concluded that after adding embryo quality as a confounding factor, miscarriage rate differences were not significant. Chuang et al. [Citation26] compared chromosomal constitutions between different TE biopsy sites and TE and ICM. The distance from ICM does not have an impact on chromosomal consistency (TE site 1 – 86.2% and TE site 2 – 89.7%), pointing that aberrant cells spread randomly in the embryo. The level of confined mosaicism observed was 14%.
Conclusion
In this article, we reviewed the pregnancy outcomes of mosaic embryo transfers regarding embryo quality, maternal age, genetic testing approach used, and recognition of artifacts. Our results and suggestions are concordant with other studies – implantation rates are lower after mosaic embryo transfers, however, such results should not be a crucial factor for avoiding the transfer. Artifacts have to be differentiated from undeniable aberrations and correct interpretation of results must be done. It is advisable to avoid transfer with embryos with mosaic aneuploidies, which are viable with congenital anomalies. Transfers of mosaic embryos should be considered more carefully and must include patient counseling and following prenatal testing if necessary.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Webster A, Schuh M. Mechanisms of aneuploidy in human eggs. Trends Cell Biol. 2017;27:55–68.
- McCoy RC, Demko Z, Ryan A, et al. Common variants spanning PLK4 are associated with mitotic-origin aneuploidy in human embryos. Science. 2015;348:235–238.
- Taylor TH, Gitlin SA, Patrick JL, et al. The origin, mechanisms, incidence and clinical consequences of chromosomal mosaicism in humans. Hum Reprod Update. 2014;20:571–581.
- Kalousek DK, Vekemans M. Confined placental mosaicism. J Med Genet. 1996;33:529–533.
- Rodriguez TA, Burgoyne PS. Evidence that sex chromosome asynapsis, rather than excess Y gene dosage, is responsible for the meiotic impairment of XYY mice. Cytogenet Cell Genet. 2000;89:38–43.
- Cram DS, Leigh D, Handyside A, et al. PGDIS Position Statement on the Transfer of Mosaic Embryos. Reprod Biomed Online. 2019;39:e1–e4.
- Harton GL, De Rycke M, Fiorentino F, et al.; European Society for Human Reproduction and Embryology (ESHRE) PGD Consortium. ESHRE PGD consortium best practice guidelines for amplification-based PGD. Hum Reprod. 2011;26:33–40.
- Yang Z, Lin J, Zhang J, et al. Randomized comparison of next-generation sequencing and array comparative genomic hybridization for preimplantation genetic screening: a pilot study. BMC Med Genomics. 2015;8:30.
- Biesecker LG, Spinner NB. A genomic view of mosaicism and human disease. Nat Rev Genet. 2013;14:307–320.
- Hardy K, Hardy PJ. 1(st) trimester miscarriage: four decades of study. Transl Pediatr. 2015;4:189–200.
- Robinson WP, Barrett IJ, Bernard L, et al. Meiotic origin of trisomy in confined placental mosaicism is correlated with presence of fetal uniparental disomy, high levels of trisomy in trophoblast, and increased risk of fetal intrauterine growth restriction. Am J Hum Genet. 1997;60:917–927.
- Johnson DS, Cinnioglu C, Ross R, et al. Comprehensive analysis of karyotypic mosaicism between trophectoderm and inner cell mass. Mol Hum Reprod. 2010;16:944–949.
- Wang BBT, Rubin CH, Williams J. Mosaicism in chorionic villus sampling: an analysis of incidence and chromosomes involved in 2612 consecutive cases. Prenat Diagn. 1993;13:179–190.
- Lin P-Y, Lee C-I, Cheng E-H, et al. Clinical outcomes of single mosaic embryo transfer: high-level or low-level mosaic embryo, does it matter? JCM. 2020;9:1695.
- Kahraman S, Cetinkaya M, Yuksel B, et al. The birth of a baby with mosaicism resulting from a known mosaic embryo transfer: a case report. Hum Reprod. 2020;35:2020.
- Franasiak JM, Forman EJ, Hong KH, et al. The nature of aneuploidy with increasing age of the female partner: A review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101:656–663.e1.
- Dahl BW, Kahan Bw CA. Studies with mouse embryonic sac cells: An approach to understanding the pattern of ontogeny of cell-mediated immune functions. In: Horton JD, editor. Develpoment and differentiation of vertebrate lymphocytes. Amsterdam (The Netherlands): Elsevier/North-Holland Biomedical Press; 1980. p. 241–253.
- Bianchi DW, Wilkins-Haug LE, Enders AC, et al. Origin of extraembryonic mesoderm in experimental animals: relevance to chorionic mosaicism in humans. Am J Med Genet. 1993;46:542–550.
- Zore T, Kroener LL, Wang C, et al. Transfer of embryos with segmental mosaicism is associated with a significant reduction in live-birth rate. Fertil Steril. 2019;111:69–76.
- Girardi L, Serdarogullari M, Patassini C, et al. Incidence, origin, and predictive model for the detection and clinical management of segmental aneuploidies in human embryos. Am J Hum Genet. 2020;106:525–534.
- Treff NR, Franasiak JM. Detection of segmental aneuploidy and mosaicism in the human preimplantation embryo: technical considerations and limitations. Fertil Steril. 2017;107:27–31.
- Scott RT, Galliano D. The challenge of embryonic mosaicism in preimplantation genetic screening. Fertil Steril. 2016;105:1150–1152.
- Victor AR, Tyndall JC, Brake AJ, et al. One hundred mosaic embryos transferred prospectively in a single clinic: exploring when and why they result in healthy pregnancies. Fertil Steril. 2019;111:280–293.
- Fiorentino F. Pregnancy outcome of transferred embryos with mosaicism and segmental variations. Reprod Biomed Online. 2019;39:e9–e10.
- Lledó B, Morales R, Ortiz JA, et al. Implantation potential of mosaic embryos. Syst Biol Reprod Med. 2017;63:206–208.
- Chuang TH, Hsieh JY, Lee MJ, et al. Concordance between different trophectoderm biopsy sites and the inner cell mass of chromosomal composition measured with a next-generation sequencing platform. Mol Hum Reprod. 2018;24:593–601.
