Abstract
Objective
To investigate the effect of the ultrasonic energy during MRI-guided high-intensity focused ultrasound ablation (HIFU) of uterine fibroids on molecular and tissue markers of endometrial receptivity in women of reproductive age.
Material and methods
A prospective cohort study of 60 women of reproductive age was conducted. The main group consisted of 32 patients suffering from the symptomatic course of uterine fibroids who received treatment with HIFU ablation of uterine fibroids. The control group consisted of 28 healthy fertile women examined voluntarily. The endometrium obtained with pipelle biopsy on days 20–22 of the cycle was examined by scanning electron microscopy and immunohistochemistry before and three months after the treatment. The results were processed by the method of variation statistics using the SPSS 22.0.
Results
The focused ultrasound rays passing through the endometrium did not cause any change in the maturation rate or the state of intercellular contacts. At the same time, a significant increase in the frequency of asynchronous maturation of pinopodia was found to be 14.28% before HIFU versus 50.00% after HIFU; p = .021 and the number of heteromorphic secretory cells 5.88% before HIFU versus 53.33% after HIFU; p = .002 in implantation endometrium. A significant decrease in the stromal expression of CD95+bright in the endometrium to the level comparable with control values was observed after HIFU (from 70.22 ± 9.77 c/s to 48.81 ± 5.47 c/s; p < .001; the control level − 47.80 ± 2.13 c/s). The ratio and expression of steroid receptors, proliferation markers, p53-dependent apoptosis and its blockers, regulators and markers of angiogenesis, LIF and LIF-R signaling molecules in the stroma and endometrial glands did not change significantly after treatment.
Conclusion
This study did not reveal any significant negative effects of HIFU ablation of uterine fibroids on endometrial receptivity in women of reproductive age.
Introduction
Uterine fibroids are a clinically relevant problem due to their high incidence: about 25% of women older than 35 years have myomas and one-third of those patients have symptoms associated with fibroids [Citation1–2]. A combination of uterine fibroids and infertility occurs in 1.2–2.4% of women, while the mechanisms of the influence of uterine fibroids on fertility today remain a subject of discussion [Citation1–6].
The problem of choosing the therapeutic tactics of uterine fibroids in patients of reproductive age planning pregnancy is associated not only with a change in the functional state of the myometrium after treatment, but also with a corresponding change in the function of the endometrium, which plays a key role in the initiation of implantation and invasion of trophoblast [Citation6,Citation7].
In the presence of indications, myomectomy with careful layer-by-layer closure of the node bed is the first-line treatment method for patients with uterine fibroids planning a pregnancy [Citation1,Citation2,Citation8–10]. In this case, the risk of uterine rupture after laparoscopic myomectomy does not exceed 0.5–1% [Citation11–12]. At the same time, when planning pregnancy for women of late reproductive age who have a certain decrease in ovarian reserve, the question of recovery time after myomectomy sometimes acquires fundamental importance. In addition, a uterine scar after myomectomy can consider an indication for abdominal delivery during subsequent pregnancy, which may increase perinatal risks. In addition, there are certain risks of pregnancy with a scar tissue on the uterus [Citation2,Citation12].
These circumstances dictate the feasibility of a thorough study of alternative methods of preparing patients with uterine fibroids for pregnancy. As an alternative, there are conservative regression methods for the treatment of uterine fibroids, including magnetic resonance imaging guided high-intensity focused ultrasound (HIFU), which allows to accelerate pre pregnancy preparation and to avoid surgical intervention with scar formation on the uterus [Citation13–14].
The mechanisms of the influence of focused ultrasound rays passing through the endometrium during the procedure of HIFU ablation on the indicators of its receptivity are not fully understood, the results of individual studies are contradictory. In this regard, it seems relevant to study the functional activity of the endometrium after treatment using HIFU ablation of uterine fibroids.
The objective of this study is to investigate the effect of the ultrasonic energy during MRI-guided high-intensity focused ultrasound ablation (HIFU) of uterine fibroids on molecular and tissue markers of endometrial receptivity in women of reproductive age.
Material and methods
A prospective cohort study of 60 women of reproductive age was conducted.
The study was approved by the local ethics committee of the Ural Science Research Institute of Maternity and Child Care of the Ministry of Health of the Russian Federation. In accordance with the provisions of the Declaration of Helsinki by the World Medical Association of the last revision, informed consent was obtained from all patients and/or their legal representatives to participate in the study and use biological material before being included in the study.
The main group consisted of 32 patients suffering from the symptoms of uterine fibroids who received treatment with HIFU ablation of uterine fibroids. The control group consisted of 28 healthy fertile women who were examined on a voluntary basis, and who did not have a history of miscarriage and had a history of normal vaginal delivery after the physiological pregnancy.
The procedure of HIFU ablation was performed on the ExAblate-2000 (InSightec, Israel), combined into a single system with a 1.5 Tesla magnetic resonance imager (GeneralElectric, USA).
The endometrium obtained with pipelle biopsy on days 20–22 of the cycle was examined by scanning electron microscopy and immunohistochemistry before and three months after the treatment.
The obtained endometrial samples were evaluated using scanning electron microscopy. For research using a scanning electron microscope, a part of the material after dehydration was dried at the critical sublimation point of carbon dioxide and examined in a Hitachi-S350 scanning electron microscope, Japan.
For the immunohistochemical study, a two-stage streptavidin-biotin-peroxidase method was used with antigen unmasking using standard sets of monoclonal and polyclonal antibodies from Bond RTU Primary, USA and DAKO, Denmark. Using the Dako Cytomation imaging system, a reaction was performed. The Super Sensitive Polymer-HPR Detection System (BioGenex, USA) bezbiotin-free detection system was used to visualize primary antibodies.
The inclusion criteria for the study of main group were as follows: women in reproductive age (18-45 years) with symptomatic uterine fibroids (pain, infertility, dysfunction of the pelvic organs); II–V types of nodes according to the FIGO classification (2011); sizes of nodes from 4 to 9 cm; ovulatory cycle; informed voluntary consent to participate in the study signed by the patient. Criteria for exclusion in the study: hyperplastic processes of the endometrium in combination with uterine myoma; the acute inflammatory process of the genitals; oncological diseases; severe extragenital pathology; pregnancy; taking hormone therapy less than 3 months before inclusion in the study; contraindications for the use of HIFU ablation.
Exclusion criteria from the study: the onset of pregnancy; severe complications during the treatment of fibroids; malignant and atypical changes according to histological examination of endometrium; refusal of the patient from further participation in the study.
Statistical analysis of the results was carried out using the programs SPSS 16.0, SPSS: An IBM Company (USA) and Statistica 10.0, StatSoft (USA). The results of the study were presented as mean ± standard error of the mean (M ± m). The significance of differences in the results was determined using a paired or unpaired student t-test and Wilcoxon's test.
Differences between statistical values were considered statistically significant at a confidence level of p < .05.
Results
The average age of the patients did not significantly differ and amounted to 37.50 ± 1.33 years in the main group and 35.85 ± 1.43 years in the control group (p > .05).Patients did not have significant differences in anthropometric indices; average growth and weight parameters were observed. BMI in the main group was 24.64 ± 3.06 kg/cm2, in the control group 23.04 ± 1.86 kg/cm2; p > .05. The main clinical manifestations in patients of the main group were menometrorrhagia (40.63%) and pain (53.13%). The frequency of infertility as a clinical manifestation of uterine fibroids was 37.5%, where in primary infertility among women of the main group accounted for 15.63%, secondary infertility – for 21.87% of women with uterine fibroids.
A dynamic analysis of the uterine fibroids clinical manifestations after treatment with HIFU ablation showed a significant decrease in the incidence of pain from 53.13% to 15.63%; p = .002 and menorrhagia from 40.63% to 12.50%; p = .002.
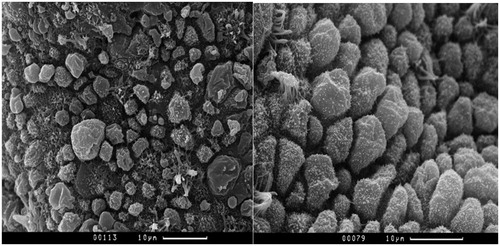
In the study of endometrial samples by scanning electron microscopy (SEM) in patients with uterine myoma by the dynamics of treatment use of HIFU ablation, a significant decrease in the frequency of maturing pinopodies was revealed − 68.75% cases, compared with the indicators before treatment in 93.75% cases, p = .024, due to the increase in the frequency of mature pinopodia after HIFU ablation and, as a consequence, the absence of significant differences in the frequency of mature pinopodia in endometrial samples after treatment of uterine fibroids compared with the control group: after treatment − 59.38%, against 71.42% in the control group, p > .05 (). This may indicate improvement of ultrastructural predictors of implantation during treatment. A higher frequency of detection of mature pinopodia was naturally accompanied by a change in the state of intercellular contacts, namely, a higher frequency of their separation.
Table 1. Ultrastructural morphological picture of the endometrium during the alleged ‘implantation window’ after treatment with HIFU ablation of uterine fibroids (20–22 of the menstrual cycle).
At the same time, after HIFU ablation in the endometrium, there was a significantly higher frequency of asynchronous maturation of pinopodia observed in 50.0% of observations compared with 14.28% of control group samples, and a significantly higher frequency of heteromorphic secretory cells in 28.13%, versus 0% in the control group (, ). This may indicate a certain influence of penetrating focused ultrasound on the functional state of individual endometrial cells and inhibition of intercellular interactions under the influence of ultrasonic wave energy.
Figure 1. The asynchronous maturation of pinopodies. Maturing pinopodes (MgP), mature pinopodes (MP) and areas of ‘calm’ epithelium (CE) without pinopodes are determined on the surface epithelium in the left hand side micrograph in patients of main group. The micrograph on the right hand side shows the uniform fields of mature pinopodia (MP) in patients of control group. Scanning electron microscopy of the endometrium.
A comparative analysis of the sex steroid receptors expression and their ratio in the endometrium in the dynamics of treatment using HIFU ablation of uterine fibroids revealed no significant differences ().
Table 2. The endometrial indicators dynamics after HIFU ablation of uterine fibroids (20–22 of the menstrual cycle; M ± m, p).
After treatment with HIFU ablation, a statistically significant decrease in the stromal expression of CD56bright + was observed in patients with uterine fibroids, to a level comparable with control values. There were no significant differences in the level of CD56bright + expression in the endometrial glands in the observation groups ().
There was a significant increase in the leukemia inhibitory factor (LIF) stromal expression in the endometrial glands to a level statistically comparable with control values after HIFU ablation of uterine fibroids (). There were no significant changes in the expression of receptor for LIF after treatment.
The expression of proliferation markers, p53-dependent apoptosis and its blockers in the stroma and endometrial glands did not significantly change after therapy with HIFU ().
At the same time, it should be noted that p53 expression indices after HIFU fibroids ablation remained significantly lower and bcl-2 on the contrary, were significantly higher than control values ().
The level of stromal expression of CD34+ in patients after HIFU uterine fibroids ablation did not significantly change, remaining significantly higher than the control values throughout the observation period ().
There were no significant changes in the expression of the angiogenesis regulators vascular endothelial growth factor A (VEGF A) & its receptor 1 (VEGF-R1) and their ratio VEGF-A/VEGF-R1 in endometrial samples after treatment. This ratio was significantly higher than the control values during the entire observation period, which demonstrates the pro-angiogenic potential of endometrial tissue in patients with uterine myoma ().
An increase in the level of CD56+brightcells in the endometrium in combination with activation of pathological neo-angiogenesis can increase the amount of bleeding in women with uterine myoma, which we observed in our study. Initially, prior to treatment, most patients with uterine myoma showed high expression of CD56+bright, CD34+and angiogenesis inducers (VEGF-A) in the endometrial stroma compared with fertile healthy women.
After HIFU, the expression of angiogenesis markers CD34+and VEGF-A in the stroma of the endometrium did not statistically significantly change, remaining at a high level. This explains the incomplete regression of complaints of menorrhagia in some patients aftertreatment using HIFU ablation.
Identified changes can be of great clinical significance. In previous studies [Citation15], we showed that HIFU ablation of uterine fibroids can be an effective procedure for preparing for pregnancy with the right choice of conditions and indications for its use.
At the same time, in our study, after the treatment of uterine fibroids with HIFU ablation, there was a statistically significant increase in the level of stromal and glandular expression of LIF receptor signaling molecules, which play a key role in the successful interaction of the blastocyst with endometrium and the adhesion of the latter on its surface.
Conclusion
The study did not reveal any significant changes in the functional state of the endometrium after HIFU ablation of uterine fibroids. The positive aspects of the influence of HIFU energy include the decrease in the excess stromal expression of uNK and an increase in glandular and stromal LIF expression in the endometrium. The HIFU ablation can be considered as the second line of treatment of uterinefibroids in women who planning pregnancy in case of the right choice of indications and conditions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Giuliani E, As-Sanie S, Marsh EE. Epidemiology and management of uterinefibroids. Int J Gynecol Obstet. 2020; 149(1):3–9.
- Adamyan LV, Andreeva EN, Artymchuk NV, et al. Uterine fibroids, diagnosis, treatment, recommendations. Moscow: Clinical recommendations, treatment protocol; 2015. (In Russian). [cited 2020 May 03]. Available from: https://aig-journal.ru/articles/Mioma-matki-diagnostika-lechenie-i-reabilitaciya-Klinicheskie-rekomendacii-protokol-lecheniya.html.
- Vlahos NF, Theodoridis TD, Partsinevelos GA. Myomas and adenomyosis: impact on reproductive outcome. Biomed Res Int. 2017; 2017:5926470.
- Parazzini F, Tozzi L, Bianchi S. Pregnancy outcome and uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2016;34:74–84.
- Kuznetsova IV, Evsyukova LV. Uterine fibroids and fertility. Gynecology. 2016;18(3):23–29. (In Russian).
- Dzhemlikhanova LK, Niauri DA, Abdulkadyrova ZK. Uterine fibroids and theeffectiveness of assisted reproductive technology programs. J Obstet Women’s Dis. 2016; 65(6):79–87. (In Russian).
- Carp HJA, editor. Recurrent pregnancy loss: causes, controversies, and treatment. 2nd ed. New York, NY: C.R.S.Press, Taylor & Francis Group; 2017.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016; 22(6):665–686.
- Wang Y, Zhang S, Li C, et al. Minimally invasive surgery for uterine fibroids. Ginekol Pol. 2020; 91(3):149–157.
- Metwally M, Raybould G, Cheong YC, et al. Surgical treatment of fibroidsfor subfertility. Cochrane Database Syst Rev. 2020;29(1):CD003857.
- Zaami S, Montanari Vergallo G, Malvasi A, et al. Uterine rupture during induced labor after myomectomy and risk of lawsuits. Eur Rev Med Pharmacol Sci. 2019; 23(4):1379–1381.
- Kundu S, Iwanuk C, Staboulidou I, et al. Morbidity, fertility and pregnancyoutcomes after myoma enucleation by laparoscopy versus laparotomy. Arch Gynecol Obstet. 2018; 297(4):969–976.
- Fan HJ, Zhang C, Lei HT, et al. Ultrasound-guided high-intensity focusedultrasound in the treatment of uterine fibroids. Medicine. 2019; 98(10):1–6.
- Nazarenko GI, Krasnova TV, Tonkogorova IV, et al. Assessment of efficacy and safety of HIFU-ablation in uterine myoma treatment considering nodes localization. Ultrasound Functional Diagnostics. 2016;1:29–39. (In Russian).
- Melkozerova OA, Shchedrina ID, Mikhel'son AA, et al. The effectiveness of focused ultrasound ablation of uterine fibroids under the control ofmagnetic resonance imaging in patients of reproductive age with a symptomatic course ofuterine fibroids. Ross Vestn Akush-Ginekol. 2019;19(5):52–60. (In Russian).
