Abstract
Objective
To evaluate serum concentration of 8-isoprostane, nitrotyrosine (NT), and total antioxidant capacity (TAC) in pregnant women with diabetes mellitus (DM) considering preconception planning and method of diabetes correction in 11–14 and 30–34 weeks.
Materials and methods
The study included 130 women: T1DM (n = 40), T2DM (n = 35), gestational diabetes (GDM, n = 40) and the control group (n = 15). The serum concentrations of NT, 8-isoprostane, and TAC were measured by ELISA methods.
Results
Elevated 8-isoprostane levels were observed in all patients with DM, but this biomarker's maximum values have been seen in T1DM and T2DM on insulin groups. A similar tendency was observed for the concentration of NT in both the 1st and 3rd trimesters. TAC levels showed a statistically relevant decrease in all DM groups compared to the control. The correlation analysis showed a direct correlation between HbA1c and serum 8-isoprostane levels in the 1st (r = .27) and 3rd (r = .3) pregnancy trimesters as well as inverse correlation with TAC level (r = −.48). Direct (NT, 8-isoprostane) and inverse correlations (TAC) were fixated for this biomarker concentration and preeclampsia rates.
Conclusion
DM in pregnancy is related to oxidative stress activation, which might lead to the development of adverse perinatal outcomes.
Introduction
Diabetes mellitus (DM) prevalence rate dramatically grows worldwide and has reached 4–6% of the general population according to the latest numbers [Citation1]. Consistently we see the growth of the number of pregnant women with different carbohydrate metabolism disorders. DM in course of pregnancy is associated with a high risk of adverse perinatal outcome: preeclampsia (PE), preterm birth, intrauterine growth restriction (IUGR), stillbirth, diabetic fetopathy, and fetal deficiencies [Citation1].
It is well known that in the course of pregnancy, we meet a variety of oxidative stress (OS) markers synthesis. These markers are important for embryo development, implantation, development, and functioning of the placenta [Citation2]. DM complicated pregnancy is related to higher OS expression due to excessive production of free radicals (peroxynitrite and nitrotyrosine), toxic products of phospholipid acidification (malondialdehyde (MDA), 8-isoprostane) and specific proteins and enzymes (asymmetric dimethylarginine (ADMA) and catalase) [Citation2]. Along with the pointed processes consistent reduction of antioxidant systems reactivity takes place. In addition, oxidative stress appears to be one of the mechanisms that are the basis of insulin resistance formation, and its expression is directly dependent on the hyperglycemia level [Citation2].
Hyperglycemia along with other DM associated factors enforces elaboration of advanced glycation end products (AGE), which interacts with proprioceptors (RAGE) stimulate reactive oxygen species (ROS) elaboration [Citation2]. Consequently, cell membrane damages occur, causing ROS additional synthesis, activation of subclinical inflammations, and strengthening the OS in case of DM. Further release of pro-inflammatory cytokines (TNF-α, IL-6, and IL-8), prostaglandins, and endothelial dysfunction mediators (endothelin-1, ADMA) leads to pro-oxidant and antioxidant systems balance disorder in the organism [Citation2].
Another dysfunction factor is the disturbance of endothelium-dependent vasodilation caused by nitrogen oxide (NO) excessive acidification [Citation3]. NO is not only responsible for vascular distention but participates in cell migration and growth processes, thrombocyte aggregation, monocyte and macrophage adhesion, and inflammation. NO and superoxide molecule (О2−) interaction peroxynitrite (ОNOO–) is formed being a powerful pro-oxidant. Its reaction with tyrosine amino-acid residue generates nitrotyrosine (NT). Hence nitrotyrosine appears to be an indicator of acid active form production in various clinical states and may be used as an OS activity marker [Citation3]. Peroxynitrite provides DNA damages that lead to cell dysfunction and apoptosis.
8-isoprostane (8-iso-PGF2α) is a prostaglandin F2 isomer obtained through cell membrane phospholipid peroxidation or low-density lipoproteins [Citation4]. 8-iso-PGF2α is considered a stable and sensitive biomarker of lipid peroxidation and its quantity is in direct ratio to the level of elaborated free radicals. The study showed a considerable increase of 8-iso-PGF2α in patients with type 1DM compared to the healthy group [Citation4].
As a counterbalance to the OS, there is an antioxidant system in the organism. The most important enzyme antioxidants are the superoxide dismutase, catalase, and glutathione peroxidase [Citation5]. Due to the cumulative activity of these enzymes, it is worth measuring the unified activity of all the antioxidants or the total antioxidant capacity level (TAC) instead of separate evaluation of each agent’s activity [Citation6]. This biomarker is used for measuring the antioxidant potential of all liquids of an organism. It was established that in diabetic pregnancy, the enzyme antioxidant defense system’s activity was disturbed [Citation5,Citation6].
Not only does DM itself influence the oxidative stress expression but also its compensation level and in a lesser degree the correction mode. Duration of glycemia condition, HbA1c level, and insulin therapy necessity are directly related to exceeded OS marker release and pregnancy outcome [Citation1].
Basing on the literature data, we can assume that OS biomarkers concentration is elevated in the plasma of pregnant women with poor controlled DM, meanwhile, the activity of the antioxidant system is decreased. Nevertheless, there is neither detailed analysis of serum 8-isoprostane, NT, and TAC expression considering of preconception planning and methods for the correction of diabetes, nor their assessment in different DM types during the gestation process.
Our study objective was to evaluate the serum levels of 8-isoprostane, nitrotyrosine, and total antioxidant capacity in pregnant women with different types of DM considering the DM correction mode and preconception planning in 11–14 and 30–34 weeks of pregnancy. The obtained data were compared to the mentioned biomarkers expression in healthy pregnant women in the same weeks of gestation.
Materials and methods
It was a prospective cohort study. The recruiting was done at D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductive Medicine, Saint Petersburg, Russia. The appropriate informed consent was obtained from each patient. The local ethical committee approved the study design and other statures.
130 pregnant women were divided into 7 groups of comparison:
T1DM
Group I – women without preconception planning, HbA1c > 6.5% (n = 20);
Group II – women underwent preconception planning not later than 6 months before conception, HbA1c < 6.5% (n = 20);
T2DM
Group III – diet (n = 15);
Group IV – insulin (n = 20);
GDM
Group V – diet (n = 20);
Group VI – insulin (n = 20);
Control group (CG)VII – (n = 15)
The diagnosis of gestational diabetes mellitus set up according to the International Association of Diabetes and Pregnancy Study Groups (IADPSG) thresholds, and preeclampsia – according to the definition of the International Society for the Study of Hypertension in Pregnancy (ISSHP).
The level of glycosylated hemoglobin (HbA1c) was evaluated by Bio-Rad D-10 (Bio-Rad, Hercules, CA). The levels of nitrotyrosine, 8-isoprostane, and blood plasma total antioxidant capacity were measured twice: in weeks 11–14 and 30–34. Nitrotyrosine levels (pmol/ml) in plasma were measured by enzyme-linked immunosorbent assay (ELISA) using Hbt Nitrotyrosine test systems (Hycult Biotech, Netherlands). 8-isoprostane(pg/ml) in peripheral serum was evaluated by 8-Isoprostane (Cayman Chemical, Ann Arbor, MI). The blood plasma total antioxidant capacity (mM) was evaluated by Antioxidant Assay Kit (Cayman Chemical).
Statistical analysis
The statistical data processing was performed by SPSS V. 23.0 and Prism 8-GraphPad (San Diego, CA). The sample distribution parameters were evaluated by Kolmogorov–Smirnov test. The one-way analysis of variance (ANOVA) with 95% confidence interval was applied to determine the statistically relevant difference of normally distributed data quantity parameters. For non-parametric data distribution, the Kruskal–Wallis, median, and Dunn tests were applied. The statistical processing of qualitative indicators was performed by applying the χ2criterion. For the correlational analysis, Spearman’s rank correlation coefficient was applied. The median value equality hypothesis was negated in the case of p < .05 value level.
Results
The characteristics of the studied groups are presented in . Women’s average age in T2DM and GDM groups was higher than in the control (p = .01). The same tendency was observed concerning women’s BMI (p = .001). Children born to mothers with T1DM and GDM had the biggest weight (). Women with T1DM (65%) and the control group (60%) were nulliparous, while those with T2DM and GDM were predominantly multiparous. Diabetic vasculopathy was detected in the majority of women with T1DM. Every third patient with T1DM from the I group had diabetic nephropathy (35%). The highest HbA1c level was observed in groups I and IV, which indicated serious metabolic disorders in these women.
Table 1. Clinical characteristics for study groups.
Such pregnancy complications as gestational arterial hypertension (GH) and PE were more frequently spotted in patients with various types of DM. Every fourth woman (25%) of the I group had moderate preeclampsia, and 15% had a severe form of PE with early-onset (). 6 patients (30%) of II group had only moderate preeclampsia with late-onset. PE was detected in half of the patients with T2DM on insulin: 6 (30%) had moderate form and 4 (20%) – severe signs. In 10% of cases, it was an early-onset of PE and 40% – the late-onset. Only 2 patients of GDM-d (10%) had moderate preeclampsia in late pregnancy. In the GDM group on insulin the preeclampsia cases had late-onset; 4 patients (20%) had moderate form and 10% the severe form. No preeclampsia cases were registered in the control group. High frequency of preterm delivery was observed in DM groups, while all the deliveries in the control group were at term ().
Fetal macrosomia was more frequent in patients with DM compared to the control (p = .043): T1DM – 27.5%, T2DM – 3 (8.6%), GDM – 11 (27.5%). Among all the studied patients, only one case of IUGR was observed in a woman with GDM on insulin therapy (p = .04). A tendency of small-for-gestational-age fetuses (SGA) was more often seen in patients with various DM types, while no SGA cases were detected in control (р = .049).
The studied biomarkers evaluation showed a statistically significant difference between the group of pregnant women with DM and the control (; ). Nitrotyrosine values in the first pregnancy trimester were higher in I (16.8 pmol/ml) and IV (13.0 pmol/ml) groups. This tendency was observed for the third pregnancy trimester in all groups of patients with DM compared to the control (p < .05). Exceeded levels of 8-isoprostane were seen in the 1st and 3rd pregnancy trimesters in patients with DM (p < .05). The maximal value of this marker was spotted in patients of I group (1st trimester – 645.2 pg/ml; 3rd – 625.1 pg/ml), which authentically differed from indexes of the group: III – 392.9 pg/ml, and V – 383.7 pg/ml in the 3rd trimester respectively (p = .001). TAC levels manifested relevant reduction in all groups with DM compared to the control, both in the 1st and 3rd pregnancy trimesters (; ). Our study did not discover any authentic difference of these marker levels in the 1st and 3rd pregnancy trimesters.
Figure 1. Maternal serum levels of NT, 8-iso-PGF2α, TAC in 11–14 and 30–34 weeks in the study groups.
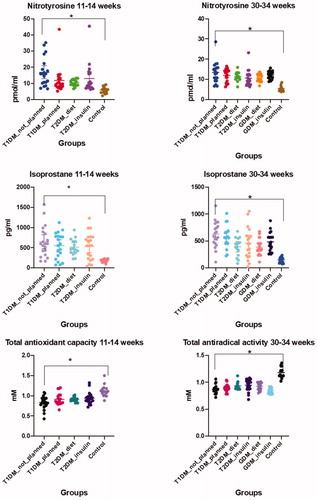
Table 2. Expression of nitrotyrosine, 8-isoprostane, and TAC and correlation analysis in the study groups.
Correlation analysis showed an inverse correlation with TAC (r = −.48, p = .0001), and a direct correlation between 8-isoprostan level and HbA1c in the 1st (r = .27, p = .013) and 3rd (r = .3, p = .01) pregnancy trimesters. Direct (NT, 8-Isoprostane) and an inverse correlation was established for these biomarkers and rate of preeclampsia ().
Discussion
Our data show that DM is associated with the activation of oxidative stress processes. The degree of OS occurrence is directly dependent on metabolic control before conception as well as in the first period of pregnancy.
8-isoprostane is elaborated during cell membrane phospholipid peroxidation and appears to be the most sensitive and specific oxidative stress marker [Citation2]. The increase of the 8-isoprostane level was observed in all diabetic groups in the 1st and 3rd pregnancy trimesters. Remarkably, a higher level of this biomarker was spotted in T1DM patients without preconception planning. Our data correspond to some studies which established 8-iso-PGF2a increase in the case of any DM in pregnancy. Răchişan et al. [Citation4] showed a considerable 8-iso-PGF2a increase in patients with T1DM compared to the control group. It was also established that in patients with good glycemic control, the concentration of 8-iso-PGF2a decreased alongside with HbA1c level [Citation4]. Coughlan et al. [Citation7] established that 8-isoprostane placental synthesis correlates with the degree of glycemic control, and it was by several folds greater in women with GDM. Similarly, Lappas et al. [Citation2] showed that 8-isoprostane expression of an embryo and maternal tissues increased in GDM cases. Shang et al. [Citation8] confirmed that the concentration and activity of oxidative stress markers (8-iso-PGF2a, MDA) in maternal blood also increased in GDM cases. In addition, Li et al. [Citation9] detected not only plasma level increase of 8-isoprostane and other markers in pregnant women with GDM but also consistent concentration growth of the pointed markers along the trimesters. Rueangdetnarong et al. showed that 8-iso-PGF2a, TNF-a, and IL-10 plasma concentration was excessive in the GDM group. Interestingly, good glycemic control achievement in these patients did not decrease OS degree [Citation10].
We established that nitrotyrosine levels also increased in the 1st and 3rd pregnancy trimesters in women with DM. Szabó et al. [Citation11] showed a nitrotyrosine immune reactivity increase in patients with T2DM. The considerable correlation was also seen between nitrotyrosine immunostaining intensity and glucose blood fasting level, HbA1c, ICAM, and VCAM adhesion molecules [Citation11]. Horvath et al. [Citation3] showed that exceeded nitrosative stress and consequent cell damage play an important part in complications of diabetes and pregnancy. It was established that nitrotyrosine level in maternal plasma in 16–29 and 36–40 weeks, cord blood, and placenta correlated with gestation terms and was higher in women with GDM requiring insulin therapy. Gelisgen et al. [Citation12] also detected exceeded levels of nitrotyrosine in blood plasma and of other oxidative stress markers in pregnant women with GDM, while the activity of other antioxidant enzymes declined.
Our results manifest a significant decline of TAC in pregnant women with various types of DM, which witnessed for antioxidant systems decompensation in long term hyperglycemia. Toescu et al. [Citation1] established that the TAC index in pregnant women with T1DM, T2DM, and GDM was considerably lower in each pregnancy trimester than in the control group. Also, Shang et al. [Citation8] detected a relative decrease of antioxidant systems (SOD, GPX) and TAC in the placenta and plasma of 68 women with carbohydrate metabolism disorder. According to Ozler et al. [Citation5], who evaluated TNF-a and TAC levels in patients with GDM depending on correction mode (insulin or diet), both indexes proved their prognostic significance. TAC decrease was detected in the GDM group and appeared to be an independent predictor of insulin therapy necessity. Zygula et al. [Citation13] in a study embracing 89 pregnant women (59 – GDM, 30 – control) showed that the TAC level authentically decreased not only in plasma but also in saliva in patients with pregnancy diabetes while other oxidative stress markers manifest exceeded levels. It was also established that TAC levels in cases of diet corrected GDM were higher than in patients with insulin correction, which witnesses a direct relation between adequate glycemic control and the biomarker expression level.
Contrary data were obtained by Usluogullari et al. [Citation14] who detected higher values of TAC in plasma in women with GDM. Zamani-Ahari et al. [Citation6] evaluated saliva TAC in women with GDM and pregnant women without diabetes. They showed that saliva TAC levels were much higher in the GDM group. A similar study by Surdacka et al. [Citation15] also demonstrated exceeded TAC levels in saliva of patients with GDM.
The strong point of our study was one that involved patients with all types of DM. Separately the degree of glycemic control and the correction mode for DM were evaluated. The study was performed in dynamics and considered oxidative stress marker levels in the first and third pregnancy trimesters.
The limitations of the present study mainly lie in a relatively small sample number. Apart from that, DM is a multifactorial disease, and its etiology embraces a variety of factors, including genetics, nutrition, and environment. The evaluated indexes may also be affected by other factors, such as smoking, pregnant woman’s hormonal profile, concomitant diseases, psychic distress level, etc. Hence further investigations are necessary with a more significant sample number and combined risk factors evaluation.
Conclusion
DM in pregnancy is directly related to exceeded OS. Our results manifest that levels of 8-isoprostane, nitrotyrosine, and TAC in diabetic pregnancy may be applied as an index for oxidative stress degree. These biomarkers might have a good prognostic value in terms of assessment of pregnancy risks. We can assume that exceeded oxidative stress may be relevant to adverse perinatal outcomes as well as the pathogenesis of DM and its complications.
Ethical approval
The local ethics committee approved the study.
Acknowledgments
We thank all patients who participated in this study and the staff who assisted with sample collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Toescu V, Nuttall SL, Martin U, et al. Changes in plasma lipids and markers of oxidative stress in normal pregnancy and pregnancies complicated by diabetes. Clin Sci. 2004;106(1):93–98.
- Lappas M, Permezel M, Rice GE. Release of proinflammatory cytokines and 8-isoprostane from placenta, adipose tissue, and skeletal muscle from normal pregnant women and women with gestational diabetes mellitus. J Clin Endocrinol Metab. 2004;89(11):5627–5633.
- Horvath EM, Magenheim R, Kugler E, et al. Nitrative stress and poly(ADP-ribose) polymerase activation in healthy and gestational diabetic pregnancies. Diabetologia. 2009;52(9):1935–1943.
- Răchişan AL, Hruşcă A, Căinap S, et al. The activity of 8-iso-prostaglandin F2alpha as an oxidative stress marker in vivo in paediatric patients with type 1 diabetes mellitus and associated autoimmunities. Clin Lab. 2014;60(2):253–259.
- Ozler S, Oztas E, Uygur D, et al. The value of total antioxidant status and serum tumor necrosis factor-α levels at 24-28 weeks of gestation in the prediction of optimal treatment protocol in gestational diabetes mellitus. Exp Clin Endocrinol Diabetes. 2019;127(7):485–491.
- Zamani-Ahari U, Zamani-Ahari S, Fardi-Azar Z, et al. Comparison of total antioxidant capacity of saliva in women with gestational diabetes mellitus and non-diabetic pregnant women. J Clin Exp Dent. 2017;9(11):e1282–e1286.
- Coughlan MT, Vervaart PP, Permezel M, et al. Altered placental oxidative stress status in gestational diabetes mellitus. Placenta. 2004;25(1):78–84.
- Shang M, Zhao J, Yang L, et al. Oxidative stress and antioxidant status in women with gestational diabetes mellitus diagnosed by IADPSG criteria. Diabetes research and clinical practice. 2015;109(2):404–410.
- Li H, Yin Q, Li N, et al. Plasma markers of oxidative stress in patients with gestational diabetes mellitus in the second and third trimester. Obstet Gynecol Int. 2016;2016:3865454.
- Rueangdetnarong H, Sekararithi R, Jaiwongkam T, et al. Comparisons of the oxidative stress biomarkers levels in gestational diabetes mellitus (GDM) and non-GDM among Thai population: cohort study. Endocr Connect. 2018;7(5):681–687.
- Szabó C, Zanchi A, Komjáti K, et al. Poly(ADP-Ribose) polymerase is activated in subjects at risk of developing type 2 diabetes and is associated with impaired vascular reactivity. Circulation. 2002;106(21):2680–2686.
- Gelisgen R, Genc H, Kayali R, et al. Protein oxidation markers in women with and without gestational diabetes mellitus: a possible relation with paraoxonase activity. Diabetes Res Clin Pract. 2011;94(3):404–409.
- Zygula A, Kosinski P, Zwierzchowska A, et al. Oxidative stress markers in saliva and plasma differ between diet-controlled and insulin-controlled gestational diabetes mellitus. Diabetes Res Clin Pract. 2019;148:72–80.
- Usluogullari B, Usluogullari CA, Balkan F, et al. Role of serum levels of irisin and oxidative stress markers in pregnant women with and without gestational diabetes. Gynecol Endocrinol. 2017;33(5):405–407.
- Surdacka A, Ciężka E, Pioruńska-Stolzmann M, et al. Relation of salivary antioxidant status and cytokine levels to clinical parameters of oral health in pregnant women with diabetes. Arch Oral Biol. 2011;56(5):428–436.
