Abstract
Object
To evaluate the efficacy of dydrogesterone for the treatment of premenopausal patients with endometrial polyps (EPs).
Methods
A single-center, open-label, prospective, single-arm clinical treatment trial was conducted in patients of reproductive age with EP(s). Patients were prescribed dydrogesterone from day 15 to day 24 of the menstrual cycle over a period of 3 months. At the 3-month follow-up, the efficacy of dydrogesterone was evaluated based on changes in self-report symptoms and ultrasonographic characteristics. The predictive factors of efficacy as well as the predictive value of the significant factors were also assessed.
Results
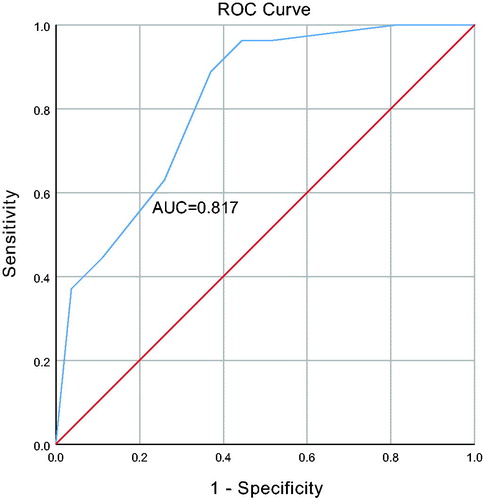
A total of 60 patients were included. Improvements in both symptoms and ultrasound findings occurred in 31 patients, achieving an efficacy rate of 51.67%. Of 41 patients with clinical presentations, 39 (95.1%) reported improvements in symptoms. In terms of ultrasound findings, 33 (55%) of patients demonstrated improvements. Significant decreases were observed in the mean endometrial thickness (1.17 ± 0.33 cm vs 0.90 ± 0.35 cm, p < .001) and polyp size (1.10 ± 0.34 cm vs 0.74 ± 0.65 cm, p = .001) after the application of dydrogesterone. Age (p = .006), polyp size (p = .006), and blood flow within polyps (p = .035) were significant predictors of dydrogesterone efficacy. These factors, when combined, demonstrated a good predictive value ([area under the curve (AUC)=0.81]).
Conclusion
Dydrogesterone is effective in the management of EPs in premenopausal patients. Age, polyp size and blood flow should be taken into consideration when prescribing dydrogesterone for this population of women.
摘要
目的:评价地屈孕酮治疗绝经前患者子宫内膜息肉的疗效。
方法:在患有EP(s)的育龄患者中进行一项单中心, 开放标签, 前瞻性, 单臂临床治疗试验。患者从月经周期的第15天到第24天服用地屈孕酮, 治疗3个月。在3个月的随访中, 根据症状的主诉和超声特征的变化来评估地屈孕酮的疗效。并对疗效预测因素和重要因素的预测值进行了评估。
结果:研究一共纳入了60名患者。其中31例患者的症状和超声检查结果均有改善, 有效率达51.67%。在41名有临床表现的患者中, 39名(95.1%)报告症状改善。就超声检查结果而言, 有33(55%)名患者表现出改善。应用地屈孕酮后, 平均子宫内膜厚度(1.17±0.33cm vs 0.90±0.35cm, p < .001)和息肉大小(1.10±0.34 cm vs 0.74±0.65cm, p = .001)显著降低。年龄(p= 0.006)、息肉大小(p= 0.006)和息肉内血流(p =.035)是地屈孕酮疗效的重要预测因子。这些因素结合起来, 显示出良好的预测值([曲线下面积(AUC) 0.81])。
结论:地屈孕酮对绝经前患者的EPs治疗有效。在为这部分妇女开地屈孕酮时, 应考虑年龄、息肉大小和血流情况。
Introduction
Endometrial polyps (EPs) refer to the localized overgrowth of endometrial tissue consisting of stroma, glands, and potentially blood vessels [Citation1]. Sessile or pedunculated, it is a frequently encountered disorder in gynecology clinics, with a prevalence ranging from 7.8% to 34.9% depending on the population [Citation2].The incidence of EPs is rising in part due to rapid developments in ultrasound technology.
The precise molecular mechanisms underlying this common disease remain unclear. Published studies have proposed several different hypotheses, such as gene mutation, overexpression of endometrial aromatase, and monoclonal endometrial hyperplasia, all of which need further confirmation [Citation3].
Hysteroscopic resection has long been considered the ‘gold standard’ for the treatment of Eps [Citation4]. Nevertheless, medical treatment may be an alternative choice, especially for premenopausal patients who have concerns about the risks of surgical intervention yet do not wish to be relegated to the ‘wait and see’ category of patients. Progesterone has been found to promote cellular apoptosis of the endometrial tissue[Citation5], and certain progesterone-based agents may offer promising options for treating EPs. However, few studies have focused on the cost-saving strategy of this therapeutic option in the management of EPs.
Dydrogesterone (6-dehydro-retroprogesterone) is a selective progesterone receptor agonist with excellent oral bioavailability and potent progestogenic activity, while having no androgenic, glucocorticoid or estrogenic activity, making it a good option for progestin therapy[Citation6]. In this single-arm study, we aimed to assess the efficacy of dydrogesterone for premenopausal patients with EPs as well as, identify factors that may impact treatment efficacy.
Materials and methods
Study design and patient characteristics
This was a prospective, single-center, open-label, single-arm study of premenopausal patients with a diagnosis of EPs on transvaginal ultrasonography (TVUS) who were treated in the clinic at the Peking Union Medical College Hospital from February 2019 to September 2019. The study is registered on ClinicalTrials.gov as NCT 03790215.
The work flow is illustrated in . Inclusion criteria were: (1) premenopausal women aged from 20 to 50 years old; (2) with or without the following menstrual changes: (a) menostaxis (longer than 7 days); (b) menorrhagia(more than twice as much as usual); (c) intermenstrual bleeding; (3) with one of the following signs on TVUS on day 10 of a menstrual cycle: (a) typical signs consistent with EP: (median/high) echo with a regular contour within the uterine lumen [Citation7]; (b) atypical signs consistent with EP: punctate cystic areas within the endometrium [Citation7] and an endometrial thickness >1 cm; (4) not allergic to progesterone hormone drugs.
Exclusion criteria were: (1) no menses within half a year; (2) surgery or drug treatment of endometrial lesions in the past half year; (3) current intrauterine device; (4) use of hormone drugs in the recent half year; (5) acute gynecological inflammation; (6) clinically suspected/diagnosed malignant tumors or a history of tumors; (7) abnormal liver and kidney function; (8) benign breast tumors; (9) pregnant participants.
Demographic and clinical information were obtained from all patients including age, parity, gravidity, delivery mode, presence and types of symptoms, history of endometrial polypectomy and ultrasonographic characteristics.
Participants enrolled were administered dydrogesterone (Duphaston, Abbott Healthcare Products B.V., Weesp, the Netherlands) tablet of 10 mg twice a day, from day 15 to day 24 of the menstrual cycle over a period of 3 months.
Outcome assessments
The primary endpoints of the study were collected after 3 months of therapy (also on day 10), when the efficacy of dydrogesterone was evaluated based on improved symptoms and ultrasound findings. To avoid the inter-observer variability, the same person performed the ultrasound at start of treatment and after 3 months. An improvement in symptoms was defined as the disappearance of symptoms. Improvements in ultrasound findings were defined as ≥50% reduction in polyp size or a decrease in the number of polys (for polyps with typical signs) and an endometrial thickness < 1 cm (for patients with atypical findings). The longest diameter of the polyp was used to determine polyp size.
To identify factors which may influence treatment efficacy, we divided the cohort into two groups based on the response to the treatment, an effective treatment group and ineffective treatment group.
Safety assessments
The incidence of drug-related adverse events (AEs) throughout the study was also evaluated based on patient self-reports.
Statistical analysis
Statistical analyses were conducted using SPSS (version 26.0; SPSS Inc, Chicago, IL). Intergroup comparisons were performed using the Mann-Whitney U test or Student t test for continuous variables and chi-square test/Fisher’s test for categorical variables. Cox regression models were used to perform univariate and multivariate analyses. Receiver–operating characteristics (ROC) curves were performed (plot of sensitivity vs. 1 - specificity) with the estimated areas under the curves (AUCs). A p value <.05 was considered significant.
Results
Patient characteristics
Details of the included cohort are shown in Supplementary Table 1. A total of 60 patients were included in the present study, with a mean age of 35.9 ± 6.4 years. With respect to symptoms, intermenstrual bleeding and menostaxis were two major manifestations with similar rates of occurrence, 33.3% (20/60) and 26.7% (16/60), respectively. Asymptomatic patients accounted for 31.7% (19/60), all of whom were diagnosed during regular physical examinations. Totally, 48 patients presented with typical features of EPs with a mean size of 1.1 ± 0.34 cm, while the remaining 12 patients presented with punctate cystic areas.
Changes after administration of dydrogesterone
Changes in symptoms
When we focused on changes in symptoms among patients who were symptomatic before the treatment, 95.1% (39/41) of the included patients reported improvements. Two patients who experienced persisted menostaxis after treatment underwent surgery.
Changes in ultrasound findings
In total, 55.0% (33/60) of patients showed improvements in ultrasound findings based on the criteria mentioned above.
The mean endometrial thickness decreased significantly (1.17 ± 0.33 cm vs 0.90 ± 0.35 cm, p < .001) compared to baseline, with a mean change of −0.27 ± 0.41 cm. In our study cohort, 46 patients presented with decreased endometrial thickness, 6 with no changes and 8 with increased endometrial thickness ().
Table 1. Changes in signs on transvaginal ultrasonography at follow-up.
Similarly, we observed a significant reduction in mean polyp size (1.10 ± 0.34 cm vs 0.74 ± 0.65 cm, p = .001) compared to baseline, with a mean change of −0.36 ± 0.58 cm. Of the 48 patients with typical findings, 31 ultimately showed a decrease in the size of polyps (with 15 of the patients showing complete regression), 3 patients showed no changes, and 14 patients had an increase in size ().
With regard to the number of EPs, 21 patients had fewer polyps, 26 experienced no changes, and one patient showed increase in the number of polys.
Comprehensive outcomes
Improvements in both symptoms and ultrasound findings occurred in 31 patients, achieving an efficacy rate of 51.67%. Of the remaining 29 patients, 2 patients reported no improvements in symptoms, and there were no improvements in ultrasound findings in 27 patients.
Comparative analysis
We further investigated factors that may have impacted treatment efficacy. As illustrated in , patients in the effective treatment group were much younger than those in the ineffective treatment group (32.00[28.00–39.00] years vs 39.00[35.00–42.50] years, p = .007). Significant differences also existed between the effective and ineffective treatment groups with respect to parity (p = .029), polyp size (p = .019), and the presence of blood flow within the polyps (p = .036).
Table 2. Comparison of patient characteristics between groups.
presents the univariate and multivariate logistic regression models that were used to evaluate predictive factors for the efficacy of dydrogesterone. In the univariate analysis, potential predictive factors included were age, gravidity, parity, number of endometrial polyps, polyp size, endometrial thickness, echoes, blood flow within the polyps, symptoms, and a previous history polypectomy. In this analysis, age (p = .006), gravidity (p = .035), parity (p = .032), polyp size (p = .004) and blood flow (p = .027) were significantly associated with the efficacy of dydrogesterone.
Table 3. Risk factors for efficacy of dydrogesterone after univariate and multivariate analysis.
Subsequent multivariate analysis indicated that age (HR 7.618, 95% CI 1.781–32.581; p = .006), polyp size (HR 7.101, 95% CI 1.744–28.916; p = .006), and blood flow (HR 5.902, 95% CI 1.128–30.874; p = .035) were significant predictors of dydrogesterone efficacy after adjusting for other factors. When combined, this factors demonstrated a good predictive value (AUC = 0.81) for the efficacy of this drug ().
Safety assessments
No adverse events were identified based on the patient self-reports at the follow-up visits.
Discussion
In our single-arm study, we administered dydrogesterone for the first time to patients with EPs and obtained relatively satisfying results, with an effective rate of 51.67% based on improvements in symptoms and ultrasound findings. Age, polyp size and blood flow within polyps were found to be independent predictive factors for dydrogesterone efficacy.
The high prevalence of asymptomatic patients, low risk of malignant transformation, and possibility of spontaneous regression of EPs suggest that regular polypectomy may be not the best choice for some premenopausal patients. To some extent, polypectomy may even be considered as overtreatment for this cohort, and may be considered a therapeutic approach with high healthcare costs that also carries with it the risks associated with anesthesia and surgery [Citation3]. A multicenter, prospective observational trial reported a rate of 2.27% for surgical complications in patients treated with hysteroscopic polypectomy [Citation8].
Studies have investigated the role of estrogen and progesterone in the development of polyps because these hormones are important regulators that maintain the balance between proliferation and apoptosis in normal endometrium, but no consensus has been reached [Citation9–11]. Recently, Feng et al. reported that progesterone increases H19 expression, thus upregulating Wnt1 and downregulating miR‐152 in a dose‐dependent manner [Citation12]. The suppression of Wnt signaling results in downregulation of Bcl‐2, a protein family known for its role in apoptosis [Citation13]. The authors proposed that the administration of progesterone could be helpful in treating the EPs by promoting apoptosis [Citation12].
Medical-based treatment of EPs is still in the research stage. Roberta and his colleagues investigated the efficacy of three cycles of subcutaneous progesterone administered during the luteal phase in reproductive-aged women with endometrial polyps. A regression rate of 47.5% was observed, which was significantly higher than that seen in the ‘wait and see’ group [Citation14]. Oguz et al. evaluated three different protocols of hormone replacement therapy on EP formation and observed that progestogen played an important preventive role [Citation15]. A randomized trial reported the efficacy of a levonorgestrel releasing intrauterine device in reducing the incidence of tamoxifen-induced endometrial polyps [Citation16]. Several studies have also suggested that post-hysteroscopic progesterone hormone therapy is effective in preventing the recurrence of Eps [Citation17].
In our study, patients complained of AUB accounted for 65%, implying the treatment was associated with a negative impact on quality of life. However, 95.1% (39/41) of the symptomatic patients reported an improvement in their symptoms. In another randomized clinical trial, symptomatic improvement was observed in 93.3% (7/75) of patients in the polypectomy group and 37.3% (28/75) of patients in the observation group [Citation18].
With regard to ultrasound findings, 55% (33/60) achieved success based on the criteria set in our study. We observed a significant decrease in the median endometrial thickness and polyp diameter after the administration of dydrogesterone. It is worth mentioning that all TVUSs were performed in the proliferative phase of the menstrual cycle, which is likely to provide the most reliable results [Citation19]. Another study focusing on the efficacy of post-hysteroscopic progesterone hormone therapy also reported a significant reduction in endometrial thickness [Citation20].
Age, polyp size and blood flow within polyps were identified as independent predictive factors for dydrogesterone efficacy, and when combined, demonstrated a good predictive value for the efficacy of this drug. These three parameters may offer valuable tools for evaluating patients who choose medical management. This type of analysis is rare among published studies. Gu and his group reported that the number of endometrial polyps, the presence of endometriosis, and a history of previous polypectomy were independent risk factors for recurrent Eps [Citation21].
One possible explanation for the lack of studies evaluating hormone therapy for the treatment of EPs may be the concerns surrounding side effects. However, when compared with other progestin agents, dydrogesterone has not been associated with any unwanted metabolic side effects due to the absence of estrogenic, androgenic and glucocorticoid activity [Citation6]. The various uses of dydrogesterone in published studies have supported its safety profile [Citation22]. In our study, the participants reported no side effect, emphasizing the safety of dydrogesterone in clinical practice.
The relatively small sample size is a limitation of our study. Furthermore, we chose TVUS instead of hysteroscopy and guided biopsy as a screening tool because of the noninvasive nature of this standard diagnostic method. It is also a limitation of our study that histologic confirmation of EP was not obtained prior to treatment, however, for obvious reasons, this type of information would be difficult to obtain in routine clinical practice.
Conclusion
Our present study indicates that dydrogesterone is effective in the management of EPs in premenopausal women. Age, polyp size and blood flow within polyps should be taken into consideration when prescribing dydrogesterone in this population of women. Large, multicenter, randomized controlled trials with long-term follow-up are needed to further evaluate the efficacy of dydrogesterone for the treatment of EPs.
Informed consent
Written informed consents were obtained from all participants.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by Ethics Committee of Peking Union Medical College Hospital, Beijing, China.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in connieluyinxi/EP at https://github.com/connieluyinxi/EP.
Additional information
Funding
References
- Kim KR, Peng R, Ro JY, et al. A diagnostically useful histopathologic feature of endometrial polyp: the long axis of endometrial glands arranged parallel to surface epithelium. Am J Surg Pathol. 2004;28(8):1057–1062.
- Kanthi JM, Remadevi C, Sumathy S, et al. Clinical study of endometrial polyp and role of diagnostic hysteroscopy and blind avulsion of polyp. J Clin Diagn Res. 2016;10(6):QC01–QC04.
- Tanos V, Berry KE, Seikkula J, et al. The management of polyps in female reproductive organs. Int J Surg. 2017;43:7–16.
- Nappi C. ADSS. State-of-the-art hysteroscopic approaches to pathologies of the genital tract. Tuttlingen (Germany): Endo Press; 2014.
- Xie YL, Yang YJ, Tang C, et al. Estrogen combined with progesterone decreases cell proliferation and inhibits the expression of Bcl-2 via microRNA let-7a and miR-34b in ovarian cancer cells. Clin Transl Oncol. 2014;16(10):898–905.
- Schindler AE. Dydrogesterone-a unique progestogen. Maturitas. 2009;65(Suppl 1):S1.
- Schorge JS, Schaffer JI, Halvorson LM, et al. Abnormal uterine bleeding. In: Schorge JO, Schaffer JI, Halvorson LM, Hoffman BL, Bradshaw KD, Cunningham FG, editor. Williams gynecology. Columbus: McGraw-Hill Professional; 2008.
- Luerti M, Vitagliano A, Di Spiezio Sardo A, et al. Effectiveness of hysteroscopic techniques for endometrial polyp removal: the Italian multicenter trial. J Minim Invasive Gynecol. 2019;26(6):1169–1176.
- Sant’Ana de Almeida EC, Nogueira AA, Candido dos Reis FJ, et al. Immunohistochemical expression of estrogen and progesterone receptors in endometrial polyps and adjacent endometrium in postmenopausal women. Maturitas. 2004;49(3):229–233.
- Lopes RG, Baracat EC, de Albuquerque Neto LC, et al. Analysis of estrogen- and progesterone-receptor expression in endometrial polyps. J Minim Invasive Gynecol. 2007;14(3):300–303.
- Zitao L, Kuokkanen S, Pal L. Steroid hormone receptor profile of premenopausal endometrial polyps. Reprod Sci. 2010;17(4):377–383.
- Feng M, Zhang T, Ma H. Progesterone ameliorates the endometrial polyp by modulating the signaling pathway of Wnt and β-catenin via regulating the expression of H19 and miR-152. J Cell Biochem. 2019;120(6):10164–10174.
- Knight T, Luedtke D, Edwards H, et al. A delicate balance - the BCL-2 family and its role in apoptosis, oncogenesis, and cancer therapeutics. Biochem Pharmacol. 2019;162:250–261.
- Venturella R, Miele G, Cefali K, et al. Subcutaneous progesterone for endometrial polyps in premenopausal women: a preliminary retrospective analysis. J Minim Invasive Gynecol. 2019;26(1):143–147.
- Oguz S, Sargin A, Kelekci S, et al. The role of hormone replacement therapy in endometrial polyp formation. Maturitas. 2005;50(3):231–236.
- Gardner FJ, Konje JC, Bell SC, et al. Prevention of tamoxifen induced endometrial polyps using a levonorgestrel releasing intrauterine system long-term follow-up of a randomised control trial. Gynecol Oncol. 2009;114(3):452–456.
- Hua W, Zou W, Liu DD, et al. Effective observation on different kinds of drug for preventing recurrence after hysteroscopic resection of endometrial polyp. Mod Med Health Care. 2013;29:672–674.
- Lieng M, Istre O, Sandvik L, et al. Clinical effectiveness of transcervical polyp resection in women with endometrial polyps: randomized controlled trial. J Minim Invasive Gynecol. 2010;17(3):351–357.
- Nalaboff KM, Pellerito JS, Ben-Levi E. Imaging the endometrium: disease and normal variants. Radiographics. 2001;21(6):1409–1424.
- Li F, Wei S, Yang S, et al. Post hysteroscopic progesterone hormone therapy in the treatment of endometrial polyps. Pak J Med Sci. 2018;34(5):1267–1271.
- Gu F, Zhang H, Ruan S, et al. High number of endometrial polyps is a strong predictor of recurrence: findings of a prospective cohort study in reproductive-age women. Fertil Steril. 2018;109(3):493–500.
- Trivedi N, Chauhan N, Vaidya V. Effectiveness and safety of dydrogesterone in regularization of menstrual cycle: a post-marketing study. Gynecol Endocrinol. 2016;32(8):667–671.