Abstract
Aims
Our study was to evaluate the benefits of human umbilical cord mesenchymal stem cells (hUCMSCs) for the prevention of premature ovarian failure (POF) in a rat model.
Materials and methods
80 female SD rats aged between 6 and 8 weeks were randomly divided into 4 groups A, B, C and D. Rats in group A is normal control group; group B, C and D received zona pellucida glycoprotein 3 (pZP3) administration to induce POF model. Among these, group B is model control group; group C received PBS injection in ovaries and group D received hUCMSCs injection in ovaries, all injections were performed after modeling on the same day. Estrus cycle; serum hormone level of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) and amount of ovarian follicles were detected 20 days after treatment.
Results
We successfully injected hUCMSCs in the ovary tissue of a POF rat. The estrus cycle and hormone expression of the rats in group D tends to be normal. Histological studies indicated that hUCMSCs transplantation increased the amount of ovarian follicles.
Conclusions
This study shows that hUCMSCs may have a preventive effect on POF rats.
摘要
目的:本研究旨在评价人脐带间充质干细胞 (hUCMSCs) 在预防大鼠卵巢早衰 (POF) 中的作用。
材料与方法:将80只6∼8周龄雌性SD大鼠随机分为A、B、C、D四组, A组为正常对照组, B、C、D组注射透明带糖蛋白3 (pZP3) 诱导POF模型。其中B组为模型对照组, C组卵巢注射PBS, D组卵巢注射hUCMSCs, 所有注射均于造模当天进行。治疗20 d后检测动情周期, 血清黄体生成素 (LH)、卵泡刺激素 (FSH) 水平及卵泡数量。
结果:成功地将hUCMSCs注射到POF大鼠的卵巢组织中。D组大鼠动情周期和激素表达趋于正常。组织学研究表明, hUCMSCs移植后卵泡数量增加。
结论: hUCMSCs对POF大鼠有一定的预防作用。
Introduction
POF, with a morbidity rate of 1.0%–1.8% in women, refers to menolipsis in women younger than 40 years old due to the failure of ovarian function [Citation1–3]. The pathogenesis of POF remains unclear, though it is believed that immunological factors play very important roles in the process of disease initiation and development. Studies reveal that up to 30% POF is associated with autoimmunity, and pZP3 has previously been reported to induce autoimmune ovarian disease and POF in neonatal rats [Citation4,Citation5]. Up to now, there is no valid treatment to protect or recovery ovarian function, and clinically, sex hormone replacement therapy is mainly adopted to maintain ovarian function [Citation6]. Some researchers advocate gene therapy and immune etiological treatment, but still lack breakthrough in this field so far [Citation7,Citation8]. Therefore, it is essential to discover efficient treatment for POF patients. Mesenchymal stem cells (MSCs) existing in a variety of tissues take on multi-lineage differential potential [Citation9–11]. In recent years, studies of MSCs on POF have made fundamental progress [Citation12–15]. hUCMSCs show great differential potential, strong proliferation ability, low occurrence of immunological rejection, and are easy to obtain with lower ethical arguments, which make hUCMSCs promising in clinical use as multi-potent stem cells [Citation16–20]. The study about the efficacy of hUCMSCs on POF and ovarian function was insufficient currently. We have established a rat model of POF with immunological injury and tested the effect of hUMCSMs in this model, providing important information about the role and safety of hUCMSCs in vivo treatment.
Material and methods
Animal grouping
80 Female SD rats aged between 6 and 8 weeks with normal estrus cycle were obtained from animal laboratory of Shanghai Tongji Hospital and housed at specific pathogen free (SPF) grade laboratory animal facility. All rats were randomly divided into 4 groups (A, B, C and D). Rats in Group A is normal control group without any operation or treatment. Group B, C and D received pZP3 administration to induce POF model. As model group, group B does not receive any processing after modeling. Group C received PBS injection in ovaries, and Group D received hUCMSCs injection in ovaries on the same day of modeling.
Source and identification of hUCMSCs
hUCMSCs purchased from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences were cultured in 60 mm culture dish (353002, BD falcon, U.S) with a cell density of 0.8 × 106/mm2 after thawed in 37 °C water bath and suspended in dulbecco’s modified eagle medium (DMEM) (Gibco, U.S). Cell passage was carried on after hUCMSCs got confluence and covered the bottom of the petri dish. hUCMSCs of the third generation were harvested for identification. Mesenchymal progenitor cell related antigens CD44, CD77, CD90 and CD105 were selected to confirm phenotype and purity of the cells using flow cytometry (FCM) [Citation21].
POF modeling
Immunological injury POF mice model was established according to the literature [Citation4,Citation5,Citation14,Citation22]: 400 μL pZP3 suspension (Zhongtai Biochemical Co. Ltd, Hangzhou, China) was injected subcutaneously into bilateral hind soles of rats in group B, C and D.
hUCMSCs transplantation
20 rats in group D were injected with hUCMSCs right after modeling. 1 × 105/μL hUCMSCs of the third generation were selected as transplantation cells. Rats were anesthetized with nembutal and underwent surgery under sterile environment. Bilateral ovary were exposed and the surrounding adipose tissues were carefully stretched to stabilize ovary. 5 μL concentrated cell suspension was extracted by a micro-syringe and was injected into each ovary. A local upheaval was invisible after injection, the needle stayed in the injection site for 30 s followed by gently pulling out. A gentle and soft operation was required during the procedure to avoid injury to ovarian blood vessels. Post-injection observation was required to avoid bleeding and the spillover of hUCMSCs suspension. Meanwhile, 20 rats in group C were treated with PBS instead of MSC. The day of modeling and cell transplantation refers to Day 0.
Estrus cycle observation
All rats were given vaginal smear once a day at 8 a.m. from Day 0 to Day 20 to observe the estrus cycle. A cotton swab bedewed with normal saline was gently sticked into rat vagina followed by smearing on a microslide. After air-dry, fixation with methyl alcohol and staining with Giemsa, the microslide was observed under light microscope. The estrous cycle can be divided into 4 stages according to the changes in the proportion of epithelial cells and leukocytes in vaginal smears: proestrus, estrus, metestrus and diestrus [Citation23]. We took the day with the most nucleated epithelial cells on the vaginal smear as the first day of the estrous stage and counted a complete estrous cycle from the first day of the first estrous stage until the first day of the next estrous stage. The rat was excluded if a complete estrous cycle was not monitored within 20 days.
Serum sex hormone detection
20 days after modeling/injection, the tail vein blood of all rats was taken for sex hormone detection. The expression of LH, FSH and E2 was detected by enzyme-linked immunosorbent assay (ELISA) following the protocol of ELISA kits of LH, FSH and E2 (Xinqidi Biotech, China).
Ovarian follicles counting and detection by hematoxylin-eosin (HE) staining
All rats were sacrificed 20 days after modeling/injection. Ovary tissues were dissected after perfusion followed by fixation, dehydration, paraffin embedding, slicing and dewaxing. Dewaxed slides were stained with hematoxylin for 5 min followed by rinsing with running water. Slides were then incubated with 1% hydrochloric acid ethanol solution for 30 s to differentiate followed by rinsing with running water. 1% ammonium hydroxide solution was added for 30 s to turn blue followed by rinsing with running water. Then the slides incubated with eosin for 1 min followed by dehydration, transparency, mounting and incubation with 95% ethyl alcohol 1 (5 min); 95% ethyl alcohol 2 (5 min); 100% ethyl alcohol 1 (5 min); 100% ethyl alcohol 2 (5 min); xylene 1 (5 min) and xylene 2 (10 min) orderly and then were covered with neutral balsam. We counted all follicles of 4 groups of rats respectively and calculated the average number of follicles per rat.
Statistical analysis
All data were expressed as mean ± standard deviation (SD). Statistical significance was assessed using t-test. p < .05 was considered statistically significant.
Results
Identification of hUCMSCs
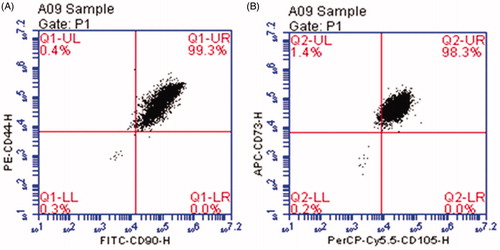
The results of FCM revealed that the hUCMSCs express positive mesenchymal progenitor markers with CD44, CD73, CD90 and CD105. The purity of the isolated cells reached over 98% (). According to the report issued by Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, the hUCMSCs can differentiate into a variety of mesoderm-type cells, including osteoblasts, chondrocytes, adipocytes, cardiomyocytes, skeletal muscle cells, and endothelial cells.
Situation of rats after modeling/injection
For rats in group C and D, the general condition was normal with good wound healing: no bleeding occurred 2 h after surgery, no wound swollen observed 3 days after surgery. Rats were weak on the operation day, but appeared normal compared to the rats in group A and B 2 days after operation.
Transplantation of hUCMSCs in ovary ameliorates estrus cycle disorder
All rats underwent a complete estrous cycle during the entire observation period, we observed that there was significant statistical difference between group B and group A in estrus cycle (p < .05) which proved the effect of POF modeling. In group C and group D, we found that the estrous cycle was shorter than that in group B, but there was no significant statistical difference between group C and group B (p < .1) and there was statistical difference between Group D and group B (p < .05) which suggested that hUCMSCs transplantation facilitates the amelioration of sexual cycle disorder ().
Table 1. Evaluation of ovarian function between 4 groups.
Transplantation of hUCMSCs in ovary regulates the sex hormone expression
Serum sex hormone values of all rats in 4 groups 20 Days after modeling/injection were listed in . Briefly, serum LH and FSH expression in group B was significantly higher than that of group A (p < .05) indicating further injury of ovarian function after modeling. The values of LH and FSH were both significantly different (p < .05) between groups A and D, and between groups B and D which suggested that hUCMSCs transplantation regulates the aberrant hormone expression in immunological injury POF rats but still above the normal levels. Moreover, there was no statistical difference of LH and FSH between groups C and B (p > .1) indicating PBS injection did not improve sex hormone expression. As for the serum E2 level, there was no statistical difference in between groups B and D compared with group A and between groups B and C.
Transplantation of hUCMSCs improves rat ovarian function
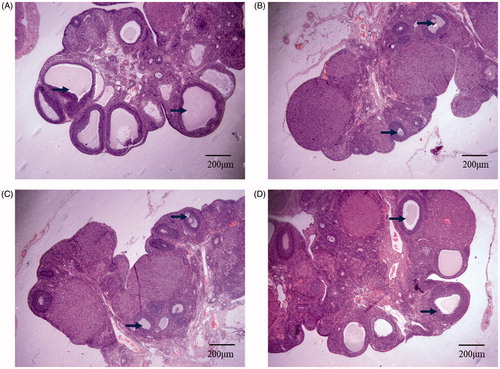
We performed HE staining to observe the structure of ovary 20 days after modeling/injection and counted all follicles of 4 groups of rats respectively. The results revealed that there was a significant decline in the number of follicle in group B compared to that of group A (p < .05). After transplantation, the average number of follicles in group D was higher than that in group B (p < .05), though it did not reach the level of group A (p < .05). We also observed that the average number of follicles in group C was lower than that in group B. These results indicate that hUCMSCs transplantation may restore ovarian function to some extent (, ).
Figure 2. HE staining of follicles of 4 groups of rats. (A) normal control group: Follicles between 200 microns and 400 microns in diameter are evenly distributed in the ovarian cortex; (B) model control group: Almost no follicular development in ovarian cortex; (C) PBS injection group: Almost no follicles in ovarian cortex, and ovarian tissue is damaged to some extent; (D) hUCMSCs transplantation group: The ovarian cortex has follicles between 100 microns and 200 microns in diameter, and the ovarian tissue is partially damaged. The black arrow points to the follicles included in the count. Scale bar: 200 μm.
Discussion
In our study, we transplanted hUCMSCs into the ovaries of POF rats, obtaining the following results: hUCMSCs transplantation improves the disorder of sexual cycle, modulates the serum hormone expression to a better state and restores ovarian function. These results proved that hUCMSCs transplantation can exert beneficial and preventive effect on POF model of rats.
Mesenchymal stem cells (MSCs) therapy is of promising potential for clinic use, with bone marrow mesenchymal stem cells (BMSCs) being most widely studied worldwide. However, there are some critical shortcomings in the application of BMSCs. First, the process of obtaining BMSCs will bring suffering to patients. Second, the efficacy of BMSCs transplantation fails to meet clinical expectation as the amount as well as the differentiation and proliferation ability decreases as the patients grow old. Most vitally, allo-transplantation of BMSCs may result in severe immunological rejection [Citation24,Citation25]. Taken together, these problems limited the extensive application of MSCs in clinic. hUCMSCs, by contrast, are stem cells isolated from tissues of umbilical cord. These cells not only maintain the biological properties of mesenchymal stem cells, but also possess great ability to proliferate and differentiate. The immunological competence of hUCMSCs is relatively low compared to BMSCs, which decreases the possibility to trigger systematic immune response and graft-versus-host disease. Besides, the collection of hUCMSCs does little harm or injury to the mother or the infant, which will not trigger ethical dispute. Therefore, hUCMSCs may be an ideal replacement of BMSCs to be promoted in clinical use with promising potential.
As for hUCMSCs transplantation surgery, it should be performed with caution to avoid any injury to ovary. Notably there is dense and interweaved blood capillary network covering the surface of ovary, which is very easy to get cracked during surgery or injection. Vascular rupture may result in insufficient blood supply of ovary and the remained blood stasis may trigger elevated ovarian inflammatory response. In our process of hUCMSCs injection, we stretched the surrounding adipose tissue of ovary to stabilize ovary for injection, rather than touching ovary itself. To avoid injury to ovary resulted from mass liquid injection, we resuspended 1 × 105 cells into 5 μL DMEM followed by injection into ovary. Since the size of rat ovary is far too small, some of the ovaries inevitably bled after injection and it may exacerbate ovarian inflammatory response to some extent. This may be the reason that the ovarian function in group C seems even worse compared to group B.
In our following studies, we will further discover new methods to increase the efficacy of hUCMSCs by potentiating its ability to survive, enter into ovary and secrete to make them more promising in clinical application.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67(4):604–606.
- Wu XY, Cai H, Kallianpur A, et al. Impact of premature ovarian failure on mortality and morbidity among chinese women. PLoS One. 2014;9(3):e89597.
- Jankowska K. Premature ovarian failure. Prz Menopauzalny. 2017;16(2):51–56.
- Fu L, Feng W, Li SR, et al. ZP3 peptides administered orally suppress murine experimental autoimmune ovarian disease. J Reprod Immunol. 2007;75(1):40–47.
- Rhim SH, Millar SE, Robey F, et al. Autoimmune disease of the ovary induced by a ZP3 peptide from the mouse zona pellucida. J Clin Invest. 1992;89(1):28–35.
- Sassarini J, Lumsden MA, Critchley HO. Sex hormone replacement in ovarian failure – new treatment concepts. Best Pract Res Clin Endocrinol Metab. 2015;29(1):105–114.
- Slopien R, Warenik-Szymankiewicz A. Premature ovarian failure: diagnosis and treatment. Clin Exp Obstet Gynecol. 2014;41(6):659–661.
- Ebrahimi M, Asbagh FA. The role of autoimmunity in premature ovarian failure. Iran J Reprod Med. 2015;13(8):461–472.
- Strong AL, Neumeister MW, Levi B. Stem cells and tissue engineering: regeneration of the skin and its contents. Clin Plast Surg. 2017;44(3):635–650.
- Vats A, Bielby RC, Tolley NS, et al. Stem cells. Lancet. 2005;366(9485):592–602.
- Han Y, Li X, Zhang Y, et al. Mesenchymal stem cells for regenerative medicin. Cells. 2019;8(8):886.
- Chu D-T, Phuong TNT, Tien NLB, et al. An update on the progress of isolation, culture, storage, and clinical application of human bone marrow mesenchymal stem/stromal cells. Int J Mol Sci. 2020;21(3):708.
- Wang S, Yu L, Sun M, et al. The therapeutic potential of umbilical cord mesenchymal stem cells in mice premature ovarian failure. Biomed Res Int. 2013;2013:690491.
- Yin N, Zhao W, Luo QQ, et al. Restoring ovarian function with human placenta-derived mesenchymal stem cells in autoimmune-induced premature ovarian failure mice mediated by Treg Cells and associated cytokines. Reprod Sci. 2017;33:193371911773215.
- Takehara Y, Yabuuchi A, Ezoe K, et al. The restorative effects of adipose-derived mesenchymal stem cells on damaged ovarian function. Lab Invest. 2013;93(2):181–193.
- Ghadami M, El-Demerdash E, Zhang D, et al. Bone marrow transplantation restores follicular maturation and steroid hormones production in a mouse model for primary ovarian failure. PLoS One. 2012;7(3):e32462.
- Zhang Z, Feng R, Niu L, et al. Human umbilical cord mesenchymal stem cells inhibit T follicular helper cell expansion through the activation of iNOS in Lupus-Prone B6.MRL-Faslpr mice. Cell Transplant. 2017;26(6):1031–1042.
- Decot V, El Omar R, Beroud J, et al. Umbilical cord mesenchymal stem cells: the new gold standard for mesenchymal stem cell based therapies? Tissue Eng Part B, Rev. 2014;20(5):523–544.
- Liu G, Wang L, Pang T, et al. Umbilical cord-derived mesenchymal stem cells regulate thymic epithelial cell development and function in Foxn1(–/–) mice. Cell Mol Immunol. 2014;11(3):275–284.
- Song D, Zhong Y, Qian C, et al. Human umbilical cord mesenchymal stem cells therapy in cyclophosphamide-induced premature Ovarian failure rat model. Biomed Res Int. 2016;2016:2517514.
- Zhang X, Hirai M, Cantero S, et al. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J Cell Biochem. 2011;112(4):1206–1218.
- Ghannam S, Bouffi C, Djouad F, et al. Immunosuppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Res Ther. 2010;1(1):2.
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62(4A):609–614.
- Zhang L, Tan X, Dong C, et al. In vitro differentiation of human umbilical cord mesenchymal stem cells (hUCMSCs), derived from Wharton’s jelly, into choline acetyltransferase (ChAT)-positive cells. Int J Dev Neurosci. 2012;30(6):471–477.
- Lu Z, Ye D, Qian L, et al. Human umbilical cord mesenchymal stem cell therapy on neuromyelitis optica. Curr Neurovasc Res. 2012;9(4):250–255.