Abstract
Objective
This study aimed to investigate the effect of adding L-Carnitine to the gonadotropins on ART outcome in frozen-thawed embryo transfer cycles among PCOS women.
Methods
In this randomized clinical trial, 83 patients with PCOS were randomized to either L-Carnitine supplemented (n = 42) or control (n = 41) groups. The L-Carnitine group was given 3000 mg of oral L-Carnitine daily until the final day of ovulation. The numbers of metaphase II (MII) oocytes, 2-pronuclears (2PNs), oocyte maturity rate, fertilization rate, fertilization proportion as well as implantation, chemical and clinical pregnancy rates were compared between the two groups.
Results
Even though the duration of stimulation and endometrial thickness were comparable between groups (p > .05), serum estradiol level on the day of oocyte triggering, was significantly higher in the L-Carnitine group compared to the control group (p < .05). In contrast, the number of retrieved and MII oocytes as well as the number of 2PNs and obtained embryos were similar between groups (p > .05). Moreover, oocyte maturity rate (0.85 ± 0.38 vs. 1.02 ± 0.90), fertilization proportion (0.62 ± 0.44 vs. 0.80 ± 0.86), fertilization rate (0.70 ± 0.22 vs. 0.76 ± 0.19) along with implantation rate (18.1 vs. 13.7%), chemical (26.8 vs. 30.7%) and clinical (24.3 vs. 25.6%) pregnancy rates, were all comparable between L-Carnitine and control groups respectively (p > .05).
Conclusions
Our result showed that oral L-Carnitine administration during induction of ovulation among PCOS women could not improve laboratory and pregnancy outcome.
Introduction
Polycystic ovarian syndrome (PCOS) is one of the common endocrine disorders in women of reproductive age presented with increased androgen, chronic anovulation, and insulin resistance [Citation1]. PCOS was reported in 5–18% of women at the age of reproduction [Citation2]. Ovarian dysfunction, which is characterized by irregular menstruation, oligo ovulation, or anovulation; is the main fertility-related aspect of PCOS. The most current approaches in PCOS women are medication, hormonal protocols, or assisted reproductive techniques (ART). Regulation of the menstrual cycle and improving fertility could be managed through weight loss and exercise along with the use of ovulation inducers such as clomiphene citrate, gonadotropins and, insulin-sensitive drugs [Citation3]. In addition, it is shown that nutrition-associated signaling pathways have a key role in the regulation of ovarian function and normal ovulation [Citation4]. Among nutritional supplements, L-Carnitine is reported to have a low serum level among women with PCOS [Citation5]. L-Carnitine is a quaternary ammonium compound synthesized from the two amino acids; lysine and methionine which transmits fatty acids from the cytosol to the mitochondria in the living cells [Citation6]. L-Carnitine is also involved in energy production, cellular apoptosis, and glucose metabolism [Citation7]. In addition, it is evidenced that L-Carnitine supplementation could improve oocyte quality and oocyte maturation rate [Citation8]. Preliminary studies on human and animals revealed the valuable effect of L-Carnitine on folliculogenesis, ovulation rate and pregnancy outcome in PCOS [Citation9,Citation10]. Therefore, this study aimed to investigate the effect of adding L-Carnitine to the gonadotropins on ART outcome in frozen-thawed embryo transfer cycles among PCOS women.
Materials and methods
The Ethics Committee of Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences approved the study protocol (IR.SSU.MEDICINE.REC.1398.279). All participants signed a written informed consent. The study was registered in IRCT (Iranian Registry of Clinical Trials) (IRCT20110509006420N21).
Subjects
A total of 83 individuals with PCOS-related infertility aged between 18 and 40 years old, who were candidates for ART treatment, were included in the study. The study was implemented at Yazd Reproductive Sciences Institute during a 5-month period from January to May 2020. The diagnosis of PCOS was based on the Rotterdam criteria [Citation11] including polycystic ovaries on ultrasonography (≥12 small follicles measuring 2–9 mm in at least one ovary and/or ovarian volume >10 cm3)., oligomenorrhea and/or anovulation (delayed menstruation more than 35 days, or less than 8 natural hemorrhagic periods per year), and biochemical and/or clinical signs of hyperandrogenism. Signs of hyperandrogenism, such as acne and hirsutism, were evaluated on physical examination and total testosterone level higher than the adult female normal values was considered a feature of biochemical hyperandrogenism. Women with a history of severe endometriosis, other endocrine disorders, and azoospermia in their husbands were excluded from the study. Subjects were randomized to either L-Carnitine supplemented (n = 42) or control (n = 41) groups using computer-generated random numbers in sealed, unnamed envelopes each holding a single number. The participants, nurses, and physicians were not blinded to the allocated group.
Ovarian stimulation protocol
In order to reduce the risk of ovarian hyperstimulation syndrome (OHSS) developing among women with PCOS, all PCOS patients undergoing infertility treatment in our clinic were hyperstimulated using the GnRH antagonist protocol. On the second day of the cycle, all women received 150 IU of the recombinant human follicle-stimulating hormone rFSH (Cinal F) (Cinagen, Tehran, Iran) subcutaneously for 5 days. In the L-Carnitine supplemented group all patients received 3000 mg L-Carnitine orally from the second day of the cycle until the day of oocyte triggering. In both groups when the dominant follicle reached 12–13 mm, Cetrotide (Cetrorelix, Merck Serono Laboratories, Aubonne, Switzerland) was injected subcutaneously by the dose of 0.25 mg daily. Once the dominant follicle of 17 mm was observed in the vaginal ultrasonography, final oocyte triggering was done using 10,000 IU intramuscular hCG (human chorionic gonadotropin) (Pregnyl, Organon, the Netherlands) and 0.2 mg GnRH-a (Decapeptyl, Ferring Co., Germany) subcutaneously. Transvaginal oocyte pick up was performed after 36 h. Oocytes were fertilized by conventional in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI). According to our clinics' freeze-all policy for PCOS women, all embryos were vitrified in the cleavage stage on day 2 after oocyte retrieval. Embryo vitrification and warming procedures were implemented as described earlier [Citation12]. Frozen-thawed embryos were transferred two months later.
Endometrium preparation
All women in both groups received 6 mg/day estradiol valerate (Aburaihan Co., Tehran, Iran) orally from the second day of the menstrual cycle. Endometrial thickness was measured on day 13 of the menstrual cycle by vaginal ultrasonography. When endometrial thickness reached ≥ 8 mm, 400 mg of vaginal peccaries (Cyclogest®, Cox Pharmaceuticals, Barnstaple, UK) was administered twice a day. Embryo transfer was done three days after the initiation of progesterone consumption. Estradiol and progesterone continued for up to 8 weeks after pregnancy confirmation.
Hormone assay
Serum levels of anti-Mullerian hormone (AMH), FSH and luteinizing hormone (LH), along with total testosterone concentration were measured in a venous blood sample collected from all participants on the day 3 of their menstrual cycle. AMH level was measured using a commercial ELISA kit (AMH/MSI ELISA; AnshLabs, TX, USA), FSH and LH concentrations were determined using a commercial ELISA kit (JTC Diagnosemittel UG, Voehl, Germany). The assessment of total testosterone level was done by a commercial ELISA kit (Diagnostic Biochem Canada Inc., Ontario, Canada). Serum estradiol level on the day of oocyte triggering was measured using an ELISA kit (AccuBind® ELISA, CA, USA).
Pregnancy outcomes
The primary outcome was considered clinical pregnancy that was confirmed by detecting fetal heartbeats 2 weeks following the positive β hCG. The secondary outcomes include chemical pregnancy: serum β hCG > 50 IU/L two weeks after embryo transfer; implantation rate: the percentage of intrauterine gestational sacs divided by transferred embryos; oocyte maturity rate: the number of metaphase II (MII) oocytes divided by the number of oocytes retrieved; fertilization proportion: the number of 2-pronuclears (2PNs) divided by the number of oocytes retrieved; fertilization rate: the number of 2PNs divided by the total number of MII oocytes.
Statistical analysis
The sample size of 40 cases in each arm was estimated to permit the detection of a 10% increase in clinical pregnancy rate as our primary outcome after L-Carnitine administration with 80% power at 5% alpha level. The SPSS software (Statistical Package for the Social Sciences, version 20, Chicago, IL, USA) was used for data analysis. For comparison of parametric and non-parametric continuous and also categorical variables between the two groups, Student’s t-test, Mann–Whitney U test and, Chi-square test were used respectively. p-value < .05 was considered the significance level.
Results
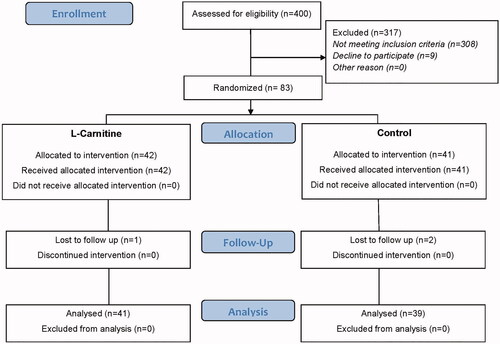
At first, 400 women were evaluated for enrollment. Three hundred and seventeen participants were excluded from the study: 308 women did not meet the inclusion criteria and 9 women declined to participate ().
A total of 83 women was randomized to the L-Carnitine (n = 42) and control (n = 41) groups. After embryo transfer, one woman in the L-Carnitine group and 2 patients in the control group were lost to follow up. Finally, 41 and 39 women were analyzed in the L-Carnitine and control group respectively.
Baseline features including female age, male age, type and duration of infertility body mass index (BMI) as well as AMH day 3 FSH and LH, and also total testosterone were similar between the two groups (p > .05) (). With regard to the cycle outcome, even though the duration of stimulation and endometrial thickness were comparable between the groups (p > .05), serum estradiol level on the day of oocyte triggering was significantly higher in the L-Carnitine group compared to the control group (p < .05) (). In contrast, the number of retrieved and MII oocytes as well as the number of 2PNs and obtained embryos were similar between the groups (p > .05). Moreover, the percentages of mild to moderate OHSS development was similar between the two groups (p > .05) (). There was no case of severe OHSS in either group. Regarding reproductive outcome, oocyte maturity rate (0.85 ± 0.38 vs. 1.02 ± 0.90), fertilization proportion (0.62 ± 0.44 vs. 0.80 ± 0.86), fertilization rate (0.70 ± 0.22 vs. 0.76 ± 0.19) along with implantation rate (18.1 vs. 13.7%), chemical (26.8 vs. 30.7%) and clinical (24.3 vs. 25.6%) pregnancy rates, were all comparable between L-Carnitine and control groups respectively (p > .05) ().
Table 1. Basic characteristics of ‘L-Carnitine’ group versus control group.
Table 2. ART cycle characteristics of ‘L-Carnitine’ group versus control group.
Table 3. ART outcomes of ‘L-Carnitine’ group versus control group.
Discussion
The results of the present study showed that the addition of L-Carnitine to the ovarian stimulation protocol had no beneficial effect on the number of retrieved and mature oocytes, 2PNs, and embryos compared with the control group. Moreover, fertilization and implantation rates as well as chemical and clinical pregnancy were similar between the L-Carnitine and control groups.
It was previously stated that L-Carnitine supplementation improves PCOS symptoms through an antagonistic effect on insulin resistance and subsequent reducing blood glucose level [Citation13]. It is also indicated that adding L-Carnitine to the regimen of PCOS women may regulate male and female hormonal unequal ratio and decrease hyperandrogenism effects [Citation5]. In addition, there is evidence regarding the improvement of ovulation and pregnancy rate using L-Carnitine in PCOS women [Citation14]. Based on the mentioned theories, we assumed that adding L-Carnitine to the stimulation protocol may improve ART outcomes in infertile women with PCOS.
In contrast with our findings, the result of an RCT that evaluated the effect of adding L-Carnitine to clomiphene citrate on pregnancy outcome, showed improvement in both ovulation and cumulative pregnancy rates in clomiphene-resistant women with PCOS. In addition, the number of follicles >17 mm, estradiol concentration and endometrial thickness were significantly higher in the L-Carnitine group. The women were asked to have intercourse every other day for one week from the day of hCG injection. The spontaneous clinical pregnancy rate was significantly higher in women who received L-Carnitine [Citation14]. In a similar RCT with a smaller sample size, Abd-Elfattah et al confirmed that adding L-Carnitine to clomiphene citrate in the follicular phase and continued administration to the luteal phase in clomiphene-resistant patients could improve ovulation quality and increase chemical and clinical pregnancy rates [Citation15]. In contrast, another RCT found that adding L-Carnitine to clomiphene citrate in women with PCOS could improve the number and quality of mature oocytes, but the pregnancy rate did not change significantly [Citation16].
Due to obesity and insulin-resistance that are very common in PCOS women, Sharkwy and colleagues examined a regimen that included clomiphene-citrate, L-Carnitine, and metformin in clomiphene-resistant obese PCOS women. The results of RCT showed significant enhancement in the menstrual regularity, ovulation and pregnancy rate in the group supplemented with L-Carnitine compared to the clomiphene-citrate plus metformin and the placebo group [Citation17]. Likewise, another RCT confirmed the beneficial effect of L-Carnitine together with letrozole on the ovulation, chemical and clinical pregnancy rates in PCOS women [Citation10].
Only one ‘before and after study’ assessed the influence of oral L-Carnitine on embryo quality and pregnancy outcome in IVF cycle. A total of 214 infertile women including 5 PCOS patients with the history of previous IVF failure were enrolled. There were no significant differences in the total number of retrieved oocytes, the oocyte maturity rate, and the fertilization rate before and after L-Carnitine supplementation. However, the embryo quality on day 3 and day 5 was significantly increased after L-Carnitine administration [Citation18].
There are several studies that indicated the valuable effects of L-Carnitine in PCOS women in terms of weight loss, balancing lipid profile as well as menstrual regulation and, reducing hirsutism [Citation13,Citation19]. Another possibility is that L-Carnitine antioxidant property could improve and maintain mitochondrial activity in oocytes, leading to retrieving good quality oocytes with high developmental capacity [Citation20,Citation21].
To the best of our knowledge, this is the first randomized clinical trial that compared the influence of L-Carnitine on the improvement of ART outcome between the two groups of PCOS women with and without L-Carnitine supplementation.
In conclusion, our result showed that oral L-Carnitine administration during the induction of ovulation among PCOS women could not improve laboratory and pregnancy outcome. Further clinical trials with larger sample sizes are needed to evaluate the impact of different dosages and duration of L-Carnitine supplementation on ART outcomes.
Acknowledgements
This study was extracted from the MD thesis of Arezoo Sheida. We acknowledge the staff of the ART laboratory and the operating room personnel at the Yazd Reproductive Sciences Institute for their great contribution to all the laboratory work and data gathering. This study was financially supported by Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Rocha AO, Azevedo RC, Silva VA, et al. Recent advances in the understanding and management of polycystic ovary syndrome. F1000Res. 2019;8:565–511.
- Ding T, Hardiman PJ, Petersen I, et al. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: a systematic review and meta-analysis. Oncotarget. 2017;8(56):96351–96358.
- Bates GW, Jr., Propst AM. Polycystic ovarian syndrome management options. Obstet Gynecol Clin North Am. 2012;39(4):495–506.
- Agarwal A, Sengupta P, Durairajanayagam D. Role of L-carnitine in female infertility. Reprod Biol Endocrinol. 2018;16(1):5
- Fenkci SM, Fenkci V, Oztekin O, et al. Serum total L-carnitine levels in non-obese women with polycystic ovary syndrome. Hum Reprod. 2008;23(7):1602–1606.
- Steiber A, Kerner J, Hoppel CL. Carnitine: a nutritional, biosynthetic, and functional perspective. Mol Aspects Med. 2004;25(5–6):455–473.
- Vanella A, Russo A, Acquaviva R, et al. L-propionyl-carnitine as superoxide scavenger, antioxidant, and DNA cleavage protector. Cell Biol Toxicol. 2000;16(2):99–104.
- Miyamoto K, Sato EF, Kasahara E, et al. Effect of oxidative stress during repeated ovulation on the structure and functions of the ovary, oocytes, and their mitochondria. Free Radic Biol Med. 2010;49(4):674–681.
- Kalhori Z, Mehranjani MS, Azadbakht M, et al. L-Carnitine improves endocrine function and folliculogenesis by reducing inflammation, oxidative stress and apoptosis in mice following induction of polycystic ovary syndrome. Reprod Fertil Dev. 2019;31(2):282–293.
- Wf G. The effect of adding L-Carnitine to induction of ovulation with letrozole among PCOS patients. Austin J Obstetr Gynecol. 2019;6(3):1141–1144.
- Guideline E. Revised consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2003;19(1):41–47.
- Aflatoonian N, Pourmasumi S, Aflatoonian A, et al. Duration of storage does not influence pregnancy outcome in cryopreserved human embryos. Iran J Reprod Med. 2013;11(10):843–846.
- Samimi M, Jamilian M, Ebrahimi FA, et al. Oral carnitine supplementation reduces body weight and insulin resistance in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol. 2016;84(6):851–857.
- Ismail AM, Hamed AH, Saso S, et al. Adding L-carnitine to clomiphene resistant PCOS women improves the quality of ovulation and the pregnancy rate. A randomized clinical trial. Eur J Obstet Gynecol Reprod Biol. 2014;180:148–152.
- Abd-Elfattah AH, Elomda FAE, Megahed HI. Effect of adding L-Carnitine to clomiphene resistant PCOs women on the ovulation and the pregnancy rate. Egyptian J Hosp Med. 2019;76(5):4138–4143.
- Kortam M, Abdelrahman R, Fateen H. Carnitine and clomiphene citrate for induction of ovulation in women with polycystic ovary syndrome: randomized controlled trial. Evidence Based Women's Health J. 2020;10(1):1–7.
- El Sharkwy I, Sharaf El-Din M. l-Carnitine plus metformin in clomiphene-resistant obese PCOS women, reproductive and metabolic effects: a randomized clinical trial. Gynecol Endocrinol. 2019;35(8):701–705.
- Kitano Y, Hashimoto S, Matsumoto H, et al. Oral administration of l-carnitine improves the clinical outcome of fertility in patients with IVF treatment. Gynecol Endocrinol. 2018;34(8):684–688.
- Salehpour S, Nazari L, Hoseini S, et al. Effects of L-carnitine on polycystic ovary syndrome. JBRA Assist Reprod. 2019;23(4):392–395.
- Moawad AR, Tan SL, Xu B, et al. L-carnitine supplementation during vitrification of mouse oocytes at the germinal vesicle stage improves preimplantation development following maturation and fertilization in vitro. Biol Reprod. 2013;88(4):104
- Moawad AR, Xu B, Tan SL, et al. L-carnitine supplementation during vitrification of mouse germinal vesicle stage-oocytes and their subsequent in vitro maturation improves meiotic spindle configuration and mitochondrial distribution in metaphase II oocytes. Hum Reprod. 2014;29(1010):2256–2268.