Abstract
Infertility concerns 15% of the couples. Management of female infertility requires a complete history of the patient followed by a physical, gynecological and endocrine examination. Infertility etiology will be investigated thanks to different tests including ovarian function and reserve assessment, search for uterine abnormalities and evaluation of tubal permeability. Polycystic ovarian syndrome (PCOS) is a predominant cause of infertility and a common gyne-endocrine disorder affecting 7 to 15% of women in reproductive age. Behavioral, medical and surgical treatments have been evaluated in order to improve the fertility of women with PCOS. Lifestyle modifications (stop smoking, physical exercise and weight loss when necessary) are of the utmost importance. Clomiphene citrate remains the first line of medical treatment of infertility in women with PCOS in absence of other male or female causes of infertility. Use of metformin solely for infertility is not recommended in absence of metabolic anomaly and new treatment as myoinositol is emerging. Surgical techniques aiming to enhance ovulation and pregnancy rate are an option when medical treatment failed. Ovarian drilling by laparoscopy or by transvaginal hydrolaparoscopy is taking a larger place in the treatment of infertility. In vitro maturation and fertilization remain the third-line of treatment in PCOS.
多囊卵巢综合征与不孕:对假定治疗方案的概述和见解 摘要
15%的夫妇患有不孕症。女性不孕症的治疗要求患者有完整的病史, 然后进行身体、妇科和内分泌检查。通过卵巢功能和储备功能评估、子宫异常检查和输卵管通透性评估等不同检查, 将探讨不孕症的病因。多囊卵巢综合征(PCOS)是不孕的主要原因, 也是影响7-15%育龄妇女的常见妇科内分泌疾病。为了提高PCOS妇女的生育能力, 已经对行为、内科和外科治疗进行了评估。改变生活方式(戒烟、必要时进行体育锻炼和减肥)至关重要。在没有其他男性或女性原因的不孕情况下, 克罗米芬仍然是PCOS女性不孕的一线治疗药物。在没有代谢异常和新的治疗方法如肌醇出现的情况下, 不建议仅将二甲双胍用于不孕症。当药物治疗失败时, 旨在提高排卵率和妊娠率的手术治疗是一种选择。在不孕症的治疗中, 通过腹腔镜或经阴道腹腔镜进行卵巢钻孔正在发挥更大的作用。体外成熟和受精仍然是PCOS的三线治疗
Introduction
Polycystic ovary syndrome (PCOS) is an endocrine and reproductive disorder affecting 7 to 15% of women of reproductive age. It was first described by Stein and Leventhal in 1935. PCOS etiology is complex, including genetic, environmental and lifestyle factors and remains controversial. PCOS is defined by the presence of at least two of the Rotterdam criteria: oligo-anovulation, clinical or biological hyperandrogenism, and micropolycystic syndrome (ovarian volume > 10 ml and/or more than 12 follicles by the ovary) [Citation1–3].
Patients suffering from PCOS will consult for different degrees of hyperandrogenism (hirsutism, seborrhea, acne), dysfunction of the menstrual cycles, overweight, infertility
A delayed diagnostic has been well documented in the literature explained in part by the lateness of the referral to a specialist, often for infertility [Citation4]. PCOS is the first cause of anovulatory infertility nowadays and infertility is found in 70 to 80% of affected women [Citation5].
The purpose of this article is to overview different available options to improve fertility in PCOS. A specific emphasis will be given to ovarian drilling, a less commonly used surgical technique that has some added values.
Evaluation of infertility in PCOS patients
Evaluation of infertility (or subfertility) is recommended after 12 months without pregnancy in couples having regular sexual intercourse (2 or 3 times/week) [Citation1].
PCOS is the first cause of female infertility but the definite diagnosis should be given after exclusion of other etiologies of infertility such as other endocrine disorders (thyroid dysfunction, hyperprolactinemia, congenital adrenal hyperplasia, Cushing syndrome, premature ovarian insufficiency, …), anatomical dysfunctions (endometriosis, pelvic inflammatory diseases, …) or iatrogenic causes (surgery, chemotherapy, radiations, …).
The patient’s personal medical and surgical as well as familial history, followed by a complete physical and gynecological examination is mandatory when exploring infertility. A thorough table with the infertility factors to search can be found in the supplemental data.
Additional diagnostic tests including blood samples, ultrasonography and hysteroscopy among others are recommended.
Anti-müllerian hormone (AMH) is a useful test in the evaluation of infertility. It is produced by granulosa cells of early follicles and helps to evaluate the ovarian reserve. Because its production is gonadotrophin-independent, AMH levels remain consistent during the menstrual cycle [Citation1]. Women with PCOS present AMH levels 2 to 3 folds higher than non-PCOS women because of an increased number of preantral and small antral follicles. The increased AMH level seems correlated with the severity of PCOS [Citation6].
Transvaginal ultrasonography (TVUS) will evaluate the antral follicle count, endometrium thickness and diagnose uterine anomaly (polyps, myomas and congenital malformation).
Treatment
Non-pharmacologic measures remain the first line and the most effective in the treatment of infertility. We already know the positive effects of clomiphene citrate letrozole and gonadotropins in the treatment of infertility in PCOS. Myo-inositol and ovarian drilling are new lines of therapeutic and will be discussed in this review. This review includes articles until March 2021.
Non-pharmacological measures
Lifestyle changes are the first line of treatment for women with PCOS. In all cases, smoking should be ceased and physical exercise encouraged. Weight loss is recommended in overweight and obese women [Citation7].
Several studies [Citation2,Citation8] have shown that a 5 to 10% weight loss in overweight and obese women may be sufficient to restore regular menstruation and ovulation [Citation9]. This weight loss also enhances the effect of the ovulation-inducing agents. Altogether, weight loss in obese women with PCOS improves pregnancy rate in addition to the known metabolic benefits [Citation10].
Bariatric surgery can be considered when BMI is above 35 kg/m2 and lifestyle changes conducted for more than 1 year have failed [Citation11]. A meta-analysis showed that obese women suffering from PCOS with bariatric surgery presented a significant decrease in testosterone levels associated with a resolution of hirsutism in 53% and of menstrual irregularities in 96% of the subjects [Citation12]. However, there is currently no strong evidence suggesting that bariatric surgery improves the pregnancy rate in women with PCOS. Moreover, surgical complications, intestinal incarceration in Petersen space and lack of vitamins should not be neglected [Citation13,Citation14].
Metformin
Due to the key role of insulin resistance in PCOS, off-label use of metformin (an insulin sensitizer) has been considered as a first-line treatment for PCOS for many years. Several studies have suggested that metformin (alone or in association with Clomiphene) increased ovulatory cycles in women with PCOS [Citation15]. However, pooled analyses also showed that it does not increase the live birth rate [Citation16,Citation17]. Therefore, the current guidelines do not recommend the use of metformin for ovulation induction and limit the use of this drug to insulin resistance in PCOS and type 2 diabetes [Citation3].
Treatment already confirmed
First line of treatment
Clomiphene citrate
Clomiphene citrate (CC) remains the first-line ovulation induction drug in women with PCOS suffering from infertility [Citation15]. CC is an anti-estrogen therapy that blocks estrogen receptors in the hypothalamus and, with the negative feedback mechanism, leads to a stimulation of the follicular development [Citation5,Citation18,Citation19]. Administration of CC has to be monitored (by ultrasound and endocrine blood sample) to appreciate the day of the ovulation and to prevent multiple pregnancies (rate of 11% risk) [Citation20–23]. As monitoring, ultrasound evaluation is done on days 11 to 14, and measures of the follicular growth and endometrial thickness are also performed [Citation20–23].
Letrozole
Letrozole is a member of the aromatase inhibitors family. Aromatase inhibitors result in lower E2 levels. This strongly reduces the risk of multiple follicle development. This is one of the main advantages of letrozole among CC. The other advantage is that letrozole does not affect endometrial estrogen receptors, and therefore does not exert any deleterious effect on endometrial thickness and cervical mucus [Citation24,Citation25]. Mejia and al suggest a higher ovulation rate with letrozole but there is no evidence of a higher pregnancy rate with this therapy [Citation26] However, recommendations remain that letrozole is a second-line of treatment for women who have CC resistance or failure without another infertility factor [Citation27].
Second line therapeutic options: gonadotropin therapy
A second-line therapeutic option is gonadotropin therapy associated with timed intercourse. For women with PCOS, all recommendations now agree to use a low-dose step-up regimen to prevent ovarian hyperstimulation syndrome (OHSS) and multiple pregnancies. The high number of antral follicles in women with PCOS predispose them to develop OHSS [Citation28,Citation29].
Third line of treatment: in vitro fertilization and in vitro maturation
When the second-line treatment options have failed, more complex therapies should be proposed, mainly in vitro fertilization (IVF) and, more recently, in vitro maturation (IVM).
IVF protocols use the combined action of gonadotropins with gonadotropin-releasing hormone agonist and antagonist.
Complications of this technique are multiple pregnancies and OHSS. After ovulation, multiple luteinized cysts are present within the ovaries leading to larger ovaries with higher vascular permeability resulting in a third space because of the shift of the fluids. Estrogens, progesterone and local cytokines are released as a vascular endothelial growth factor which encourages vascular hyperpermeability. The creation of a third space can lead to hypovolemia, renal insufficiency, and death. Women with PCOS are predisposed to develop OHSS because of the high number of antral follicles [Citation30].
The IVF-associated high risk of OHSS and multiple pregnancies in women with PCOS may be avoided with the in vitro maturation (IVM) procedure. IVM involves a short duration of gonadotrophin stimulation without a trigger injection [Citation31]. Oocytes are retrieved from smaller follicles than what is done in conventional IVF. Oocyte meiosis and maturation to metaphase II occurs in vitro. Particularly appealing for PCOS women, this technique is also an opportunity to minimize exposure to high E2 doses in women with breast cancer or with thrombophilia [Citation28].
New treatments
Myoinositol
Inositol is one of the therapeutic alternatives which has recently been investigated [Citation23]. It acts as the first line of treatment. There are nine different stereoisomers of inositol. Myoinositol is widely found in nature in plants and animal tissues. D-chiro-inositol is the second common isomer. Inositol can be produced in human cells from glucose and is directly involved in insulin cellular signaling [Citation32]. It acts as an intracellular second messenger to regulate hormones like TSH, FSH and insulin. Inositol plays different roles with these stereoisomers, one enhancing cell glucose transportation through the stimulation of the glucose-transporter 4 (GLUT4) translocation to the cell membrane, a second one downregulating the release of free fatty acids from adipose tissue. D-chiro-inositol upregulates the pyruvate dehydrogenase enzyme leading to the production of adenosine triphosphate (ATP), glycogen synthesis and in the ovaries, regulating the insulin-induced androgen synthesis. Myoinositol and D-chiro-inositol promote the enzyme-inducing the conversion from glucose to glycogen.
Myoinositol modulates the activation of glucose utilization and glucose transporters and regulates glucose uptake and FSH signaling in the ovaries.
Administration of D-chiro-inositol has been proved to reduce insulin resistance. Inositol improves the metabolic profile of PCOS patients. Some studies report that the dose needed is 1 g D-chiro-inositol + 400 mcg folic acid per day to reduce metabolic syndrome and to increase the glycemia/insulin ratio [Citation33]. Regidor et al. studied the effect of combined treatment with 2 × 2000 mg myo-inositol and 2 × 200 mcg folic acid per day for 2 and 3 months. They suggested that a myo-inositol therapy in women with PCOS induced a better fertilization rate and better embryo quality. Therefore, the use of myo-inositol is recommended as an improvement in IVF protocols for patients with PCOS with a dose of 4000 mg per day [Citation34].
Studies also demonstrated a role of inositol in metabolic disorders and human reproduction as in ovulation [Citation35]. Pundir et al. [Citation36], in their review, found that inositol supplementation appeared to increase the rates of ovulation and the frequency of menstrual cycles. Nevertheless, no studies found a better live birth rate.
Ovarian drilling
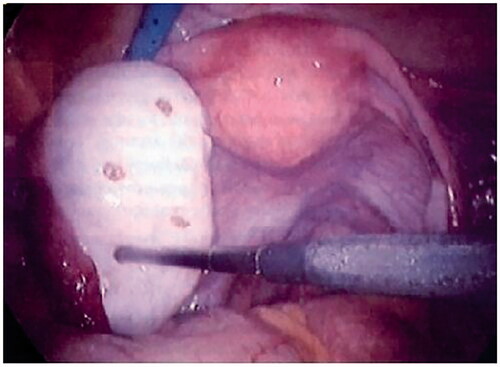
An alternative treatment is laparoscopic or transvaginal ovarian drilling, also called ovarian diathermy or electrocoagulation. It acts as a second line of treatment. Indeed, this technique can be proposed after 4 to 6 cycles of CC and before treatment with gonadotrophins [Citation5]. Ovarian drilling is a technique consisting of puncturing the ovaries. The aim of the technique is to penetrate the ovarian capsule with an electrosurgical probe at a number of points (3 to 6 punctures). Each point measuring 4 mm in diameter and 5–7mm in-depth and the duration of the penetration is 5 s. This technique replaces cuneiform resection of the ovaries. Cuneiform resection leads to adherences and surgical complications and was performed before the beginning of the ovarian drilling technique. [Citation37] ().
The first laparoscopic ovarian drilling was performed in 1984 and the first transvaginal laparoscopy in 2001. Both techniques can be used [Citation38,Citation39].
Technique
Laparoscopic ovarian cautery is performed with a traditional endoscope and two additional trocars. The whole length of the unipolar needle electrode is inserted into the ovary with a setting of 40 W for 4–5 s. The laparoscopic technique is the recommended procedure for ovarian drilling.
Transvaginal hydrolaparoscopy is performed with saline solution in the peritoneal cavity. The endoscope has an angle of 30 degrees and permits examination of the entire pelvic cavity.
Salpingoscopy with fallopian tube and fimbriae inspection can also be performed by transvaginal laparoscopy (THL) [Citation40]. Bilateral ovarian drilling was performed, with about 10 perforations in each ovary and a depth of insertion of 10 mm. This technique can be realized with spinal anesthesia [Citation41].
The risks associated with ovarian drilling are those linked to any surgical procedure and the risk of creating adhesion. The choice of the technique (laparoscopy versus THL) may influence safety. Giampaolino et al. compared the benefits of laparoscopy versus THL [Citation40]. Results showed that THL is faster, reduces adhesions, and is better tolerated by the patients (less post-operative pain). The mechanisms of these benefits could be the installation of saline solution, the shorter duration, the use of bipolar diathermy and the reduction of bleeding due to lower manipulation of the ovaries than during a laparoscopy.
THL is also an easier approach for women with obesity [Citation40,Citation42]. Finally, Giampaolino et al. also suggest that the learning curve with THL is shorter than with laparoscopy.
Nevertheless, the risk of this technique is a perforation of the rectum, (0.5% risk) [Citation43]. Should this happen, it is usually managed by conservative treatment and antibiotics. A second-look laparoscopy will be performed to confirm the peritoneal integrity.
Mechanism of action
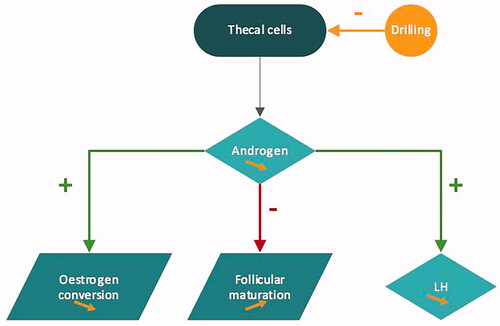
The mechanism of action of ovarian drilling is still poorly understood. It seems that ovarian drilling impairs local androgen synthesis and reduces intraovarian androgen level which decreases the inhibitory effect on follicular maturation. Lower levels of androgens diminish the peripheral conversion of androgen to estrogen and decrease the positive feedback on LH secretion. Recruitment of new follicles is permitted spontaneously or with exogenous FSH stimulation [Citation44] ().
This technique improves hirsutism and acne, irregular cycles, the occurrence of ovulation and pregnancy [Citation41,Citation45].
Ovarian drilling does not require the monitoring of ultrasound and avoids the risk of multiple pregnancies or OHSS [Citation41]. In 2020, a Cochrane review showed moderate-quality evidence that laparoscopic ovarian drilling probably reduces the number of multiple pregnancies and may result in less OHSS [Citation46].
The efficacy of ovarian drilling is variable in the literature. Ovulation and pregnancy rates of 30 to 90% and 13 to 80%, respectively, have been reported [Citation47]. Factors increasing the efficacy of the technique are high LH concentration (>10UI/l), short infertility duration (<3 years), age (<35 years) and low antral follicle count (<50) [Citation48]. High BMI (>35kg/m2), insulin resistance and high testosterone concentration are negative factors for the effectiveness of this treatment [Citation41].
The choice of the technique may also influence the results. In the first 6 months following THL, Giampaolino et al. demonstrated in 2018 that ovulation occurred in 82.9% of the patients associated with a pregnancy rate of 70% [Citation41].
Besides its efficacy in terms of pregnancy rate, the other big advantage of ovarian drilling is the long-term maintenance of its benefits. Indeed, studies have shown that improvement/resolution of the symptoms will persist up to 20 years after the procedure in more than 60% of the patients. This long-term efficacy is not achieved with the other treatment options of PCOS (excepted lifestyle modifications when they are maintained through the years) [Citation30]. Debras et al. showed in 2020 in a retrospective study that ovarian drilling permits spontaneous pregnancy and has a long-term effect. The predictive factors for this effectiveness were a normal body mass index (BMI), an infertility period of less than three years, an AFC of less than 50, and an age of less than 35 [Citation44].
Conclusion
PCOS is a frequent syndrome and the most frequent cause of infertility. PCOS is defined as a syndrome with at least two of three of the Rotterdam criteria. A complete evaluation of the infertility is needed to exclude other causes of infertility. PCOS treatment is still controversial but three lines of therapies were discussed. The first line of treatment remains lifestyle modifications and bariatric surgery-associated or not with metformin and myoinositol. Clomiphene citrate and letrozole are considered also as the first line of treatment. Gonadotrophin therapy and ovarian drilling are the second line of treatment. Nevertheless, the place of transvaginal hydrolaparoscopic ovarian drilling is still not well clarified. Further studies are necessary to encourage this technique. If the patient is still resistant to those therapies, a third-line of treatment is proposed as in vitro fertilization and in vitro maturation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril. 2013;99(1):63.
- Pasquali R, Antenucci D, Casimirri F, et al. Clinical and hormonal characteristics of obese amenorrheic hyperandrogenic women before and after weight loss. J Clin Endocrinol Metab. 1989;68(1):173–179.
- Tarlatzis BC, Fauser BCJM, Legro RS, et al. Consensus on infertility treatment related to polycystic ovary syndrome. Hum Repro. 2008;23(3):462–477.
- Gibson-Helm ME, Lucas IM, Boyle JA, et al. Women’s experiences of polycystic ovary syndrome diagnosis. Fam Pract. 2014;31(5):545–549.
- Melo AS, Ferriani RA, Navarro PA. Treatment of infertility in women with polycystic ovary syndrome: approach to clinical practice. Clinics. 2015;11:765–769.
- Sahmay S, Aydogan Mathyk B, Sofiyeva N, et al. Serum AMH levels and insulin resistance in women with PCOS. Eur J Obstet Gynecol Reprod Biol. 2018;224:159–164.
- Artini PG, Obino MER, Sergiampietri C, et al. PCOS and pregnancy: a review of available therapies to improve the outcome of pregnancy in women with polycystic ovary syndrome. Expert Rev Endocrinol Metabol. 2018;13(2):87–98.
- Kiddy DS, Hamilton‐Fairley D, Bush A, et al. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol. 1992;36(1):105–111.
- Balen AH, Dresner M, Scott EM, et al. Should obese women with polycystic ovary syndrome receive treatment for infertility? BMJ. 2006;332(7539):434–435.
- Crosignani PG, Colombo M, Vegetti W, et al. Overweight and obese anovulatory patients with polycystic ovaries: parallel improvements in anthropometric indices, ovarian physiology and fertility rate induced by diet. Hum Reprod. 2003;18(9):1928–32.
- Malik SM. Defining the role of bariatric surgery in polycystic ovarian syndrome patients. World J Diabetes. 2012;3(4):71–79.
- Escobar-Morreale HF, Santacruz E, Luque-Ramírez M, et al. Prevalence of “obesity-associated gonadal dysfunction” in severely obese men and women and its resolution after bariatric surgery: A systematic review and meta-analysis. Hum Reprod Update. 2017;23(4):390–408.
- Mahawar KK, Parmar C, Graham Y. One anastomosis gastric bypass: key technical features, and prevention and management of procedure-specific complications. Minerva Chir. 2019;74(2):126–136.
- Arterburn DE, Telem DA, Kushner RF, et al. Benefits and risks of bariatric surgery in adults: a review. JAMA. 2020;324(9):879–887.
- Morley LC, Tang T, Yasmin E, et al. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev. 2017;11(11):CD003053.
- Legro RS, Barnhart HX, Schlaff WD, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356(6):551–566.
- Johnson NP, Stewart AW, Falkiner J, et al. PCOSMIC: a multi-centre randomized trial in women with polycystic ovary syndrome evaluating metformin for infertility with clomiphene. Hum Reprod. 2010;25(7):1675–1683.
- Kettel LM, Roseff SJ, Berga SL, et al. Hypothalamic-pituitary-ovarian response to clomiphene citrate in women with polycystic ovary syndrome. Fertil Steril. 1993;59(3):532–538.
- Kerin JF, Liu JH, Phillipou G, et al. Evidence for a hypothalamic site of action of clomiphene citrate in women. J Clin Endocrinol Metab. 1985;61(2):265–268.
- Kumar P, Sait S. Luteinizing hormone and its dilemma in ovulation induction. J Hum Reprod Sci. 2011;4(1):2–7.
- Dehbashi S, Parsanezhad ME, Alborzi S, et al. Effect of clomiphene citrate on endometrium thickness and echogenic patterns. Int J Gynecol Obstet. 2003;80(1):49–53.
- Zhao J, Zhang Q, Li Y. The effect of endometrial thickness and pattern measured by ultrasonography on pregnancy outcomes during IVF-ET cycles. Reprod Biol Endocrinol. 2012;10(1):100.
- Unfer V, Facchinetti F, Orrù B, et al. Myo-inositol. effects in women with PCOS: A meta-analysis of randomized controlled trials. Endocr Connect. 2017;6(8):647–658.
- Huang S, Du X, Wang R, et al. Ovulation induction and intrauterine insemination in infertile women with polycystic ovary syndrome: A comparison of drugs. Eur J Obstet Gynecol Reprod Biol. 2018;231:117–121.
- Mejia RB, Summers KM, Kresowik JD, et al. A randomized controlled trial of combination letrozole and clomiphene citrate or letrozole alone for ovulation induction in women with polycystic ovary syndrome. Fertil Steril. 2019;111(3):571–578.e1.
- Casper RF. Letrozole versus clomiphene citrate: which is better for ovulation induction? Fertil Steril. 2009;92(3):858–859.
- Balen AH, Morley LC, Misso M, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update. 2016;22(6):687–708.
- Helmy M, Saleh S, El-Khouly N, et al. Transvaginal needle versus laparoscopic ovarian drilling in drug-resistant polycystic ovary syndrome: a randomized, controlled study. Menoufia Med J. 2019; 32(2):436–440.
- Weiss NS, Kostova E, Nahuis M, et al. Gonadotrophins for ovulation induction in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2019;1(1):CD010290.
- Gjønnaess H. Late endocrine effects of ovarian electrocautery in women with polycystic ovary syndrome. Fertil Steril. 1998;69(4):697–701.
- Walls ML, Hart RJ. In vitro maturation. Best Pract Res: Clin Obstetr Gynaec. 2018;53:60–72.
- Fauser BCJM, Tarlatzis BC, Rebar RW, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97(1):28–38.e25.
- Laganà AS, Rossetti P, Sapia F, et al. Evidence-based and patient-oriented inositol treatment in polycystic ovary syndrome: changing the perspective of the disease. Int J Endocrinol Metab. 2017;15(1):e43695.
- Atay V, Cam C, Muhcu M, et al. Comparison of letrozole and clomiphene citrate in women with polycystic ovaries undergoing ovarian stimulation. J Int Med Res. 2006;34(1):73–76.
- Baillargeon JP, Iuorno MJ, Jakubowicz DJ, et al. Metformin therapy increases insulin-stimulated release of D-Chiro-inositol-containing inositolphosphoglycan mediator in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89(1):242–249.
- Pundir J, Psaroudakis D, Savnur P, et al. Inositol treatment of anovulation in women with polycystic ovary syndrome: a meta-analysis of randomised trials. BJOG. 2018;125(3):299–308.
- Laffargue P, Gares R, Luscan R, et al. Syndrome de Stein-Leventhal d’origine congénitale probable guéri par résection cunéiforme des ovaires et cortisone; hyperplasie fonctionnelle du stroma ovarien [Stein-Leventhal syndrome of probable congenital origin cured by cuneiform resection of ovaries & cortisone; functional hyperplasia of the ovarian stroma]. Bull Fed Soc Gynecol Obstet Lang Fr. 1957;9(3):317–322.
- Mitra S, Nayak PK, Agrawal S. Laparoscopic ovarian drilling: an alternative but not the ultimate in the management of polycystic ovary syndrome. J Nat Sci Biol Med. 2015;6(1):40–48.
- Ferraretti AP, Gianaroli L, Magli MC, et al. Transvaginal ovarian drilling: a new surgical treatment for improving the clinical outcome of assisted reproductive technologies in patients with polycystic ovary syndrome. Fertil Steril. 2001;76(4):812–816.
- Giampaolino P, Morra I, Tommaselli GA, et al. Post-operative ovarian adhesion formation after ovarian drilling: a randomized study comparing conventional laparoscopy and transvaginal hydrolaparoscopy. Arch Gynecol Obstet. 2016;294(4):791–796.
- Debras E, Fernandez H, Neveu ME, et al. Ovarian drilling in polycystic ovary syndrome: Long term pregnancy rate. Eur J Obstet Gynecol Reprod Biol X. 2019;4:100093.
- Giampaolino P, De Rosa N, Della Corte L, et al. Operative transvaginal hydrolaparoscopy improve ovulation rate after clomiphene failure in polycystic ovary syndrome. Gynecol Endocrinol. 2018;34(1):32–35.
- Ezedinma NA, Phelps JY. Transvaginal hydrolaparoscopy. JSLS. 2012;16(3):461–465.
- Lebbi I, Ben Temime R, Fadhlaoui A, et al. Ovarian drilling in PCOS: is it really useful? Front Surg. 2015;2:30.
- Lemieux S, Lewis GF, Ben-Chetrit A, et al. Correction of hyperandrogenemia by laparoscopic ovarian cautery in women with polycystic ovarian syndrome is not accompanied by improved insulin sensitivity or lipid-lipoprotein levels. J Clin Endocrinol Metab. 1999;84:4278–4282.
- Bordewijk EM, Ng KYB, Rakic L, et al. Laparoscopic ovarian drilling for ovulation induction in women with anovulatory polycystic ovary syndrome. Cochrane Database Syst Rev. 2020;2:CD001122.
- Seow KM, Juan CC, Hwang JL, et al. Laparoscopic surgery in polycystic ovary syndrome: reproductive and metabolic effects. Semin Reprod Med. 2008;26(1):101–110.
- Practice Committee of American Society For Reproductive M.Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril. 2012;98(2):302–307.
Appendix
Gynecological history including duration of infertility, menstrual cycle length and menstrual bleeding characteristics has to be noted.
Physical examination includes the body mass index, blood pressure, signs of hyperandrogenism, acanthosis, exophthalmos, developments of second sexual characters, … hyperandrogenism is often characterized by acne (on more than 2 sites on the body) and hirsutism. Hirsutism is diagnosed by an excess of hair on androgenic regions well defined by Ferriman and Gallway score. Androgenic alopecia and acanthosis can also be highlighted.
Tenderness of posterior cul-de-sac, uterosacral ligaments, tumors, uterine abnormalities have also to be noticed during the gynecological examination.
The most important items to cover during anamnesis and examination are listed in .
Table A1. Exploration of infertility.