Abstract
Objective
This study aimed to investigate serum levels of adiponectin, and the mRNA expression of forkhead box C2 (FOXC2) and glucose transporter-4 (GLUT4) in visceral adipose tissue obtained from patients with gestational diabetes mellitus (GDM) and healthy pregnant women.
Methods
Venous blood samples were obtained from 60 pregnant women with gestational normal glucose tolerance (GNGT) and 21 patients with GDM. Visceral adipose tissues were obtained from 11 women with GDM and 30 with GNGT. Serum adiponectin levels were detected by enzyme-linked immunosorbent assay, and FOXC2 and GLUT4 mRNA expression were detected by quantitative polymerase chain reaction.
Results
Serum adiponectin concentrations were lower in the women with GDM than in the controls (p < .05). FOXC2 and GLUT4 mRNA expression were decreased in visceral adipose tissue of GDM women than in the controls (p < .05). Correlation analyses showed that FOXC2 tended to have a positive correlation with GLUT4 in GDM patients' visceral adipose tissue (p =.0564).
Conclusion
Our results revealed that decreased adiponectin, FOXC2, and GLUT4 expression were associated with increased risk of GDM and the regulation mechanism of GLUT4 mediated by FOXC2 would be the focus of further studies.
摘要
目的:本研究旨在研究妊娠期糖尿病(GDM)患者和健康孕妇内脏脂肪组织中血清脂联素水平以及叉头框C2(FOXC2)和葡萄糖转运体4(GLUT4)的mRNA表达。 方法:抽取60例妊娠期糖耐量正常孕妇(GNGT)和21例GDM患者的静脉血。内脏脂肪组织取自11例GDM患者和30例GNGT患者。采用酶联免疫吸附法检测血清脂联素水平, 定量聚合酶链反应法检测FOXC2、GLUT4 mRNA表达。 结果:GDM患者血清脂联素浓度低于对照组(p<0.05)。与对照组相比, GDM患者内脏脂肪组织中FOXC2和GLUT4的mRNA表达降低(p<0.05)。相关性分析显示, 在GDM患者的内脏脂肪组织中, FOXC2与GLUT4呈正相关(p=0.0564)。 结论:此研究显示, 脂联素、FOXC2和GLUT4表达降低与GDM发病风险升高相关, FOXC2介导的GLUT4调控机制将是今后研究的重点。
Introduction
Gestational diabetes mellitus (GDM) is defined as glucose intolerance first occurring during pregnancy, which can lead to adverse pregnancy outcomes [Citation1,Citation2] and predispose the mother and the offspring to type 2 diabetes and metabolic syndrome later in life [Citation3,Citation4]. The pathogenesis of GDM remains incompletely understood. Several studies have found that the occurrence and development of GDM is related to the levels of some inflammatory factors and adipocytokines such as adiponectin, tumor necrosis factor-α, leptin [Citation5–7].
Adiponectin is the most abundant peptide secreted by adipocytes that plays a vital role in preventing insulin resistance/diabetes. Recently, clinical studies have revealed that hypoadiponectinemia is associated with a marked increase in risk for development of GDM [Citation8]. Adiponectin deficiency induces hyperglycemia and other metabolic defects of GDM in pregnant adiponectin gene knockout (Adipoq−/−) mice [Citation9]. Glucose transporter-4 (GLUT4) is an essential glucose transporter in the skeletal muscle membrane and is an important factor of glucose intolerance. The overexpression of GLUT4 significantly improves insulin-signaling in GDM, leading to improved glycemic control and increased insulin secretion in spontaneous gestational diabetic C57BLKS/J Lepr(db/+) mice [Citation10]. Forkhead box C2 (FOXC2) is a winged helix/forkhead transcription factor gene and plays an important role in adipocyte metabolism [Citation11]. The human FOXC2 gene is located on chromosome 16q24.3, and the genetic variation of the FOXC2 gene is associated with triglyceride levels [Citation12]. Reduced expression of FOXC2 and brown adipogenic genes such as MASK in human is associated with insulin resistance [Citation13].
What remains unclear is that the expression of FOXC2 in GDM and its role in the occurrence and development of GDM. The aim of this study was to investigate the expression of FOXC2 and GLUT4 in adipose tissue and the level of adiponectin in serum of GDM patients to provide new ideas for the prevention of GDM.
Materials and methods
Study population and inclusion criteria
This study was carried out prospectively in the department of Obstetrics and Gynecology of The First Hospital of Changsha from 2018 until 2020 and all participants provided informed consent. The Institutional Ethics Committee of The First Hospital of Changsha, complying with the ethical guidelines of the 1975 Declaration of Helsinki, approved the collection of samples. Venous blood samples were obtained from 21 patients with GDM and 60 pregnant women with gestational normal glucose tolerance (GNGT) and age-matched pregnancy group. The samples of visceral adipose tissue were obtained from 11 women with GDM and 30 with GNGT who underwent cesarean section. Both the study groups were recruited from the same hospital and during the same time period.
Pregnant women receiving systematic periodic examination and women with GDM under strict glucose control were recruited at the time of an elective surgical procedure. Exclusion criteria included acute inflammation, hypertension during pregnancy, liver and kidney disease, cardiovascular disease, thyroid disease, polycystic ovary syndrome, or tumors.
Real-time PCR
Tissue specimens stored at liquid nitrogen were added with trizol (Takara) to extract the total RNA, and 1 μg of RNA was reverse transcribed into cDNA with PrimeScript RT reagent Kit (Takara). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed to detect genes expression by using TB Green real-time qPCR Kit (Takara) on LightCycler® 480 system (Roche Diagnostics). The relative expression of FOXC2 and GLUT4 was calculated and normalized using the 2−ΔΔCt method relative to GAPDH. The primer sequences are shown in Supplemental Table S1.
Table 1. Clinical characteristics of the study population.
Enzyme-linked immunosorbent assay
Venous blood samples obtained before surgery were centrifuged to separate plasma immediately and stored at −80 °C until detected. Serum samples were diluted by 1:4000 for the detection of adiponectin. Adiponectin was measured in duplicate in GDM and GNGT samples according to the manufacturer’s protocol of the commercial ELISA kit (Proteintech).
Statistical analysis
Clinical characteristics data, conforming to the normal distribution, was presented as the mean ± SD, otherwise expressed as median and interquartile range. Statistical analysis was performed using the SPSS version 18.0 (IBM Corporation). The Student’s t-test or Mann–Whitney test was used to analyze the differences between the two groups. p <.05 were considered statistically significant. Correlation analysis was conducted to examine the possible correlations of gene expressions and adiponectin level and other clinical indicators.
Results
Comparison of clinical characteristics between pregnant females with and without GDM
The characteristics of patients from whom the serum samples were obtained are shown in . Homeostasis model assessment of insulin resistance (HOMA-IR) and triglyceride levels (p<.05) were higher in the GDM group compared to the GNGT group (). Total cholesterol levels (p<.05) and high-density lipoprotein cholesterol levels were significantly lower in GDM compared to GNGT women (). However, there were no significant difference between normal pregnant females and those with GDM regard to age, gestational age, body mass index, fasting plasma glucose levels, fasting insulin levels, HemoglobinA1c levels, and low-density lipoprotein cholesterol levels.
Serum levels of adiponectin in GDM and GNGT
In order to detect the serum adiponectin levels of females with GDM, Elisa were performed. Serum adiponectin (p <.05, ) was significantly lower in the GDM group (4.624 ± 1.356 μg/mL) compared with the GNGT group (6.030 ± 2.697 μg/mL).
Correlations between adiponectin serum levels with clinical parameters
The correlations in all, GNGT, and GDM women between adiponectin serum levels and maternal clinical parameters are shown in .
Table 2. Correlations between adiponectin serum levels and clinical parameters in all women, GNGT, and GDM women.
In all women, adiponectin expression tended to have a negative correlation with age (p = .0507), and were significantly negatively correlated with BMI and HOMA-IR (all p<.05). Serum adiponectin levels had positive correlations with TC and HDL-C (all p<.05) in all and GNGT women and tended to negatively correlate with BMI (p = .0574) in GNGT women. However, there were no significant correlation between serum adiponectin levels and other clinical parameters.
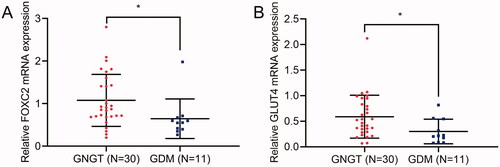
FOXC2 and GLUT4 expression is decreased in adipose tissues of females with GDM
In order to detect the levels of FOXC2 and GLUT4 in adipose tissues of females with GDM, qRT-PCR was performed. The results indicated that the mRNA expression of FOXC2 and GLUT4 were both downregulated in GDM adipose specimens (n = 11) compared with that in GNGT women (n = 30) ().
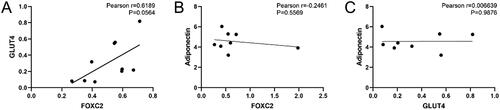
Correlations among FOXC2, GLUT4, and adiponectin serum levels in GDM women
Correlations among FOXC2, GLUT4, and adiponectin were analyzed by Pearson correlation and further by linear regression analysis. As shown in , analysis indicated that FOXC2 tended to have a positive correlation with GLUT4 (r = 0.6189, y = 1.060x − 0.2273, p = .0564). While there was no significant correlation between serum adiponectin levels with mRNA expression of FOXC2 and GLUT4 ().
Discussion
Adiponectin almost exclusively secretes from adipose tissue, and circulates into the bloodstream in three main oligomeric forms. In the last years, investigations demonstrated the involvement of adiponectin in GDM [Citation14]. Low pre-pregnancy adiponectin concentration was associated with increased risk of developing GDM, and these associations might be independent of known metabolic risk factors for GDM [Citation8]. Our results indicated that serum adiponectin levels were lower in GDM compared to control women, which was similar to a previous finding [Citation15–17]. Furthermore, HOMA-IR, TC, TG, and HDL-C levels in our study were comparable between the GDM and the GNGT group, and serum adiponectin levels had positive correlations with TC and HDL-C in all and GNGT women rather than GDM women.
FOXC2 expressed in the adipose tissues and skeletal muscles of humans, and the adipose tissues of adult mice [Citation18]. Several studies identified that FOXC2 regulated adipocyte metabolism associated with whole body insulin sensitivity by involving in beta-adrenergic-cAMP-protein kinase A signaling pathway [Citation11,Citation19,Citation20]. The expression level of FOXC2 protein and mRNA in the visceral adipose tissue of T2DM patients was significantly lower than that of nondiabetic patients [Citation21]. GLUT4 is the main gene involved in adipose differentiation and glucose metabolism, which plays an important role in the production of insulin resistant. Both adipose tissue and skeletal muscle from women with GDM displayed decreased GLUT4 mRNA and protein expression compared normal glucose-tolerant pregnant control [Citation22]. FOXC2 could promote the expression of GLUT4, and FOXC2 participates in the regulation of insulin resistance by regulating insulin-resistance-related genes GLUT4 [Citation23]. We speculate that FOXC2 might involve in the development of GDM through GLUT4.
Our study showed decreased FOXC2 mRNA expression in the visceral adipose tissue of GDM patients as compared with the healthy. At the same time, the expression of GLUT4 in GDM was also lower than in the controls, which is consistent with previous finding [Citation22,Citation24,Citation25]. In addition, FOXC2 mRNA expression was also positively related to GLUT4 mRNA expression in visceral adipose tissue of GDM.
In summary, low serum adiponectin concentrations, low FOXC2, and GLUT4 mRNA expression were associated with increased risk of GDM and may be a critical factor for the pathogenesis of GDM. Lower FOXC2 expression may affect GLUT4 levels in visceral adipose tissue, eventually leading to GDM. However, more research is needed in order to determine the role and precise mechanism(s) of FOXC2 in the pathogenesis of GDM.
Supplemental Material
Download MS Word (15.2 KB)Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- O'Sullivan JB, Gellis SS, Dandrow RV, et al. The potential diabetic and her treatment in pregnancy. Obstet Gynecol. 1966;27(5):683–689.
- O'Sullivan JB, Gellis SS, Dandrow RV, et al. The potential diabetic and her treatment in pregnancy. Obstet Gynecol. 2003;102(1):7–9.
- Hummel S, Much D, Rossbauer M, et al. Postpartum outcomes in women with gestational diabetes and their offspring: POGO study design and first-year results. Rev Diabet Stud. 2013;10(1):49–57.
- Aviram A, Guy L, Ashwal E, et al. Pregnancy outcome in pregnancies complicated with gestational diabetes mellitus and late preterm birth. Diabetes Res Clin Pract. 2016;113:198–203.
- Lappas M. Activation of inflammasomes in adipose tissue of women with gestational diabetes. Mol Cell Endocrinol. 2014;382(1):74–83.
- Fasshauer M, Blüher M, Stumvoll M. Adipokines in gestational diabetes. Lancet Diabetes Endocrinol. 2014;2(6):488–499.
- Fonseca MJ, Santos AC. Umbilical cord blood adipokines and newborn weight change. Arch Gynecol Obstet. 2015;291(5):1037–1040.
- Hedderson MM, Darbinian J, Havel PJ, et al. Low prepregnancy adiponectin concentrations are associated with a marked increase in risk for development of gestational diabetes mellitus. Diabetes Care. 2013;36(12):3930–3937.
- Qiao L, Wattez JS, Lee S, et al. Adiponectin deficiency impairs maternal metabolic adaptation to pregnancy in mice. Diabetes. 2017;66(5):1126–1135.
- Ishizuka T, Klepcyk P, Liu S, et al. Effects of overexpression of human GLUT4 gene on maternal diabetes and fetal growth in spontaneous gestational diabetic C57BLKS/J Lepr(db/+) mice. Diabetes. 1999;48(5):1061–1069.
- Cederberg A, Grønning LM, Ahrén B, et al. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106(5):563–573.
- Pajukanta P, Allayee H, Krass KL, et al. Combined analysis of genome scans of Dutch and Finnish families reveals a susceptibility locus for high-density lipoprotein cholesterol on chromosome 16q. Am J Hum Genet. 2003;72(4):903–917.
- Yang X, Enerbäck S, Smith U. Reduced expression of FOXC2 and brown adipogenic genes in human subjects with insulin resistance. Obes Res. 2003;11(10):1182–1191.
- de Gennaro G, Palla G, Battini L, et al. The role of adipokines in the pathogenesis of gestational diabetes mellitus. Gynecol Endocrinol. 2019;35(9):737–751.
- Catalano PM, Hoegh M, Minium J, et al. Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia. 2006;49(7):1677–1685.
- Lowe LP, Metzger BE, Lowe WL, Jr, et al. Inflammatory mediators and glucose in pregnancy: results from a subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. J Clin Endocrinol Metab. 2010;95(12):5427–5434.
- Xu J, Zhao YH, Chen YP, et al. Maternal circulating concentrations of tumor necrosis factor-alpha, leptin, and adiponectin in gestational diabetes mellitus: a systematic review and meta-analysis. Scient World J. 2014;2014:926932.
- Nakae J, Kitamura T, Kitamura Y, et al. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell. 2003;4(1):119–129.
- Di Gregorio GB, Westergren R, Enerback S, et al. Expression of FOXC2 in adipose and muscle and its association with whole body insulin sensitivity. Am J Physiol Endocrinol Metab. 2004;287(4):E799–803.
- Ridderstråle M, Carlsson E, Klannemark M, et al. FOXC2 mRNA expression and a 5' untranslated region polymorphism of the gene are associated with insulin resistance. Diabetes. 2002;51(12):3554–3560.
- Nian X, Zhang X, Wang Y, et al. Correlations of FOXC2 gene expression and polymorphism with type 2 diabetes mellitus. Clin Lab. 2016;62(5):781–791.
- Colomiere M, Permezel M, Lappas M. Diabetes and obesity during pregnancy alter insulin signalling and glucose transporter expression in maternal skeletal muscle and subcutaneous adipose tissue. J Mol Endocrinol. 2010;44(4):213–223.
- Zhang X, Wang Y, Zuo F, et al. Effect of insulin-regulated FOXC2 expression in adipocyte differentiation and insulin resistance. DMSO. 2020;13:2801–2809.
- Tumurbaatar B, Poole AT, Olson G, et al. Adipose tissue insulin resistance in gestational diabetes. Metab Syndr Relat Disord. 2017;15(2):86–92.
- Feng C, Jin Z, Sun L, et al. Endogenous SHBG levels correlate with that of glucose transporters in insulin resistance model cells. Mol Biol Rep. 2019;46(5):4953–4965.