Abstract
Objective: The objective of the present document was to review/summarize reported outcomes compared between menopausal hormone therapy (MHT) containing estradiol (E2) versus other estrogens and MHT with progesterone (P4) versus progestins (defined as synthetic progestogens).
Methods: PubMed and EMBASE were systematically searched through February 2021 for studies comparing oral E2 versus oral conjugated equine estrogens (CEE) or P4 versus progestins for endometrial outcomes, venous thromboembolism (VTE), cardiovascular outcomes, breast outcomes, cognition, and bone outcomes in postmenopausal women.
Results: A total of 74 comparative publications were identified/summarized. Randomized studies suggested that P4 and progestins are likely equally effective in preventing endometrial hyperplasia/cancer when used at adequate doses. E2- versus CEE-based MHT had a similar or possibly better risk profile for VTE and cardiovascular outcomes, and P4- versus progestin-based MHT had a similar or possibly better profile for breast cancer and cardiovascular outcomes. E2 may potentially protect better against age-related cognitive decline and bone fractures versus CEE; P4 was similar or possibly better versus progestins for these outcomes. Limitations are that many studies were observational and some were not adequately powered for the reported outcomes.
Conclusions: Evidence suggests a differential effect of MHT containing E2 or P4 and those containing CEE or progestins, with some evidence trending to a potentially better safety profile with E2 and/or P4.
摘要
目的
本文的目的是回顾/总结已有结果, 比较雌二醇(E2)与其他雌激素的绝经激素治疗(MHT), 以及黄体酮(P4)与其他孕激素(定义为合成孕激素)的MHT。
方法
2021年2月在PubMed和EMBASE进行系统的文献检索, 比较口服E2与口服结合雌激素(CEE)或P4与孕激素对绝经后女性子宫内膜、静脉血栓(VTE)、心血管结局、乳腺、认知和骨骼的影响。
结果
74篇文献可用于鉴定/总结。随机研究显示, 如果剂量足够, P4和其他孕激素在预防子宫内膜增生/癌方面, 可能同样有效。E2和基于CEE的MHT比较, 对于VTE和心血管结局具有相似或更好的风险特征, 而P4与基于其他孕激素的MHT相比, 对于乳腺癌和心血管结局具有相似或更好的风险特征。和CEE相比, E2可能可以更好地预防与年龄相关的认知下降和骨折;在这些方面, P4与其他孕激素相似或可能更好。局限性在于许多研究是观察性的, 有些研究没有充分证明所报告的结果。
结论
证据显示, 含E2或P4的MHT与含CEE或其他孕激素的MHT具有不同效果, 一些证据显示含E2和/或P4的MHT可能具有更好的安全性。
Introduction
Menopausal hormone therapy (MHT) with estrogen alone or in combination with a progestogen is widely used to prevent or treat menopause-related hot flushes, night sweats, dyspareunia, vaginal dryness, and osteoporosis in postmenopausal women [Citation1]. The most commonly prescribed estrogens are estradiol (E2) and conjugated equine estrogens (CEE), while the common progestogen choices include natural progesterone (P4) and those derived from progesterone or testosterone, such as medroxyprogesterone acetate (MPA) and norethisterone acetate (NETA) (i.e. progestins, defined as synthetic progestogens).
Results from the Women’s Health Initiative (WHI) study of CEE plus MPA raised safety concerns of MHT [Citation2, Citation3]. Many women discontinued MHT, especially oral estrogens or estrogens/progestins, following these publications [Citation4], and use of E2- and P4-containing MHT increased [Citation5].
The objective here was to review and summarize clinical data from studies/publications directly comparing the effects of oral E2 versus CEE and P4 versus progestins on endometrial outcomes, venous thromboembolism (VTE), cardiovascular disease (CVD) outcomes, stroke, breast outcomes, cognition, bone outcomes, vasomotor symptom (VMS), and quality of life (QOL).
Methods
Data sources and searches
The concept of this review stemmed from a request by the US Food and Drug Administration to provide data supporting a therapeutic benefit of low-dose estradiol/progesterone over low-dose estrogen/progestin products. PubMed and EMBASE were systematically searched from inception through February 2021, using keywords: endometrium, endometrial, cardiovascular, coronary, VTE, thrombosis, myocardial infarction (MI), coronary artery bypass graft (CABG), stroke, coagulation, lipids, hypertension, blood pressure (BP), mortality, breast, cognition, bone, hot flush, VMS, QOL, weight, metabolic, and sleep, in conjunction with (E2 and menopause) or (P4 and menopause). Relevant studies were also identified from review articles.
Study selection
English-language clinical studies, including meta-analyses, comparing formulations with different estrogens or different progestogens were included. Only studies on oral E2 and oral CEE were included for estrogen comparisons. Some large observational studies comparing risks for different formulations side-by-side were included given the breadth of data. Studies of transdermal estrogens were included only if they compared oral P4 with another progestogen during transdermal estrogen use. Comparisons of P4 versus progestins were limited to oral regimens as transdermal P4 does not provide adequate endometrial protection [Citation6].
Data extraction and summary
Four authors/reviewers independently screened the search results for eligible studies. One author extracted details of study design, patient population, interventions, and targeted outcomes for effects of E2 versus CEE and P4 versus progestins, and a medical writer quality checked the accuracy of data extraction.
Data from the identified studies were not reported/published in a way appropriate to generate a meta-analysis. Results from all identified trials with comparisons were summarized based on study quality by study design with any randomized controlled trials (RCTs) summarized first followed by observational studies, then by study size. Tables of evidence were generated, studies were organized by main outcome, and conclusions were made based on the overall trend of the reported data.
Results
Article review
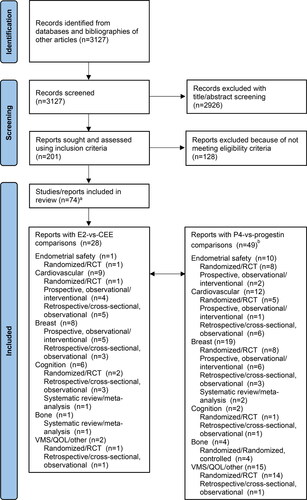
Twenty-eight articles comparing oral E2 versus CEE [Citation7–34] and 48 articles comparing oral P4 versus progestins were identified (3 studies had both comparisons; ) with relevant outcomes [Citation21, Citation28, Citation34–79]. Among these articles, 32 reported findings from prospective, randomized studies or RCTs [Citation7, Citation22–25, Citation51–77]; 19 from prospective, observational/interventional trials [Citation11–18, Citation26, Citation33, Citation36, Citation43–50]; 18 from retrospective or cross-sectional, observational studies [Citation8, Citation19–21, Citation27–32, Citation34, Citation35, Citation37–40, Citation78, Citation79]; and 4 from meta-analyses [Citation9, Citation10, Citation41, Citation42]. A recently presented retrospective observational study comparing E2/P4 versus CEE/MPA was also included [Citation80].
Figure 1. Study selection follow chart. CEE: conjugated equine estrogens; E2: estradiol; P4: progesterone; QOL: quality of life; VMS: vasomotor symptoms. aThree articles reported outcomes with both E2 versus CEE and P4 versus progestins. bThirteen articles reported multiple outcomes with P4 versus progestins.
Endometrial outcomes
Details of the clinical studies included in this section can be found in .
Table 1. Studies comparing endometrial outcomes with E2 versus CEE and P4 versus synthetic progestogens.
Thickness, hyperplasia and cancer
The effect of unopposed estrogens on endometrial thickness was evaluated in one randomized study, which showed that estrogen has a dose-dependent effect on the endometrium, with the 1 mg E2 and 0.625 mg CEE having similar estrogenic effects [Citation7].
Three randomized studies, including the Postmenopausal Estrogen/Progestin Interventions (PEPI) trial [Citation51], showed that the addition of 200 mg P4 to estrogens versus other progestogens (10 mg MPA, 10 mg dydrogesterone [DYD], 5 mg nomegestrol acetate, 10 mg chlormadinone acetate [CMA]) similarly counteracted the effects of the estrogens on endometrial thickness, hyperplasia, and other endometrial biopsy observations [Citation51, Citation53, Citation54, Citation56]. Another randomized study reported a similar proportion of women with increased endometrial thickness ≥5 mm with estradiol valerate plus 100 mg P4 or 4 mg MPA [Citation63].
Endometrial cancer risk was evaluated in two large European (1992–2000) [Citation43] and French (1990–2008) [Citation44] observational studies, which reported increased risks with current or ever use [Citation43, Citation44] or >5 years use (but not ≤5 years) [Citation44] of estrogens plus P4 versus no hormone use, while estrogens combined with progestins were not associated with an increased risk [Citation43, Citation44]. However, P4 doses were not reported in either study, a limitation of these observational studies.
Uterine bleeding
One randomized study directly compared unopposed E2 or CEE and reported similar unscheduled vaginal bleeding with 1.0 mg E2 and 0.625 mg CEE, but significantly less with 0.5 mg E2 (versus CEE) [Citation7]. Two randomized studies showed that women using 200 mg P4 cyclically with oral CEE (0.625 mg) had fewer days of bleeding [Citation52, Citation55], had less blood flow [Citation52, Citation55], and more were amenorrheic [Citation52] than when using MPA (5 mg). Two other randomized studies showed that when P4 (200 mg, cyclic) was used with transdermal E2 (50 µg), irregular bleeding occurred more frequently than with cyclic MPA (10 mg) [Citation56], nomegestrol acetate (5 mg) [Citation56], DYD (10 mg) [Citation56], or CMA (10 mg) [Citation53]. However, amenorrhea rates were 37.1% with P4 and 11.9% with CMA in one study [Citation53], while the proportions of amenorrhea cycles were similar between all progestogens in the other study [Citation56].
Cardiovascular and thrombotic outcomes
Details of the design and results of the clinical studies included in this section can be found in .
Table 2. Studies comparing cardiovascular outcomes with E2 versus CEE and P4 versus synthetic progestogens.
VTE and coagulation factors
Risk for VTE was similar or lower with oral E2 than with CEE in 4 observational studies () [Citation12, Citation27, Citation31, Citation32]. In the Million Women Study (MWS), a similar but significant increase in VTE risk was observed with oral E2 (RR 1.45) or CEE alone (RR 1.46) when comparing current vs never users [Citation12]. Similarly, another case-control study (n = 80,396) showed an increase in VTE risk with E2 (OR 1.27) or CEE (OR 1.49) alone when comparing current users vs non-users [Citation31]. When directly comparing CEE with E2, two case-control studies reported a significantly greater VTE risk with CEE than with E2 [Citation27,Citation31]. However, a recent retrospective cohort study found a similar VTE risk with MHT containing oral E2 or CEE, which did not support a differential effect of E2 compared with CEE on VTE [Citation32].
Figure 2. Outcomes with E2 versus CEE and P4 versus synthetic progestogens. (A) Cardiovascular outcome and breast cancer risks with estrogens. (B) Cardiovascular outcome risk with progestogens. (C) Breast cancer risk with progestogens.
AF: atrial fibrillation; CEE: conjugated equine estrogens; CHD: coronary heart disease; CI: confidence interval; CMA: chlormadinone acetate; CPA: cyproterone acetate; CVD: cardiovascular disease; der: derivatives; DYD: dydrogesterone; E: estrogen; E2: estradiol; Es: estrogens; HR: hazard radio; LNG: levonorgestrel; MACE: major adverse cardiac event; MDG: medogestone; MPA: medroxyprogesterone acetate; NETA: norethisterone acetate; NOMAC: nomegestrol acetate; OR: odds ratio; P: progestin; P4: progesterone; PMG: promegestone; RR: relative risk; TD: transdermal; VTE: venous thromboembolism.
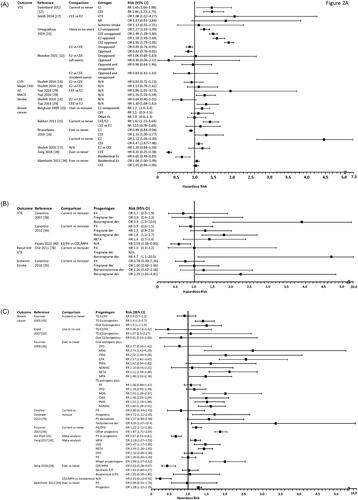
Surrogate markers of coagulation were reported in some studies. Furthermore, a cross-sectional study (n = 282) of coagulation parameters found a more pro-thrombotic profile with CEE versus E2 [Citation13]. This is in contrast to an older and smaller (n = 35) randomized study, which showed the opposite with increased plasminogen activity with CEE (0.625 and 1.25 mg) but not E2 (1 and 2 mg), and decreased thrombin time with 1 mg E2 but not with other regimens versus placebo [Citation24].
When evaluating the effects of progestogens on VTE, three large observational studies (Estrogen and Thromboembolism Risk [ESTHER], Etude Epidémiologique de femmes de l’Education Nationale [E3N], and Menopause, Estrogen, and Veins [MEVE]) found increased VTE risk with MHT with norpregnane derivatives but not with P4 or pregnane derivatives () [Citation36, Citation38, Citation78]. A recently presented retrospective cohort study found a lower VTE risk in women who initiated E2/P4 versus CEE/MPA () [Citation80].
Hemostasis data, other surrogate measures, with transdermal estrogens plus different progestogens were consistent with clinical VTE risk data [Citation37, Citation39]. One French study found that norpregnanes were associated with lower activated protein C (APC) sensitivities and higher prothrombin fragment 1 + 2 concentrations [Citation37], and another that progestins increased thrombin generation [Citation39]; no significant changes on any hemostatic or thrombin generation parameters were seen with P4 in either study [Citation37, Citation39].
CVD Outcomes
Various CVD risks with CEE or E2 were reported in three observational studies () [Citation14, Citation27, Citation29]. Risks for major coronary heart disease (CHD), stroke, or total CVD with oral E2 formulations were not significantly different from those with CEE (0.625 mg) in the WHI observational study (WHI-OS) [Citation14]. Similarly, no differences in risk for MI and ischemic stroke were observed between CEE or E2 in a case-control study [Citation27]. Finally, a population-based, cohort study found women had higher risk of new-onset atrial fibrillation (AF), stroke, and major adverse cardiac events when using CEE than E2 [Citation29].
One case-control comparative study examining CVD risk among progestogens showed that current users of E2 plus norpregnane derivatives had an increased risk for ischemic stroke, but this was not observed with E2 plus P4, pregnane derivatives, or nortestosterone derivatives () [Citation35].
Lipids
Two prospective studies investigated plasma lipids, a surrogate marker for CVD risk, with different oral estrogens. The first study found an improved lipid profile and decreased homocysteine level in women using E2 or CEE alone versus no use [Citation11]. The other study was small (n = 6 per group) and found no significant lipid changes with any estrogen, except for significant decreases in low-density lipoprotein cholesterol (LDL-c) from baseline with CEE (0.625 mg) and E2 (1 mg) [Citation26].
Four randomized trials showed that the use of P4 had no adverse lipid effects in postmenopausal women. In the PEPI trial, women using CEE plus cyclic P4 had similar high-density lipoprotein cholesterol (HDL-c) levels to those using CEE-alone but significantly higher levels than with CEE plus cyclic or continuous MPA; all regimens decreased LDL-c and increased triglycerides compared with placebo [Citation66]. Similarly, another study reported significant increases in HDL-c and decreases in LDL-c with CEE (0.625 mg) when combined with P4 (100 mg) or DYD (10 mg); while triglycerides only significantly increased with DYD [Citation64]. In another study using E2V, HDL-c levels remained unchanged with P4, but significantly decreased with MPA, while triglycerides did not change with either regimen [Citation63]. Finally, a study of transdermal E2 plus P4 or CMA revealed little impact of either on total cholesterol and triglycerides [Citation53].
Blood pressure
No studies comparing oral E2 with CEE on the effects on BP were identified. Five randomized studies showed similar or better effects of MHT containing P4 versus other progestogens. The PEPI trial [Citation66] as well as two other randomized trials [Citation63, Citation64] found no changes from baseline in systolic BP (SBP) and diastolic BP (DBP) with oral CEE or E2V when combined with P4 or MPA [Citation63, Citation66], or DYD [Citation64], except for a significant decrease in DBP for CEE/P4 (0.3 mg/100 mg) [Citation64]. Further, one randomized study showed that SBP and DBP decreased from baseline with E2/P4, but increased with E2/CMA [Citation53]. Finally, a RCT found that SBP and DBP reactivity during stress tended to decrease after 8 weeks of estrogen/P4, while estrogen/MPA maintained SBP reactivity and significantly increased DBP reactivity [Citation77].
Breast
Details of the clinical studies included in this section can be found in .
Table 3. Studies comparing breast outcomes with E2 versus CEE and P4 versus synthetic progestogens.
Breast cancer
Effects of MHT containing E2 or CEE on the breast were compared in six observational studies () [Citation15–17, Citation28, Citation33, Citation34]. The European Prospective Investigation into Cancer and Nutrition (EPIC) trial [Citation15], the WHI-OS trial [Citation17], and one prospective study [Citation33] showed that breast cancer risk increased in estrogen-only users but with no significant difference between CEE and E2 users. The prospective study also showed that breast cancer risk significantly increased with longer duration of E2 products, but not CEE [Citation33]. Increased breast cancer risk with E2 or CEE in current users was also observed in a Swedish retrospective study, with CEE having a higher risk than E2 [Citation16]. However, the same Swedish study found that the risk of breast cancer remained similar in ever users of CEE and decreased in ever users of E2 [Citation16]. Finally, a retrospective analysis showed a decreased breast cancer risk with either bioidentical estrogens or CEE alone [Citation28], while a more recent retrospective study found no association of MHT containing bioidentical estrogens or CEE with breast cancer [Citation34].
Various analyses of the E3N observational study, including the E3N-EPIC study, and two case-controlled studies found increased breast cancer risk with MHT containing progestins but not progesterone (). The French E3N study (n = 80,377) showed elevated breast cancer risk with transdermal estrogens plus progestins (medogestone [MDG]/CMA/promegestone [PMG]/norethisterone acetate [NETA]) but not with P4 or DYD for overall risk and by 2-year increments of MHT duration [Citation46]. Similar results were also reported in the E3N-EPIC (n = 54,548) [Citation45] and two case-control studies (n = 1555 and 475,013, respectively) [Citation34, Citation79]. When the E3N data were analyzed by years from menopause, breast cancer risk did not increase with P4 with ≤5 years of use or when initiation was >3 years after menopause, but it did increase with >5 years of duration or when MHT was initiated ≤3 years from menopause; other progestogens, except DYD, increased breast cancer risk when used for 2 to 10 years [Citation48]. When analyzed by current versus never use, breast cancer risk was found elevated in both P4/DYD and other progestin groups, while past versus never use, showed an increased risk with past use of estrogen/progestins, but not with estrogen plus P4/DYD [Citation49]. Finally, the neutral effect of estrogen plus P4 was also consistent by breast cancer subtype (ductal or lobular), while increased risk was associated with estrogen/DYD for the lobular subtype and with estrogen/other progestins for all subtypes studied [Citation47]. Two meta-analyses had results consistent with the observational studies, with P4 associated with lower breast cancer risk versus progestins [Citation41, Citation42].
Only two studies showed no difference between P4 and other progestins for breast cancer risk. One prospective French cohort study showed no increase in breast cancer risk with E2 with P4 nor progestins [Citation50], while the other showed no increase in breast cancer for synthetic estrogens/progestins or bioidentical estrogens/P4 and a significantly lower risk with CEE/MPA from baseline and when compared with bioidentical estrogens/P4 [Citation28].
Breast density and breast tenderness
One prospective study evaluated the effects of E2 vs CEE on mammographic breast density (MBD), which showed no significant difference between the two groups [Citation18].
Studies focusing on breast tissue changes revealed similar or better effects for P4- versus progestin-containing MHT on MBD [Citation68–71] and breast tenderness [Citation55, Citation58, Citation65]. In the PEPI trial, MBD analyses found that women taking CEE with MPA or P4 had significant increases in breast density compared with placebo at month 12, but with no difference between groups [Citation69–71]. Similarly, women using CEE with MPA or P4 also had higher odds of breast discomfort compared with placebo, with no difference between MPA versus P4 [Citation68,Citation71]. A randomized study comparing E2V with P4 or MPA found more breast tenderness with P4 than with MPA (36.7% vs 14.3%) at 3 months [Citation57], but not at 6 and 12 months [Citation58, Citation65]. While one small prospective study (n = 23) showed more breast tenderness cases with CEE/MPA than CEE/P4, no statistical analysis was performed, and neither P4 or MPA alone were associated with breast tenderness [Citation55].
Cognition
Observational studies and a meta-analysis comparing different estrogens on cognitive outcomes reported mostly better outcomes with E2 than CEE (Supplementary Table 1). The meta-analysis of 36 RCTs showed that cognition tended (p = .1) to be slightly worse with CEE versus E2 alone [Citation9]. A small retrospective study showed a better neural response in women using opposed CEE or opposed E2 versus MHT-naïve women; however, verbal learning and recall performance was worse in CEE than E2 users [Citation19]. Postmenopausal women at risk for dementia or Alzheimer’s disease (AD) taking MHT for ≥1 year had better cognitive outcomes with E2 versus CEE [Citation20–22], including E2 users performing significantly better in verbal memory [Citation20, Citation21] and with a significantly higher brain metabolism level versus CEE users [Citation20]. Two years after either continuing or discontinuing MHT, women who had used E2-based MHT had better verbal memory (p = .01) than those who had used CEE-based MHT [Citation23], while continued CEE use and discontinuation of E2 use significantly decreased cerebral metabolism [Citation22].
Two small studies provided head-to-head comparisons of MHT containing P4 versus progestins for their influence on cognition (Supplementary Table 1). A 12-week RCT of women using CEE plus cyclic P4 or MPA found significantly improved working memory and significantly decreased delayed verbal memory with CEE/P4; while no changes with CEE/MPA were observed [Citation72]. Verbal memory, visual memory, executive function, or attention/working memory/processing speed did not differ with ≥1-year use of MHT containing P4 versus MPA in a cross-sectional study of women with increased AD risk [Citation21].
Bone
A systematic review and meta-analysis of 28 RCTs found that MHT containing either E2 or CEE reduced bone fracture risk versus placebo with a significantly greater reduction with E2 than with CEE (Supplementary Table 1) [Citation10].
Head-to-head comparisons of MHT containing P4 or other progestins on bone mineral density (BMD) did not show significant differences (Supplementary Table 1). In the PEPI trial, CEE with P4 or MPA significantly increased spinal and hip BMD from baseline to 3 years, in contrast to the decrease with placebo; CEE with continuous MPA was significantly better than CEE with cyclic MPA/P4 for spinal BMD [Citation67]. Three other randomized, open-label studies also found improved BMD and bone metabolism from baseline with no differences between groups for transdermal E2 with continuous P4 or MPA [Citation59], or oral CEE with cyclic P4 or DYD [Citation73, Citation74].
Menopausal symptoms and QOL
Study details for this section can be found in the Supplemental Materials and . For the most part, outcomes were similar between formulations for vasomotor symptoms and body weight and composition. Limited evidence suggests that P4 may provide better QoL and sleep than other progestogens.
Discussion
This comprehensive literature review of clinical studies comparing the safety and effectiveness of MHT containing E2 versus CEE and of P4 versus progestins found some differential effects between these different formulations, suggesting a mostly similar or better safety profile with E2 and/or P4. Most of the studies revealed similar or lower risk for breast cancer, VTE, cardiovascular outcomes, and cognitive parameters for E2- or P4-based MHT compared with CEE- or progestin-based MHT, respectively. Data suggest that E2- or P4-containing formulations are as effective as other estrogens or progestogens for relieving menopausal symptoms and maintaining bone health, but with potentially better tolerability.
Concomitant progestogen use with estrogen therapy is indicated in postmenopausal women with a uterus for endometrial protection [Citation1]. Although a systematic review reported an increased risk of endometrial cancer with P4-containing MHT [Citation81] mainly based on results from the two observational studies described [Citation43, Citation44], randomized-controlled studies showed that P4 prevents endometrial hyperplasia, offering similar endometrial protection, compared with progestins when used at an adequate dose [Citation51, Citation53]. The most recent, phase 3, randomized, placebo-controlled trial, REPLENISH, demonstrated adequate endometrial protection with P4 when given with E2 for menopausal symptom control [Citation82, Citation83].
Initial publication of increased risk of CHD, VTE, and breast cancer with CEE/MPA in the WHI raised concerns of cardiovascular diseases and breast cancer with MHT use [Citation2, Citation3]. Our review of large, observational studies of MHT, including MWS, WHI-OS, EPIC, ESTHER, and E3N, showed that E2 has similar or lower risk compared with CEE on the breast and cardiovascular outcomes including VTE [Citation12, Citation14–17, Citation29, Citation31, Citation32, Citation34], and similarly, P4 had a risk profile similar to or better compared with progestins for these outcomes [Citation34–36, Citation38, Citation45–48, Citation50, Citation78, Citation79]. In addition, the randomized, placebo-controlled PEPI trial showed no negative impact of CEE/P4 on lipids [Citation66] and comparable effects on breast density or breast discomfort with CEE/P4 and CEE/MPA [Citation68–71]. A lower risk for VTE with E2/P4 versus CEE/MPA was also observed in a retrospective study [Citation80].
Whether MHT could protect against cognitive aging and dementia in postmenopausal women has been inconclusive [Citation84]. Outcomes in cognition tests from a limited number of smaller studies suggest that E2 could provide better protection against age-related cognitive decline versus CEE [Citation20–23], whereas effects of P4 were similar to or better than MPA [Citation21, Citation72]. However, additional studies are necessary to conclude whether P4 influences cognitive function differently from other progestogens.
Data from prospective, randomized trials, including PEPI, revealed similar improvements in bone health with P4-based MHT versus MPA- or DYD-based MHT [Citation59, Citation67, Citation73, Citation74], while a meta-analysis reported a greater reduction in total fractures with MHT containing E2 versus CEE [Citation10]. Moreover, comparative studies showed that the influence on VMS reduction and body weight or composition was similar for MHT containing E2 versus CEE [Citation8, Citation25] or P4 versus progestins [Citation53, Citation55, Citation60, Citation62, Citation66, Citation68, Citation75, Citation76], while QOL and sleep disorders appeared to be similarly or better improved with P4-based versus progestin-based MHT [Citation40, Citation52, Citation57, Citation60, Citation61, Citation63, Citation76, Citation77].
Our review has limitations. Studies included are of diverse designs and few RCTs directly compared E2 versus CEE or P4 versus progestins. Many studies were observational, and therefore, could not exclude selection bias. Details of hormone use, such as specific products or exact doses, are often unknown in observational studies. For example, in patients receiving P4-based MHT, different estrogen types or products could have been used, which can influence the results. Sample sizes of the studies also varied largely, and some studies may not have been adequately powered for the reported outcomes. In addition, we also acknowledge that when individualizing MHT for women, there may be certain advantages to utilizing transdermal E2 over oral estrogens because transdermal E2 bypasses the first-pass hepatic effect, especially on VTE risk [Citation85]. However, given the breadth of that literature and the aim of our review, we considered that topic beyond the scope of this article and did not include studies comparing transdermal and oral estrogens as part of our study inclusion criteria. While we also believe performing a meta-analysis of the results from the identified studies would help quantify the differences in hormones reviewed, given the inconsistencies in data reporting between studies and within outcomes, a meta-analysis was not feasible. Another limitation of the review process was having access only to PubMed and EMBASE databases for searching. Nevertheless, this is a comprehensive and systematic review of the available literature on the differential effects of E2 versus CEE and P4 versus progestins, which could provide insights in the benefit/risk profile of MHT containing E2 and P4 that could be considered when prescribing women MHT and developing related guidelines.
Conclusion
Current evidence supports that MHT containing E2 and/or P4 is a safe and effective MHT option, and provides a similar and possibly better safety profile for some endpoints compared with CEE-based or progestin-based MHT.
Supplemental Material
Download Zip (109.6 KB)Disclosure statement
Medical writing assistance provided by Hui Zhang, PhD and Dominique Verlaan, PhD was supported by TherapeuticsMD. DFA has received research support from AbbVie, Mithra, Myovant, and ObsEva; has consulted for Agile Therapeutics, Evestra, Exeltis, Lupin, Mithra, ObsEva, and TherapeuticsMD; and has stock holdings with Agile Therapeutics and InnovaGyn, Inc. JAS has received grant/research support from AbbVie, Bayer Healthcare, Endoceutics, GTx Inc, Ipsen, Myovant Sciences, ObsEva SA, TherapeuticsMD, and Viveve Medical; has consulted or advised for AbbVie, AMAG Pharmaceuticals, Bayer HealthCare, CEEK Enterprises, Covance, DEKA M.E.L.A S.r.l, Daré Bioscience, Duchesnay USA, Hologic, KaNDy/NeRRe Therapeutics Ltd, Madorra Pty Ltd, Mitsubishi Tanabe Pharma Development America, Sebela Pharmaceuticals, Shionogi Inc, Sprout Inc., and TherapeuticsMD; serves on the speaker’s bureaus of AbbVie, AMAG Pharmaceuticals, Duchesnay USA, and TherapeuticsMD; and is a stockholder (direct purchase) in Sermonix Pharmaceuticals. KMO is the owner of Precise Publications, which received fees from TherapeuticsMD for the research and medical writing services for this manuscript and other publications/presentations. SG and BB are employees of TherapeuticsMD with stock/stock options.
Additional information
Funding
References
- The NAMS Hormone Therapy Position Statement Advisory Panel. The 2022 hormone therapy position statement of the North American menopause society. Menopause. 2022;29(7):767–794.
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women’s health initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
- Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA. 2004;292(13):1573–1580.
- Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291(1):47–53.
- American College of Obstetricians and Gynecologists. Committee opinion no. 532: compounded bioidentical menopausal hormone therapy. Obstet Gynecol. 2012;120(2 Pt 1):411–415.
- Stute P, Neulen J, Wildt L. The impact of micronized progesterone on the endometrium: a systematic review. Climacteric. 2016;19(4):316–328.
- Ettinger B, Bainton L, Upmalis DH, et al. Comparison of endometrial growth produced by unopposed conjugated estrogens or by micronized estradiol in postmenopausal women. Am J Obstet Gynecol. 1997;176(1):112–117.
- Dittmar M. Comparison of soft tissue body composition in postmenopausal women with or without hormone replacement therapy considering the influence of reproductive history and lifestyle. Ann Hum Biol. 2001;28(2):207–221.
- Hogervorst E, Bandelow S. Sex steroids to maintain cognitive function in women after the menopause: a meta-analyses of treatment trials. Maturitas. 2010;66(1):56–71.
- Zhu L, Jiang X, Sun Y, et al. Effect of hormone therapy on the risk of bone fractures: a systematic review and meta-analysis of randomized controlled trials. Menopause. 2016;23(4):461–470.
- Man RY, Ting LK, Fan S, et al. Effect of postmenopausal hormone replacement therapy on lipoprotein and homocysteine levels in Chinese women. Mol Cell Biochem. 2001;225(1):129–134.
- Sweetland S, Beral V, Balkwill A, et al. Venous thromboembolism risk in relation to use of different types of postmenopausal hormone therapy in a large prospective study. J Thromb Haemost. 2012;10(11):2277–2286.
- Blondon M, van HV, Wiggins KL, et al. Differential associations of oral estradiol and conjugated equine estrogen with hemostatic biomarkers. J Thromb Haemost. 2014;12(6):879–886.
- Shufelt CL, Merz CN, Prentice RL, et al. Hormone therapy dose, formulation, route of delivery, and risk of cardiovascular events in women: findings from the women’s health initiative observational study. Menopause. 2014;21(3):260–266.
- Bakken K, Fournier A, Lund E, et al. Menopausal hormone therapy and breast cancer risk: impact of different treatments. The European prospective investigation into cancer and nutrition. Int J Cancer. 2011;128(1):144–156.
- Brusselaers N, Tamimi RM, Konings P, et al. Different menopausal hormone regimens and risk of breast cancer. Ann Oncol. 2018;29(8):1771–1776.
- Shufelt C, Bairey Merz CN, Pettinger MB, et al. Estrogen-alone therapy and invasive breast cancer incidence by dose, formulation, and route of delivery: findings from the WHI observational study. Menopause. 2018;25(9):985–991.
- Bulbul NH, Ozden S, Dayicioglu V. Effects of hormone replacement therapy on mammographic findings. Arch Gynecol Obstet. 2003;268(1):5–8.
- Gleason CE, Schmitz TW, Hess T, et al. Hormone effects on fMRI and cognitive measures of encoding: importance of hormone preparation. Neurology. 2006;67(11):2039–2041.
- Silverman DH, Geist CL, Kenna HA, et al. Differences in regional brain metabolism associated with specific formulations of hormone therapy in postmenopausal women at risk for AD. Psychoneuroendocrinology. 2011;36(4):502–513.
- Wroolie TE, Kenna HA, Williams KE, et al. Differences in verbal memory performance in postmenopausal women receiving hormone therapy: 17beta-estradiol versus conjugated equine estrogens. Am J Geriatr Psychiatry. 2011;19(9):792–802.
- Rasgon NL, Geist CL, Kenna HA, et al. Prospective randomized trial to assess effects of continuing hormone therapy on cerebral function in postmenopausal women at risk for dementia. PLoS One. 2014;9(3):e89095.
- Wroolie TE, Kenna HA, Williams KE, et al. Cognitive effects of hormone therapy continuation or discontinuation in a sample of women at risk for Alzheimer disease. Am J Geriatr Psychiatry. 2015;23(11):1117–1126.
- Notelovitz M, Kitchens CS, Ware MD. Coagulation and fibrinolysis in estrogen-treated surgically menopausal women. Obstet Gynecol. 1984;63(5):621–625.
- Utian WH, Speroff L, Ellman H, et al. Comparative controlled trial of a novel oral estrogen therapy, estradiol acetate, for relief of menopause symptoms. Menopause. 2005;12(6):708–715.
- Lobo RA, Brenner P, Mishell DR. Jr. Metabolic parameters and steroid levels in postmenopausal women receiving lower doses of natural estrogen replacement. Obstet Gynecol. 1983;62(1):94–98.
- Smith NL, Blondon M, Wiggins KL, et al. Lower risk of cardiovascular events in postmenopausal women taking oral estradiol compared with oral conjugated equine estrogens. JAMA Intern Med. 2014;174(1):25–31.
- Zeng Z, Jiang X, Li X, et al. Conjugated equine estrogen and medroxyprogesterone acetate are associated with decreased risk of breast cancer relative to bioidentical hormone therapy and controls. PLoS One. 2018;13(5):e0197064.
- Tsai WC, Haung YB, Kuo HF, et al. Hormone replacement therapy and risk of atrial fibrillation in taiwanese menopause women: a nationwide cohort study. Sci Rep. 2016;6:24132.
- Cengiz B, Atabekoglu C, Cetinkaya E, et al. Effect of hormone replacement therapy on serum levels of tumor markers in healthy postmenopausal women. Maturitas. 2003;46(4):301–306.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810.
- Blondon M, Timmons AK, Baraff AJ, et al. Comparative venous thromboembolic safety of oral and transdermal postmenopausal hormone therapies among women veterans. Menopause. 2021;28(10):1125–1129.
- Bergkvist L, Adami HO, Persson I, et al. The risk of breast cancer after estrogen and estrogen-progestin replacement. N Engl J Med. 1989;321(5):293–297.
- Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139(6):1103–1110.
- Canonico M, Carcaillon L, Plu-Bureau G, et al. Postmenopausal hormone therapy and risk of stroke: impact of the route of estrogen administration and type of progestogen. Stroke. 2016;47(7):1734–1741.
- Canonico M, Fournier A, Carcaillon L, et al. Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort study. Arterioscler Thromb Vasc Biol. 2010;30(2):340–345.
- Canonico M, Alhenc-Gelas M, Plu-Bureau G, et al. Activated protein C resistance among postmenopausal women using transdermal estrogens: importance of progestogen. Menopause. 2010;17(6):1122–1127.
- Canonico M, Oger E, Plu-Bureau G, et al. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115(7):840–845.
- Scarabin PY, Hemker HC, Clement C, et al. Increased thrombin generation among postmenopausal women using hormone therapy: importance of the route of estrogen administration and progestogens. Menopause. 2011;18(8):873–879.
- Fitzpatrick LA, Pace C, Wiita B. Comparison of regimens containing oral micronized progesterone or medroxyprogesterone acetate on quality of life in postmenopausal women: a cross-sectional survey. J Womens Health Gend Based Med. 2000;9(4):381–387.
- Asi N, Mohammed K, Haydour Q, et al. Progesterone vs. synthetic progestins and the risk of breast cancer: a systematic review and meta-analysis. Syst Rev. 2016;5(1):121.
- Yang Z, Hu Y, Zhang J, et al. Estradiol therapy and breast cancer risk in perimenopausal and postmenopausal women: a systematic review and meta-analysis. Gynecol Endocrinol. 2017;33(2):87–92.
- Allen NE, Tsilidis KK, Key TJ, et al. Menopausal hormone therapy and risk of endometrial carcinoma among postmenopausal women in the European prospective investigation into cancer and nutrition. Am J Epidemiol. 2010;172(12):1394–1403.
- Fournier A, Dossus L, Mesrine S, et al. Risks of endometrial cancer associated with different hormone replacement therapies in the E3N cohort, 1992-2008. Am J Epidemiol. 2014;180(5):508–517.
- Fournier A, Berrino F, Riboli E, et al. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114(3):448–454.
- Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107(1):103–111.
- Fournier A, Fabre A, Mesrine S, et al. Use of different postmenopausal hormone therapies and risk of histology- and hormone receptor-defined invasive breast cancer. J Clin Oncol. 2008;26(8):1260–1268.
- Fournier A, Mesrine S, Boutron-Ruault MC, et al. Estrogen-progestagen menopausal hormone therapy and breast cancer: does delay from menopause onset to treatment initiation influence risks? J Clin Oncol. 2009;27(31):5138–5143.
- Fournier A, Mesrine S, Dossus L, et al. Risk of breast cancer after stopping menopausal hormone therapy in the E3N cohort. Breast Cancer Res Treat. 2014;145(2):535–543.
- Espie M, Daures JP, Chevallier T, et al. Breast cancer incidence and hormone replacement therapy: results from the MISSION study, prospective phase. Gynecol Endocrinol. 2007;23(7):391–397.
- Writing Group for the PEPI Trial. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The postmenopausal estrogen/progestin interventions (PEPI) trial. JAMA. 1996;275(5):370–375.
- Ryan N, Rosner A. Quality of life and costs associated with micronized progesterone and medroxyprogesterone acetate in hormone replacement therapy for nonhysterectomized, postmenopausal women. Clin Ther. 2001;23(7):1099–1115.
- Pelissier C, Maroni M, Yaneva H, et al. Chlormadinone acetate versus micronized progesterone in the sequential combined hormone replacement therapy of the menopause. Maturitas. 2001;40(1):85–94.
- Jondet M, Maroni M, Yaneva H, et al. Comparative endometrial histology in postmenopausal women with sequential hormone replacement therapy of estradiol and, either chlormadinone acetate or micronized progesterone. Maturitas. 2002;41(2):115–121.
- Cummings JA, Brizendine L. Comparison of physical and emotional side effects of progesterone or medroxyprogesterone in early postmenopausal women. Menopause. 2002;9(4):253–263.
- Di Carlo C, Sammartino A, Di Spiezio SA, et al. Bleeding patterns during continuous estradiol with different sequential progestogens therapy. Menopause. 2005;12(5):520–525.
- Zheng TP, Sun AJ, Xue W, et al. Efficacy and safety of cimicifuga foetida extract on menopausal syndrome in Chinese women. Chin Med J (Engl). 2013;126(11):2034–2038.
- Wang YP, Ma D, Cheng XT, et al. Comparison of Cimicifuga foetida extract and different hormone therapies regarding in causing breast pain in early postmenopausal women. Gynecol Endocrinol. 2019;35(2):160–164.
- Sun A, Lin S, Yu W, et al. Percutaneous estrogen in prevention of early postmenopausal bone loss in Chinese women. Chin Med J (Engl). 2002;115(12):1790–1795.
- Montplaisir J, Lorrain J, Denesle R, et al. Sleep in menopause: differential effects of two forms of hormone replacement therapy. Menopause. 2001;8(1):10–16.
- Leeangkoonsathian E, Pantasri T, Chaovisitseree S, et al. The effect of different progestogens on sleep in postmenopausal women: a randomized trial. Gynecol Endocrinol. 2017;33(12):933–936.
- Deng Y, Xue W, Wang Y, et al. Effects of different menopausal hormone replacement regimens on body composition in Chinese women. Climacteric. 2018;21(6):607–612.
- Gao L, Zheng T, Xue W, et al. Efficacy and safety evaluation of Cimicifuga foetida extract in menopausal women. Climacteric. 2018;21(1):69–74.
- Xue W, Deng Y, Wang YF, et al. Effect of half-dose and standard-dose conjugated equine estrogens combined with natural progesterone or dydrogesterone on components of metabolic syndrome in healthy postmenopausal women: a randomized controlled trial. Chin Med J (Engl). 2016;129(23):2773–2779.
- Gao L, Zuo H, Zheng T, et al. Influence of hormone therapy or C. foetida extract on breast tenderness in postmenopausal women. Climacteric. 2018;21(3):292–297.
- Writing Group for the PEPI Trial. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The postmenopausal estrogen/progestin interventions (PEPI) trial. JAMA. 1995;273(3):199–208.
- Writing Group for the Pepi Trial. Effects of hormone therapy on bone mineral density: results from the postmenopausal estrogen/progestin interventions (PEPI) trial. JAMA. 1996;276(17):1389–1396.
- Greendale GA, Reboussin BA, Hogan P, et al. Symptom relief and side effects of postmenopausal hormones: results from the postmenopausal estrogen/progestin interventions trial. Obstet Gynecol. 1998;92(6):982–988.
- Greendale GA, Reboussin BA, Sie A, et al. Effects of estrogen and estrogen-progestin on mammographic parenchymal density. Postmenopausal estrogen/progestin interventions (PEPI) investigators. Ann Intern Med. 1999;130(4 Pt 1):262–269.
- Greendale GA, Reboussin BA, Slone S, et al. Postmenopausal hormone therapy and change in mammographic density. J Natl Cancer Inst. 2003;95(1):30–37.
- Crandall CJ, Karlamangla A, Huang MH, et al. Association of new-onset breast discomfort with an increase in mammographic density during hormone therapy. Arch Intern Med. 2006;166(15):1578–1584.
- Sherwin BB, Grigorova M. Differential effects of estrogen and micronized progesterone or medroxyprogesterone acetate on cognition in postmenopausal women. Fertil Steril. 2011;96(2):399–403.
- Zhu SY, Deng Y, Wang YF, et al. Bone protection for early menopausal women in China: standard or half-dose estrogen with progestin? A one-year prospective randomized trail. Gynecol Endocrinol. 2019;35(2):165–169.
- Zuo H, Sun A, Gao L, et al. Effect of menopausal hormone therapy on bone mineral density in Chinese women: a 2-year, prospective, open-label, randomized-controlled trial. Med Sci Monit. 2019;25:819–826.
- Espeland MA, Stefanick ML, Kritz-Silverstein D, et al. Effect of postmenopausal hormone therapy on body weight and waist and hip girths. Postmenopausal Estrogen-Progestin interventions study investigators. J Clin Endocrinol Metab. 1997;82(5):1549–1556.
- Gambacciani M, Ciaponi M, Cappagli B, et al. Effects of low-dose, continuous combined hormone replacement therapy on sleep in symptomatic postmenopausal women. Maturitas. 2005;50(2):91–97.
- Matthews KA, Owens JF, Salomon K, et al. Influence of hormone therapy on the cardiovascular responses to stress of postmenopausal women. Biol Psychol. 2005;69(1):39–56.
- Olie V, Plu-Bureau G, Conard J, et al. Hormone therapy and recurrence of venous thromboembolism among postmenopausal women. Menopause. 2011;18(5):488–493.
- Cordina-Duverger E, Truong T, Anger A, et al. Risk of breast cancer by type of menopausal hormone therapy: a case-control study among post-menopausal women in France. PLoS One. 2013;8(11):e78016.
- Panay N, Nappi RE, Boolell M, et al. Venous thromboembolism risk in menopausal women treated with oral estradiol/micronised progesterone versus conjugated estrogens/medroxyprogesterone: a claims data analysis in the United States. The 20th World Congress – International Society of Gynecological Endocrinology; 2022; Florence, Italy.
- Tempfer CB, Hilal Z, Kern P, et al. Menopausal hormone therapy and risk of endometrial cancer: a systematic review. Cancers (Basel). 2020;12(8):2195.
- Lobo RA, Archer DF, Kagan R, et al. A 17β-estradiol-progesterone oral capsule for vasomotor symptoms in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2018;132(1):161–170.
- Mirkin S, Goldstein SR, Archer DF, et al. Endometrial safety and bleeding profile of a 17β-estradiol/progesterone oral softgel capsule (TX-001HR). Menopause. 2020;27(4):410–417.
- Henderson VW. Gonadal hormones and cognitive aging: a midlife perspective. Womens Health (Lond). 2011;7(1):81–93.
- Rovinski D, Ramos RB, Fighera TM, et al. Risk of venous thromboembolism events in postmenopausal women using oral versus non-oral hormone therapy: a systematic review and meta-analysis. Thromb Res. 2018;168:83–95.
