Abstract
Background
Preeclampsia has a multifactorial-yet-elusive etiology. Recent reports suggest a link between preeclampsia and vitamin D (VD) metabolic axis. Genetic variations like single-nucleotide polymorphisms (SNPs) of vitamin D receptor (VDR) gene can alter the metabolic role of VD, which have been shown by several genetic association studies. However, there is discordance among these studies.
Objective
The current study aimed to investigate the association of VDR gene polymorphism (ApaI) and VD deficiency with risk of developing preeclampsia.
Patients and Method
In this case–control study, 40 preeclamptic and 40 normotensive pregnant women were compared for VD status and VDR gene polymorphism. Serum 25-hydroxyvitamin-D [25(OH) D] level was determined by enzyme-linked immunosorbent assay (ELISA) and VDR gene polymorphism Apa1 was analyzed by Allele specific polymerase chain reaction (AS-PCR) using sequence specific primers.
Results
Serum levels of 25(OH) D were very low but comparable in both preeclamptic and normotensive pregnant women. The difference between the two groups were not statistically significant (p = .423). VDR gene polymorphism ApaI (rs7975232) was found not to have significant association with the risk of developing preeclampsia. The frequencies of wild genotype (GG) in preeclamptic and normotensive women were 27.5% and 22.5% respectively. A total of 25% of preeclamptic women had mutant homozygous genotype (TT) and 17.5% of normotensive women had mutant homozygous genotype. The frequency of mutant heterozygous genotype (GT) in preeclamptic patients was 47.5% and in normotensive women was 60%. The variation of wild and mutant genotypes between the two groups was not statistically significant (p > .05).
Conclusion
This study showed that VDR gene polymorphism (ApaI) and VD deficiency are not associated with the risk of preeclampsia.
Keywords:
Introduction
Preeclampsia (PE) is a pregnancy complication of second half of pregnancy, labor, or the early puerperium with serious consequences for mother and infant [Citation1]. American college of obstetricians and gynecologists defined PE as a new onset hypertension with blood pressure ≥140/90 mmHg and proteinuria (≥0.3 g per 24 h) after 20 weeks of gestation [Citation2]. PE affects about 3%–7% of women in their 1st pregnancies with a morbidity and mortality rate of 10%–15% [Citation3,Citation4]. However, in developing countries like Pakistan, reported prevalence of PE and eclampsia is around 19% and about 1.12% women dies (1 in 89) due to maternal causes in which major cause of death is PE or eclampsia [Citation5]. Abnormal placentation, intolerance of maternal immune system to placental and fetal antigens, and endothelial cells injury are some of the proposed causes of preeclampsia [Citation6–Citation8]. However, PE is known as the ‘disease of hypotheses’ regarding its causes and pathogenesis [Citation6–Citation8]. Recent studies have linked low levels of vitamin D (VD) with the risk of PE. Being known for calcium homeostasis, it has also been suggested that VD plays important role in modulating immune and cardiovascular systems during pregnancy that have an impact on blood pressure [Citation9]. Optimum levels of VD promote healthy gestation and fetal growth [Citation10], whereas low maternal VD levels have been reported in PE by various researchers [Citation3,Citation11–Citation13]. Furthermore, studies also reported that VD supplementation lowers the risk of PE [Citation14,Citation15]. However, the recent studies based on VD and PE are conflicting and inconsistent [Citation16,Citation17].
VD exerts its biological action by binding to a high affinity receptor, which is known as VD receptor (VDR). VDR is a nuclear receptor that belongs to the super family of steroid hormone receptors, it functions as a nuclear transcription factor [Citation18]. Since, the discovery of VDR gene, genetic variations in its DNA sequence have been extensively studied and linked to multiple disorders [Citation19] including PE. However, where some researchers have found strong association of single-nucleotide polymorphisms (SNPs) in VDR gene complex with PE [Citation20,Citation21], others have shown no association [Citation21]. In our previous work, we described the role of neurokinin-B in the pathophysiology of preeclampsia [Citation22]. Here, we intend to find the role of VD and its receptor gene polymorphism in PE in our population. Thus, a case–control study was designed to investigate the level of VD and VDR gene polymorphism in patients with PE compared with healthy individuals and to find out the possible association of VD levels and VDR gene polymorphism with the risk of PE.
Material and methods
Study design and setting
This was a case–control study. A total of 80 subjects (40 preeclamptic and 40 normal pregnant women) were enrolled in the study during their third trimester of pregnancy (gestational age = 34 ± 3 weeks) from obstetrics and gynecology wards of Hayatabad Medical Complex, Peshawar and Saidu Group of Teaching Hospital, Swat. The patients were diagnosed according to American college of obstetrician and gynecology (ACOG) 2002, criteria for preeclampsia [Citation2]. According to this criteria, a women is considered as preeclamptic if she has hypertension, systolic blood pressure (SBP) ≥140 mm Hg or diastolic blood pressure (DBP) ≥90 mm Hg on 2 occasions >4 h apart after 20 weeks’ gestation in a previously normotensive woman and proteinuria ≥300 mg protein per 24-h urine. Informed written consent was taken from all the participants. Subject’s demographics were recorded on predesigned proforma and 5 mL of blood was collected from antecubital vein. About 2.5 mL was transferred to gel tube from which serum was extracted for biochemical analysis and remaining 2.5 mL to EDTA tube which was stored for genetic analysis. The experimentation was carried out in the physiology laboratory, Institute of Basic Medical Sciences and the study was approved by the Advanced Studies and Research Board of Khyber Medical University, Peshawar (Study approval No. DIR/KMU-AS&RB/CV/000037).
Inclusion–exclusion criteria
Women suffering from chronic diseases like hypertension, cardiac pathologies, bad obstetrical history, or any fetal anomalies and those who do not want to participate in the study were excluded from the study. The term bad obstetrical history was defined as female who experience any loss in her pregnancy either abortion, still birth, early neonatal death, or demise of the fetus due to any other cause related to pregnancy. Subjects were examined for height, weight, pulse, edema, fundal height (height of the uterus), fetal heart sounds, and SBP and DBP. Obstetrical ultrasound of each patient was also done to exclude any fetal anomalies. Subjects taking VD supplements were excluded from the study. The subjects were also investigated for hemoglobin, blood grouping, and urinary proteins.
Determination of vitamin D
Serum level of 25-hydroxycholecalciferol was measured by enzyme-linked immunosorbent assay (ELISA) kit (Kit Catalogue No. EQ 6411-9601) purchased from Euroimmune Medizinischelabordiagnostika AG. The ELISA kit used solid-phase competitive protein binding assay to determine the level of 25-hydroxycholecalciferol in human serum samples. All the steps of the assay were performed according to the manufacturer’s instructions. The results were analyzed by three independent observers apart from the researchers of the study who were blind to the subject status. VD levels >25 ng/mL is the optimal level, severe VD deficiency <10 ng/mL, and insufficiency 10–19 ng/mL [Citation23].
DNA extraction and VDR genotyping
Genomic DNA was extracted from peripheral blood using commercially available PureLink Genomic DNA Mini Kit (Kit Catalog No. K1820-02). The ApaI (rs7975232) VDR gene polymorphism was genotyped using allele-specific polymerase chain reaction (AS-PCR). Specific primers () were designed for VDR rs7975232 using online tools [Citation24].
Table 1. Allele flanking and specific primer sequences and their respective PCR product sizes.
The reaction was performed in total volume of 20 µL including 2 µL PCR (10×) buffer, 0.8 µL MgCl2 (50 mM), 1 µL dNTPs (10 mM), 0.4 µL DNA Taq Polymerase (5 u/µL), 0.6 µL (10 µm) of each forward, reverse, and allele specific primers, 2 µL (50 ng/µL) of DNA template and sufficient molecular grade water to make total volume of 20 µL. The reaction mixtures were amplified in automatic thermal cycler with the following conditions; initial denaturation at 95 °C for 4 min followed by 30 cycles of 94 °C for 1 min, 64 °C for 40 s, 72 °C for 1 min, and final extension at 72 °C for 7 min. The amplicon was electrophoresed on 1.5% agarose gel stained with 2 µL ethidium bromide (10 mg/mL) at 90 mA for 45 min and visualized under ultraviolet (UV) trans-illuminator. Depending upon the presence of G or T specific band along with the control band, genotypes were assigned as homozygous wild (GG), homozygous mutant (TT), and heterozygous mutant (GT) for ApaI polymorphism.
Statistical analysis
All the continuous variables were presented as mean ± standard deviation whereas categorical variables were expressed in terms of frequencies and percentages. To compare continuous and categorical variables, independent sample t test and Pearson’s chi square test of independence was applied, wherever applicable. SNP data was calculated in terms of Hardy–Weinberg equilibrium (HWE), where chi-square statistics was applied for genotype and allele frequency analysis. Regression analysis was employed to determine the impact of different variables on disease progression. The odds ratio (OR) with 95% CI was calculated to determine the risk. p value <.05 was considered significant.
Results
The mean age of the study population preeclamptic group was 33.05 ± 5.26 years while mean age of control group was 27.55 ± 4.91 years (). Statistically significant difference was observed in the BMI of disease group compared to control group (p < .001). Similarly, a statistical significant difference was observed in the pulse rate of preeclamptic and normotensive pregnant women (p = .01). Both SBP and DBP were statistically significant between the groups (both p < .001). Similarly, a statistically significant difference was obtained in the fundal height of the diseased group compared to control group (p = .03). The difference of both gravida and para of the preeclamptic women compared to normotensive control was statistically significant (both p < .001). A similar statistically significant difference was obtained in the period of gestation of preeclamptic when compared to normotensive women (p = .007). The level of VD (p = .4) and Hb (p = .1) between the two groups in this report did not attain statistical significance. All the demographics of the study population are summarized in .
Table 2. Demographic and clinical variables between normotensive (control) and preeclamptic (cases) patients.
The frequencies and percentages of different variables in the study population are shown in . To rule out the edema status, 45% of the total study population were presented with non-pitting edema, 21.3% had mild while 33.8% had moderate pitting edema. Urine albumin was absent in 50% of study cases, 30% had 30–100 mg/g urine albumin, 18.8% had 100–300 while 1.3% have above 300 mg/g urine albumin. Urinary tract infection (UTI) was evaluated based on pus cells in urine, 77.5% had normal urine pus cells while 22.5% were presented with UTI. The BMI of 23.8% individuals were in normal range, while 23.8% and 52.5% cases were overweight and obese respectively.
Table 3. Frequency and percentage distribution of different study parameters.
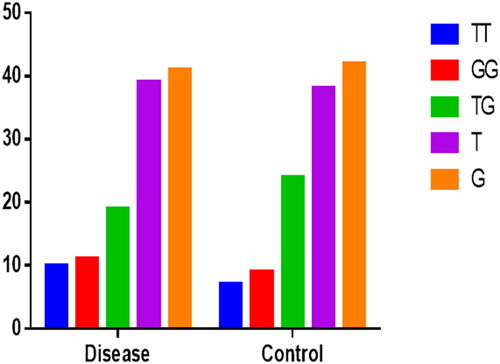
The levels of VD were normal in 2.5%, 10% were presented in VD insufficiency while 87.5% were VD deficient (Supplementary Table S1). The VDR-GG genotype was 25%, GT was 53.8%, and TT was 21.3% in study population. The overall genotype distribution between disease group and control group is also shown in . Similarly, no statistical difference was found between VDR-APA-I genotypes and study variables (preeclamptic vs. control) (p > .05). Levels of VD were low in control group but even lower in disease group though the difference was not statistically significant (Supplementary Table S2). summarizes the difference in study variables based on genotypes distribution. To find the impact of genotypes, levels of VD, and BMI on disease, logistic regression analysis was performed as shown in . The HWE was calculated using web-based online tool. Both the disease group and control group were not consistent with HWE as shown in . No statistically significant association was found between genotypes and VD levels among disease and control group. However, individual with TG genotypes were 1.8 times more likely to develop PE rather than TT genotypes. Similarly, individuals with insufficient and deficient VD levels were 3.5 times and 2.4 times more likely to develop PE rather than those with normal VD levels. Obesity was strongly associated with the development of PE with p < .001. To ascertain the effect of modifying effect of obesity on genotype association with the disease, multivariate logistic regression was performed using two independent variables, i.e. genotypes and obesity. However, our data suggest that there was no significant influence of obesity on association of VDR genetics with the disease (Supplementary Table S3 and ).
Table 4. VDR ApaI (rs7975232) genotypes distribution based on different study variables.
Table 5. Regression analysis of study variables between disease and control group.
Table 6. Hardy–Weinberg equilibrium between disease and control group.
Discussion
In the present study, we compared VD receptor gene polymorphism (Apa1) and VD status in total of 80 preeclamptic and normotensive pregnant women of the province of KPK Pakistan. In previous studies, VD has been shown to affect incidence of preeclampsia and VD supplementation also has shown some promising results in various interventional studies [Citation25]. Therefore, a case–control study was designed to address the objective of the present study. However, we found that neither VD level nor VDR SNP ApaI has any statistically significant association with the incidence of preeclampsia.
According to the present study, Apa 1 genotype distribution between preeclamptic and normotensive subjects was homozygous wild genotype (GG), present in 11 preeclamptic (27.5%) and 9 normotensive (22.5%) subjects. Heterozygous mutant genotype (GT) was present in 19 preeclamptic (47.5%) and 24 normotensive (60%) subjects. Homozygous mutant (TT) genotype was present in 10 preeclamptic (25%) and 7 normotensive (17.5%) subjects (), showing no statistically significant variation between preeclamptic and normotensive women regarding wild and mutant genotypes (p > .05). Apa-I (rs7975232) is an intron variant and doesn’t cause a change in the linear code. However, it does have a documented effect on mRNA stability and, therefore, expression of VDR gene. The phenotypic manifestation of VDR-rs7975232 is not consistent across different descents or ancestries [Citation26]. The present study is in accordance with a previous study conducted by Rezende et al., where they found no correlation between different variants of VD receptor gene (Fok1, Apa1, and Bsm1) and preeclampsia [Citation27]. Similarly, Rezavand et al. found no association of preeclampsia with VD receptor SNP Fok1 [Citation21]. Association between preeclampsia and allelic variant of VD receptor gene rs12831006 was confirmed by Baca et al., while they found no statistically significant association between SNP rs1544410 (Bsm1) and preeclampsia [Citation28]. In the current study, in preeclamptic women minor (GT, TT), genotypes were more prevalent in women with high BMI as shown in . There was no statistically significant difference between genotypes in terms of anthropometric and various clinical parameters ().
In both normotensive and preeclamptic groups, the VD levels were lower however, the level of VD was comparable in both preeclamptic and normotensive women (p = .44). As shown in , the mean value of VD in preeclamptic patients was 6.79 ng/mL and mean VD level in control group was 8.53 ng/mL. Thus, according to the results of this study 1.3% of the control group was adequate regarding VD status and also 1.3% of the preeclamptic women had adequate VD levels. In control group, 2.5% of the women were deficient and 23.7% were severely deficient regarding VD level, while 5% of preeclamptic patients were deficient and 8.8% were severely deficient. Among these 35% of preeclampsia patients and 18.8% of normotensive women had very severely deficient VD levels as shown in Supplementary Table S1. Thus 93.8% of the study group had VD deficiency. The findings of this study are consistent with a cross-sectional study conducted in Lahore Pakistan [Citation5]. They compared serum VD levels in preeclamptic and normotensive pregnant women and concluded that 95% of women were VD deficient with VD levels comparable in both groups. Another study done on a small group of pregnant women in Karachi Pakistan [Citation29] showed similar results with 90% of pregnant women having VD deficiency.
The high prevalence of VD deficiency in the study group could rise because the pregnant women were not taking VD or calcium supplements and were of low socioeconomic status having poor diet not fortified with VD and calcium supplements. Furthermore, most of these women were housewives who spent most of their time indoor at home and wearing veil and folk weave clothing and thus less exposed to sun. The findings of the current report do not associate low levels of VD with preeclampsia unlike the work done by previous researchers [Citation3,Citation12,Citation30]. Bodnar et al. conducted a nested case–control study and found that 55 nulliparous women who developed preeclampsia had VD deficiency [Citation3]. Similarly, Baker et al. also conducted a nested case–control study and reported that women having VD levels <20 ng/mL had four times greater risk of developing severe preeclampsia [Citation12]. Concomitantly, the result of our study is in agreement with previous studies conducted on vitamin D status and preeclampsia, where they found no association of VD level with preeclampsia [Citation5,Citation31]. Powe et al. conducted a nested case–control study and found no association of first trimester total 25-hydroxyvitamin-D (25 (OH) D) levels and subsequent development of preeclampsia [Citation31]. Similarly, Shand et al. conducted a case–control study on 221 pregnant women who were VD deficient however, VD deficiency was not correlated with preeclampsia or gestational hypertension [Citation32]. The discrepancy between their and the current study may be due to the different populations that were studied. Another reason of discrepancy might be the measurement of serum 25 (OH) D in third trimester of pregnancy in the present study, while Bodnar et al. assessed vitamin D level at 10 weeks of gestation. The possibility may be that third trimester VD status has no significant influence on placentation and development of preeclampsia as compared to first trimester VD concentrations. The difference between our results and Bodnar et al. may also be due to difference in weight and height measurements [Citation3]. Bodnar et al. calculated BMI from self-reported pre-pregnancy weights and heights while we measured weights and heights at time of sampling. 25 (OH) D levels are more likely associated with BMI at time of measurement than pre-pregnancy BMI. Lower circulating 25 (OH) D levels in obese subjects have been attributed to the deposition of VD in adipose tissue [Citation33]. As the risk of preeclampsia increases in obese pregnant women [Citation34], therefore it is inferred that pre-pregnancy BMI may not be a potential confounding factor as compared to BMI measured at time of 25 (OH) D measurement. The chances of preeclampsia increase with the increase in BMI as shown in our study where 97.5% of the preeclamptic patients had high BMI and only 2.5% had normal BMI. While in control group 42.5% were of normal BMI and 57.5% were of high BMI. According to the statement of American College Of Obstetricians and Gynecologists (AGOG) Technical Bulletin 219 Washington DC 1996, the risk of preeclampsia increases with high BMI by the ratio of 3:1. Association of two predictor variables, i.e. obesity and genotypes, with preeclampsia was also assessed. However, our data suggest that there was no significant influence of obesity on association of VDR genetics with the disease.
This study has certain limitations. The sample size of our study was not adequate. Therefore, including larger cohort would have represented the population more accurately. The second limitation of this study is that we did not include a control group of non-pregnant women. Inclusion of this third group of normotensive non-pregnant women would have improved our study results. Also, we only considered Apa1 polymorphism while results of the study would have improved if other vitamin D receptor gene polymorphisms (Taq1, Bsm1, and Cdx-2) were considered and their possible interaction with Apa1 evaluated.
Conclusion
This study shows that pregnant women of Pakistani population both normotensive and preeclamptic are very deficient regarding 25 (OH) D levels. Similarly, we observed no significant association of VD deficiency and VD receptor gene polymorphism (Apa1) with preeclampsia. The levels of 25 (OH) D were similar in both cases and controls and also mutant genotype frequency was similar in both groups.
Compliance with ethics guidelines
All procedures involving human participants performed in this study were in accordance to the ethical standards of Khyber Medical University’s ethical committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the institution board of study and informed consents were obtained from all individual participants included in the study.
Author contributions
AA and MS were involved in study design, execution, writing of the manuscript and analysis of the data. AJ helped in recruiting control subjects. SGV and SA helped in the organization of the dataset and in data analysis. WI and SA helped in data analysis as well as experimentation. SS helped in execution of the data and analysis of the results. IK, SGV and SA provided critical discussion. All authors reviewed and approved the final version of the manuscript.
Supplemental Material
Download Zip (77.2 KB)Disclosure statement
The authors report no conflicts of interest.
Data availability
Available from the corresponding author on reasonable request.
Additional information
Funding
References
- Hyppönen E, Cavadino A, Williams D, et al. Vitamin D and pre-eclampsia: original data, systematic review and meta-analysis. Ann Nutr Metab. 2013;63(4):1–6.
- ACOG Committee on Obstetric Practice. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American college of obstetricians and gynecologists. Int J Gynaecol Obstet. 2002;77(1):67–75.
- Bodnar LM, Catov JM, Simhan HN, et al. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007;92(9):3517–3522.
- Balogun OAA, Sibai BM. Counseling, management, and outcome in women with severe preeclampsia at 23 to 28 weeks’ gestation. Clin Obstet Gynecol. 2017;60(1):183–189.
- Umar N, Tauseef A, Shahzad F, et al. Serum 25-hydroxy vitamin D level in preeclamptic and normotensive pregnancies. J Coll Physicians Surg Pak. 2016;26(8):673–676.
- Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–799.
- Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol. 1998;179(5):1359–1375.
- Wang A, Rana S, Karumanchi SA. Preeclampsia: the role of angiogenic factors in its pathogenesis. Physiology (Bethesda). 2009;24(3):147–158.
- Shin JS, Choi MY, Longtine MS, et al. Vitamin D effects on pregnancy and the placenta. Placenta. 2010;31(12):1027–1034.
- Friedman SA, Taylor RN, Roberts JM. Pathophysiology of preeclampsia. Clin Perinatol. 1991;18(4):661–682.
- Achkar M, Dodds L, Giguère Y, et al. Vitamin D status in early pregnancy and risk of preeclampsia. Am J Obstet Gynecol. 2015;212(4):511.e1–511.e7.
- Baker AM, Haeri S, Camargo CAJr, et al. A nested case–control study of midgestation vitamin D deficiency and risk of severe preeclampsia. J Clin Endocrinol Metab. 2010;95(11):5105–5109.
- Wei S-Q, Qi H-P, Luo Z-C, et al. Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2013;26(9):889–899.
- Palacios C, Kostiuk LK, Peña-Rosas JP, Cochrane Pregnancy and Childbirth Group. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;(7):1–97.
- Ali AM, Alobaid A, Malhis TN, et al. Effect of vitamin D3 supplementation in pregnancy on risk of pre-eclampsia–randomized controlled trial. Clin Nutr. 2019;38(2):557–563.
- Stougaard M, Damm P, Frederiksen P, et al. Extra vitamin D from fortification and the risk of preeclampsia: the D-tect study. PLoS One. 2018;13(1):e0191288.
- Schneuer FJ, Roberts CL, Guilbert C, et al. Effects of maternal serum 25-hydroxyvitamin D concentrations in the first trimester on subsequent pregnancy outcomes in an Australian population. Am J Clin Nutr. 2014;99(2):287–295.
- Al-Eisa AA, Haider MZ. Vitamin D receptor gene TaqI and apal polymorphisms and steroid responsiveness in childhood idiopathic nephrotic syndrome. Int J Nephrol Renovasc Dis. 2016;9:187–192.
- Uitterlinden AG, Fang Y, van Meurs JB, et al. Vitamin D receptor gene polymorphisms in relation to vitamin D related disease states. J Steroid Biochem Mol Biol. 2004;89–90:187–193.
- Zhan Y, Liu M, You Y, et al. Genetic variations in the vitamin-D receptor (VDR) gene in preeclampsia patients in the chinese han population. Hypertens Res. 2015;38(7):513–517.
- Rezavand N, Tabarok S, Rahimi Z, et al. The effect of VDR gene polymorphisms and vitamin D level on blood pressure, risk of preeclampsia, gestational age, and body mass index. J Cell Biochem. 2019;120(4):6441–6448.
- Salman H, Shah M, Ali A, et al. Assessment of relationship of serum neurokinin-b level in the pathophysiology of pre-eclampsia: a case–control study. Adv Ther. 2018;35(7):1114–1121.
- Bischoff-Ferrari HA. “Vitamin D – why does it matter?” – defining vitamin D deficiency and its prevalence. Scand J Clin Lab Invest. 2012;72(Suppl 243):3–6.
- Ye J, Coulouris G, Zaretskaya I, et al. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinf. 2012;13(Suppl 6):1–11.
- Aguilar-Cordero M, Lasserrot-Cuadrado A, Mur-Villar N, et al. Vitamin D, preeclampsia and prematurity: a systematic review and meta-analysis of observational and interventional studies. Midwifery. 2020;87:102707.
- Jurutka PW, Whitfield GK, Hsieh J-C, et al. Molecular nature of the vitamin D receptor and its role in regulation of gene expression. Rev Endocr Metab Disord. 2001;2(2):203–216.
- Rezende VB, Sandrim VC, Palei AC, et al. Vitamin D receptor polymorphisms in hypertensive disorders of pregnancy. Mol Biol Rep. 2012;39(12):10903–10906.
- Baca KM, Govil M, Zmuda JM, et al. Vitamin D metabolic loci and preeclampsia risk in multi‐ethnic pregnant women. Physiol Rep. 2018;6(2):e13468.
- Hossain N, Khanani R, Hussain-Kanani F, et al. High prevalence of vitamin D deficiency in pakistani mothers and their newborns. Int J Gynaecol Obstet. 2011;112(3):229–233.
- Robinson CJ, Alanis MC, Wagner CL, et al. Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. Am J Obstet Gynecol. 2010;203(4):366.e1–366.e6.
- Powe CE, Seely EW, Rana S, et al. First trimester vitamin D, vitamin D binding protein, and subsequent preeclampsia. Hypertension. 2010;56(4):758–763.
- Shand A, Nassar N, Von Dadelszen P, et al. Maternal vitamin D status in pregnancy and adverse pregnancy outcomes in a group at high risk for pre‐eclampsia. BJOG. 2010;117(13):1593–1598.
- Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–693.
- DeVader SR, Neeley HL, Myles TD, et al. Evaluation of gestational weight gain guidelines for women with normal prepregnancy body mass index. Obstet Gynecol. 2007;110(4):745–751.