Abstract
Background
Polycystic ovarian syndrome (PCOS) affects up to 18% of reproductive-aged women and raises the risk of venous thromboembolic disease (VTE), due to metabolic features and an apparent fibrinolytic state. Recent studies have shown an increased risk of VTE (1.5- to 2-fold) in patients with PCOS as compared to those without PCOS. Mutations in the Protein C (PC) gene (PROC) lead to deficiency or dysfunction of the protein, Protein C deficiency is the main clotting physiological inhibitor of protein C cofactors, and is a risk factor for venous thrombosis, which can cause a variety of events, including miscarriage. This case report proposes a correlation between PCOS, protein C deficiency, venous thrombosis and inevitable miscarriage.
Case presentation
A 33-year-old Chinese woman was diagnosed with Polycystic Ovary Syndrome (PCOS) in 2015. During the course of treatment, she took ethinylestradiol and cyproterone acetate tablets for more than one year. In 2016, she was sent to a hospital for emergency care due to explosive thrombosis (thrombosis in multiple parts of the body and pulmonary thrombosis). In 2020, the patient became pregnant via natural means and came to our hospital for treatment. During the second trimester, she experienced an inevitable miscarriage. High-throughput sequencing (NGS) of peripheral blood lymphocytes revealed that the patient had a protein C deficiency resulting from a heterozygous mutation deletion of 572_574 in exon 7.
Conclusion
PC deficiency in conjunction with PCOS and the concomitant use of oral contraceptive (COC) would increase the risk of VTE, especially in the early stages of COC use.
摘要
背景
多囊卵巢综合征(PCOS)影响了18%的育龄妇女, 由于代谢特征和明显的纤维蛋白溶解状态, 增加了静脉血栓栓塞疾病(VTE)的风险。最近的研究表明, 与无PCOS的患者相比, PCOS患者的VTE风险增加(1.5- 2倍)。Protein C (PC)基因(PROC)突变导致该蛋白的缺失或功能障碍, 蛋白C缺失是蛋白C辅助因子的主要凝血生理抑制剂, 是静脉血栓形成的危险因素, 可引起包括流产在内的多种事件。本病例报告提出PCOS、蛋白C缺乏、静脉血栓形成与不可避免的流产之间的相关性。
病例介绍
2015年, 一名33岁的中国女性被诊断为多囊卵巢综合征(PCOS)。治疗期间服用炔雌醇、醋酸环丙孕酮片1年多。2016年因爆发性血栓形成(全身多部位血栓形成、肺血栓形成)入院急救。2020年, 患者通过自然方式怀孕, 来我院治疗。在妊娠中期, 她经历了不可避免的流产。外周血淋巴细胞的高通量测序(NGS)显示, 患者存在蛋白C缺乏, 原因是外显子7中572_574杂合突变缺失。
结论
PC缺陷合并PCOS和同时使用口服避孕药(COC)会增加静脉血栓栓塞的风险, 特别是在使用COC的早期。
Background
PC is a vitamin K–dependent zymogen that could be a contributor to thrombophilia, often in conjunction with other genetic or acquired risk factors [Citation1]. Partial PC deficiencies (heterozygous forms) are much more frequent (1 in 200 to 1 in 500 births) than severe PC deficiencies (homozygous or compound heterozygous forms) (1 in 500 000 to 1 in 750 000 births) [Citation2]. Hereditary PC deficiency is caused by a mutation in the PC gene (PROC) located on chromosome 2q14, and over 160 mutations in PROC have been identified as causes of PC deficiency [Citation3]. In 1981, Griffin et al. published the first study reporting that low levels of plasma protein C were associated with venous thrombosis [Citation4]. Meanwhile, thrombosis is closely related to miscarriage [Citation5]. Here, we present a case of a patient with Polycystic Ovary Syndrome (PCOS) who developed persistent hypercoagulability after taking ethinylestradiol and cyproterone acetate tablets, and later developed multi-site deep vein thrombosis, and experienced an inevitable miscarriage. High-throughput sequencing (NGS) revealed that the patient had a heterozygous mutation deletion of 572_574 in exon 7, which caused the protein C deficiency.
Case presentation
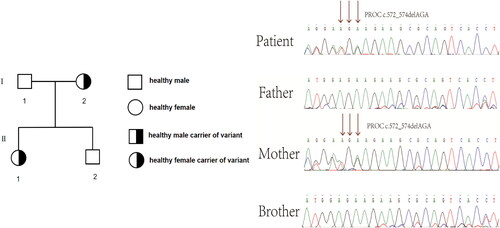
In 2015, a 33-year-old Chinese woman with height of 170 cm, weight of 64 kg and Body Mass Index (BMI) of 22.14 kg/m2 was diagnosed with PCOS in our hospital based on irregular menstruation, hyperandrogenemia and polycystic ovaries, she had no familial hypercholesterolemia (FH) and no familial history of thrombosis. The patient was treated with ethinylestradiol and cyproterone acetate tablets and metformin for more than one year. On December 28, 2016, the patient was admitted to a hospital of Guangzhou reporting chest pains and a brief fainting spell. A blood vessel color ultrasound revealed that multiple venous thrombosis had occurred in the common femoral vein, superficial femoral vein, popliteal vein, fibular vein and some lateral branches of the leg of the left lower limb. Meanwhile, Computed Tomography Angiography (CTA) indicated embolization of the pulmonary trunk and its branches (), and infection of the middle right lung and lower bilateral lung. Blood testing further indicated high D-dimer (2.45 ng/L). The patient was immediately treated with inferior vena cava filter implantation, pulmonary artery and femoral vein thrombosis aspiration, dilation and catheter-directed thrombolysis. After surgery, bridging anti-coagulation from warfarin with low molecular weight heparin (LMWH) was continued. After discharge, the patient took warfarin 3 mg once a day orally, and the international normalized ratio (INR) fluctuated within the normal range (2–3). The patient discontinued LMWH and warfarin in April 2020. Then, she became pregnant naturally in May 2020. Owing to hypercoagulability during pregnancy and pulmonary embolism, the patient came to our hospital at 8 weeks of gestation. Ultrasound indicated an intrauterine monochorionic twin pregnancy, and blood testing showed that the activate partial thromboplastin time (APTT) was 23.1 s (normal range: 23.3–32.5 s). Thus, we treated her with dydrogestrone 10 mg twice a day orally, LMWH(enoxaparin sodium) 0.6 ml twice daily, and aspirin 100 mg once daily. At 14 weeks of gestation, the patient was administered 15 mg of progesterone via injection in our hospital due to vaginal bleeding accompanied by dull pain in the lower abdomen for 2 h. Ultrasound showed that an intrauterine monochorionic double amniotic sac and old thrombosis had occurred in the left popliteal vein and superficial femoral vein (), while blood tests showed that D-dimer was 0.63 ng/L (normal range: 0–0.55 ng/L), and fibrinogen (Fbg) was 4.11 g/L (normal range: 2–4 g/L). The patient was discharged after remission and continued to take LMWH(enoxaparin sodium) and aspirin. At 22 weeks of pregnancy, the patient was readmitted to our hospital with multi-site venous thrombosis and inevitable miscarriage. Ultrasonography indicated that popliteal vein thrombosis had occurred in the left lower limb, and the patient was given an emergency cervix ligation for a 4 cm uterine opening and fetal heart monitoring of 136/156bpm and was treated for 0.6 ml low-molecular-weight heparin(enoxaparin sodium). After the operation, the patient’s weak uterine contractions were palpable, and the fetal heart rate was 132/157 bpm. Ultrasound revealed that old thrombosis had occurred in the left popliteal vein and superficial femoral vein, and blood tests showed that D-dimer was 5.63 ng/L (normal range: 0–0.55 ng/L), activate partial thromboplastin time (APTT) was 22.2 S (normal range: 23.3–32.5 s), and fibrinogen (Fbg) was 4.35 g/L (normal range: 2–4 g/L). Coagulation indexes showed that the coagulation system was abnormal. Afterward, at 26 weeks of gestation, the patient had an inevitable miscarriage and took low molecular weight heparin(enoxaparin sodium) 0.4 ml once daily. At 60 days postpartum, the patient underwent a postpartum reexamination in our hospital. According to the scale recommended by the Royal College of Obstetricians and Gynecologists (RCOG) for diagnosis and treatment of VTE during pregnancy and puerperium in 2015, the VTE score of this patient during puerperium was 1 (Preterm birth <37 + 0 weeks in current pregnancy) [Citation6]. Ultrasound showed that some old thrombosis had occurred in the left popliteal vein and the superficial femoral vein, and superficial varicose veins were present in the left lower extremities. Blood tests found that D-dimer was 5.63 ng/L (normal range: 0–0.55 ng/L), activate partial thromboplastin time (APTT) was 19.4 S (normal range: 23.3–32.5 s), thrombin time (TT) was 21.9 s (normal range: 14–21.9 s), and protein C was 38% (normal range: 70–130%). Qualitative tests for anti-cardiolipin-antibody (ACA) and anti-β2 glycoprotein 1 (Anti-β2GP1) both came back negative, indicating a hypercoagulable state. To detect the genetic causes underlying this condition, we extracted the genomic DNA of peripheral blood lymphocytes from the patient and her family members after obtaining written consent. All exons and adjacent regions of PROC were directly sequenced. We found that the patient had a heterozygous deletion variant of PROC c.572_574delAGA. The family study found that the patient had inherited heterozygous PROC c.572_574delAGA from her mother, and that her brother had not inherited PROC c.572_574delAGA from his parents ().
Figure 1. Computed Tomography Angiography (CTA) revealed embolization of the pulmonary trunk and its branches (indicated by the arrows).
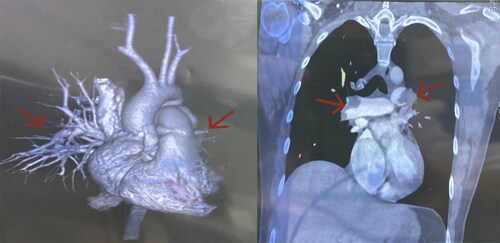
Figure 2. Ultrasonography at the 14th week of gestation showed hypoechoic filling (A, arrow) of the superficial femoral vein, which could not be closed under pressure (B, arrow). Color Doppler ultrasound showed no color flow signal (indicated by the arrow) in the superficial femoral vein (C) or popliteal vein (D).
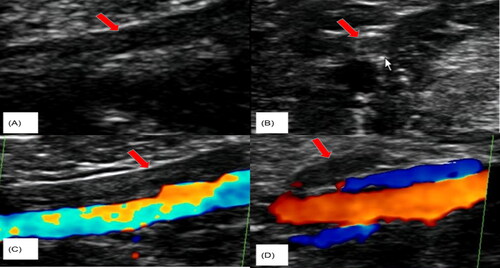
Figure 3. Genetic analysis of the PROC gene in the family. Sequencing of the PROC gene revealed that the patient carried the heterozygous deletion variant c.572_574delAGA of PROC. Family study showed that PROC c.572_574delAGA was inherited from the subject’s mother, while the subject’s brother did not inherit PROC c.572_574delAGA from his parents. The arrow indicates the mutation.
Discussion and conclusion
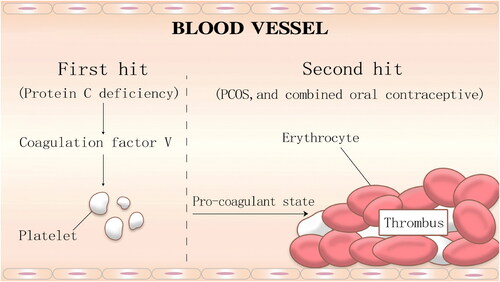
Protein C, a vitamin K-dependent glycoprotein, is synthesized in the liver [Citation7]. It can activate coagulation factors Va and VIIIa, which are required for the coagulation cascade formed by thrombin [Citation8]. Protein C deficiency is closely associated with prethrombotic conditions, which increases risks for deep venous thrombosis and pulmonary embolism [Citation9]. Protein C deficiency can be divided into acquired and hereditary forms. Acquired protein C deficiency is common, as it has often been observed in patients with anticardiolipin syndrome, disseminated intravascular coagulation, hyper-homocysteinaemia and so on [Citation10]. Hereditary protein C deficiency, including homozygous and heterozygous types, is a relatively rare autosomal dominant genetic disease with a low morbidity rate (0.2%–0.3%) [Citation11]. PROC is one of the genes encoding protein C, which is closely related to the formation of thrombi [Citation12]. PROC is located on chromosome 2q13-q14, and includes 9 exons and 8 introns. Homozygous protein C deficiency is associated with life-threatening thrombotic diseases in the neonatal period, while a patient with heterozygous deficiency C would have a higher risk of venous thromboembolism [Citation13]. The PROC mutation type has been widely reported, including PC Arg-1Cys, PC Arg9Cys, PC Val34Met and so on, with a mutation prevalence of approximately 2.36% for Chinese people [Citation14]. Protein C deficiency is associated with the heterozygous gene mutation. The deletion mutation of 3 nucleotides from 572 to 574 of the cDNA sequences leads to the deletion of translation PC product at position 192 or 193. One study illustrated that the common genetic risk factor (PROC c.572_574del) in the Chinese population conferred a 2.84-fold increased risk of developing VTE [Citation15]. An in vitro expression study demonstrated that the anticoagulant activity of the c.572_574 del AGA variant was 43.6% of that of the wild-type PC [Citation14]. In this family study, the patient who carried the PROC c.572_574delAGA, showed PC activity below standard values. VTE is the result of the interaction between one or more genetic factors and environmental risk factors in affected patients. In this case, coagulation indexes were regularly tested, and hypercoagulability of the blood still existed. Considering the patient’s long history of multi-site thrombosis and the examination data, hereditary thrombotic disease was suggested as a potential cause. Protein C deficiency caused by PROC mutation remains the main cause of multiple thrombus formation. Meanwhile, it has been reported that simple heterozygous mutations in PROC may not lead to multi-site and long-term thrombus formation [Citation16]. Thus, we speculated that thrombus was mainly caused by a first hit and second hit (first hit: protein C deficiency; second hit: PCOS, and combined oral conatraceptive) (). First, PCOS is one of the endocrine disorders affecting women of reproductive age [Citation17]; a protein C deficiency caused by PROC gene mutations (first hit), could further aggravate a high blood coagulation state in a patient with PCOS because of hyperinsulinemia, by damaging vascular endothelial cells and causing platelet aggregation[Citation18]. Meanwhile, thromboembolism would occur due to the use of ethinylestradiol and cyproterone acetate tablets, which would increase the activity of coagulation factor V [Citation19]. Moreover, the patient became pregnant naturally in 2020, which led to a hyper-coagulant state due to abnormalities in clotting factors such as AT-3 and protein C [Citation20]. Consequently, the combination of protein C deficiency, hyperinsulinemia caused by PCOS, and the use of cyproterone acetate tablets, could have led to the occurrence of multi-site thrombus and pulmonary embolism.
Our case report is similar to a previous report that initial thromboembolism in patients with protein C deficiency is associated with genetic and acquired risk factors in approximately 30% of subjects [Citation21]. Genetic thrombotic defects affect up to 16% of the population, but if fatal venous thromboembolism (VTE) is used as a clinical endpoint, general screening for genetic thrombotic defects does not seem cost-effective [Citation22]. It is not known whether routine screening for hereditary thrombosis defects before conbined oral contraceptive use is useful or feasible, even if first-degree relatives have a family history of hereditary thrombosis [Citation23]. When a woman develops a vein thrombosis in the first year of using COC, the screening test may be a sign that she has an inherited thrombotic defect. Importantly, what we learned from this case is that we should not only consider the effect of hyperinsulinemia from PCOS on blood coagulation function during treating for PCOS patients, but also pay attention to genetic diseases and carry out related genetic testing for patients and their family members. In addition, we should always emphasize the condition of the fetus and avoid the occurrence of miscarriage for pregnant women with protein C deficiency, to achieve early diagnosis and early prevention.
Currently, there are many treatment options for hereditary protein C deficiency, while the main treatments for thrombus are still coumarin anticoagulants (warfarin) or heparin therapy. It is well-accepted that patients with heterozygous mutations of inherited protein C deficiency should be given anticoagulants and protein c concentrates, while the only potential treatment for homozygous PC deficiency is liver transplantation [Citation24]. However, because warfarin reduces the production of protein C, and novel oral anticoagulants (NOACs)-rivaroxaban inhibit the likelihood of thrombosis and can be used in patients due to warfarin skin necrosis treatment, NOACs-rivaroxaban have gradually taken the place of warfarin for the treatment of patients with thrombosis [Citation25]. For postpartum patients with a history of hereditary thrombosis, clinicians should prescribe low molecular weight heparin. There are two doses of LMWH: prophylactic dose and therapeutic dose. The use of prophylactic dose is 2850 IU (0.3 ml) of an heparin calcium injection or 5000 I of heparin sodium injection U (0.5 ml) or 4000 IU (0.4 ml) of enoxaparin sodium injection subcutaneously once a day; the commonly used therapeutic dose is heparin calcium injection 0.01 ml/kg (95 IU/kg) or large heparin sodium injection 100 IU/kg or enoxaparin sodium injection 100 IU/kg, subcutaneous injection, twice a day. Prophylactic dose is recommended if there is no recent vascular embolism or related history.The therapeutic dose is recommended for patients with recent vascular embolism or related history [Citation26]. Although protein C deficiency is regarded to be closely associated with adverse pregnancy outcomes, there is no clear evidence showing that the use of heparin to treat protein C deficiency can reduce the incidence of adverse pregnancy outcomes. Therefore, heparin therapy should be considered on an individual basis [Citation27]. In addition, for pregnancy and postpartum of thrombosis in high-risk women, we should conduct caprini scoring, imaging monitoring such as ultrasound and prevention of anticoagulation therapy, so as to reduce the risk of postpartum thrombosis
In conclusion, according to the literature evaluating our case, patients with PCOS are at a very high risk of developing VTE after taking COC, especially in the early stages of COC use. Hereditary thrombogenesis defects such as mutations in the PROC gene often increase the risk of VTE. Screening for hereditary thrombosis defects should be considered in patients with venous thrombosis and PCOS, such as DVT. Lifelong oral anticoagulant therapy should be recommended in the event of life-threatening thrombosis or thrombosis at an abnormal site.
Author’s contributions
LL and MLZ contributed to the conception and design of this paper. LL, MLZ and YPT prepared the figures and wrote the manuscript. XHP and YS were responsible for clinical information collection. JHZ, XWZ and QYX contributed to the genetic sequence analysis for this case. All authors read and approved the final manuscript.
Consent for publication
The patient has written informed consent for publication of clinical details and/or clinical images was obtained from herself.
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the Guangdong Women and Children Hospital Ethics Committee. Patients provided written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Acknowledgements
We are grateful to the patient and her family who kindly consented to join the study.
Data availability statement
The data that support the findings of this study are available from corresponding author upon reasonable request.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Additional information
Funding
References
- Dahlbck B. The discovery of activated protein C resistance. J Thromb Haemost. 2010;1:3–9.
- Goldenberg NA, Manco-Johnson MJ. Protein C deficiency. Haemophilia. 2008;14(6):1214–1221.
- Kottke-Marchant K, Comp P. Laboratory issues in diagnosing abnormalities of protein C, thrombomodulin, and endothelial cell protein C receptor. Arch Pathol Lab Med. 2002;126(11):1337–1348.
- Griffin JH, Evatt BL, Zimmerman TS, et al. Deficiency of protein C in congenital thrombotic disease. J Clin Invest. 1981;68(5):1370–1373.
- Parand A, Zolghadri J, Nezam M, et al. Inherited thrombophilia and recurrent pregnancy loss. Thromb Res. 2009;123:S140.
- Royal College of Obstetricians and Gynaecologists. Thromboembolic Disease in Pregnancy and the Puerperium: acute Management. Green-top Guideline No. 37a [S/OL.] (2015-04-30) [2017-07-15].
- Stenflo JA. A new vitamin K-dependent protein. Purification from bovine plasma and preliminary characterization. J Biol Chem. 1976;251(2):355–363.
- Negoro N, Yoshikawa J. [Protein C deficiency]. Ryoikibetsu Shokogun Shirizu 1996.
- Koster T, Rosendaal FR, Briet E, et al. Protein C deficiency in a controlled series of unselected outpatients: an infrequent but clear risk factor for venous thrombosis (leiden thrombophilia study). Blood. 1995;85(10):2756–2761.
- Patel K, Chaney MA. Hypercoagulable states: thrombosis and embolism-ScienceDirect[J]. Complications in Anesthesia. 2nd ed. 2007:361–364.
- Middeldorp S, Vlieg A. Does thrombophilia testing help in the clinical management of patients? Br J Haematol. 2008;143(3):321–335.
- Wu D, Zhong Z, Chen Y, et al. Analysis of PROC and PROS1 single nucleotide polymorphisms in a thrombophilia family. Clin Respir J. 2019;13(8):530–537.
- Mahmoodi BK, Brouwer JLP, Kate M, et al. A prospective cohort study on the absolute risks of venous thromboembolism and predictive value of screening asymptomatic relatives of patients with hereditary deficiencies of protein S, protein C or antithrombin. J Thromb Haemost. 2010;8(6):1193–1200.
- Tang L, Lu X, Yu JM, et al. PROC c.574_576del polymorphism: a common genetic risk factor for venous thrombosis in the Chinese population. J Thromb Haemost. 2012;10:2019–26.
- Tang L, Wang HF, Lu X, et al. Common genetic risk factors for venous thrombosis in the chinese population. Am J Hum Genet. 2013;92(2):177–187.
- Ding Q, Shen W, Xu Y, et al. Clinical and genetic features of protein C deficiency in 23 unrelated chinese patients. Blood Cells Mol Dis. 2013;50(1):53–58.
- Gurkan B, Sezcan M, Dila Z, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841.
- Boden G, Rao AK. Effects of hyperglycemia and hyperinsulinemia on the tissue factor pathway of blood coagulation. Curr Diab Rep. 2007;7(3):223–227.
- Agrawal R, Prelevic G, Conway GS, et al. Serum vascular endothelial growth factor concentrations in postmenopausal women: the effect of hormone replacement therapy. Fertil Steril. 2000;73(1):56–60.
- Vliet H, Rodrigues SP, Snieders M, et al. Sensitivity to activated protein C during the menstrual cycle in women with and without factor VLeiden. Thromb Res. 2008;121(6):757–761.
- Dusse LM, Rios DRA, Pinheiro MB, et al. Pre-eclampsia: relationship between coagulation, fibrinolysis and inflammation. Clin Chim Acta. 2011;412(1-2):17–21.
- Kalev M, Day T, Van de Water N, et al. Screening for a prothrombotic diathesis in patients attending family planning clinics. N Z Med J. 1999;112(1096):358–361.
- Bloemenkamp KW, Rosendaal FR, Helmerhorst FM, et al. Higher risk of venous thrombosis during early use of oral contraceptives in women with inherited clotting defects. Arch Intern Med. 2000;160(1):49–52.
- Dinarvand P, Moser KA. Protein C deficiency. Arch Pathol Lab Med. 2019;143(10):1281–1285.
- Menon N, Sarode R, Zia A. Rivaroxaban dose adjustment using thrombin generation in severe congenital protein C deficiency and warfarin-induced skin necrosis. Blood Adv. 2018;2(2):142–145.
- Zhao A, Wei X, et al. Chinese expert consensus on diagnosis and treatment of recurrent abortion complicated with prethrombotic state. Chin J Reprod Contraception. 2021;41(10):861–875.
- Qi YDaH. Interpretation of ACOG guidelines for inherited thrombolic disorders in pregnancy (2018). Chin J Appl Gynecol Obstet. 2019;35:298–303.