Abstract
Introduction
Embryo implantation provides an efficient way for patients with repeated implantation failure (RIF) to achieve pregnancy. The aim of this study is to compare the implantation outcomes of RIF patients in artificial cycle to those in natural cycle, both were treated with RNA sequencing endometrial receptivity test (rsERT) based personalized embryo implantation.
Methods
The endometrial receptivity (ER) analysis was performed using rsERT followed by personalized embryo transfer at optimal window of implantation (WOI). The implantation rate (IR), clinical pregnancy rate (CPR) and live birth rate (LBR) were calculated. The expression levels of biomarkers involved in pregnancy process in the patients detected as in receptivity status were also analyzed.
Results
The rsERT shown that 44.8% (natural cycle) and 47.8% (artificial cycle) patients were in non-receptive status, which indicated a WOI displacement. After personalized embryo transfer, the IR of patients in artificial cycle was higher than those in natural cycle (52.2% vs 27.6%). The expressions of FKBP52, MUC1 and LPAR3 were significantly lower in artificial cycle than in natural cycle.
Conclusion
Using artificial cycle for personalized embryo transfer based on rsERT may yield better pregnancy outcomes for RIF patients. A gene expression analysis of FKBP52, MUC1 and LPAR3 provided a potential way to increase implantation outcomes for RIF patients.
Introduction
It is estimated that 8-12% of couples worldwide currently suffer from infertility [Citation1] and embryo implantation provides an efficient way for these couples to achieve pregnancy. The implantation is a complex process involving interactions between and blastocyst and endometrium, during which the endometrial receptivity (ER) is essential for successful implantation. Indeed, it has been estimated that ER accounts for two-thirds of implantation success [Citation2,Citation3]. The receptivity level is essential for implantation success, which period is known as the ‘window of implantation’ (WOI). It has been reported that approximately 60% of repeated implantation failure (RIF) can be attributed to WOI displacement [Citation4–7].
Various methods have been developed to determine the ER status, mainly including ultrasonography, histology and transcriptome [Citation8–10]. Among which, transcriptomic methods endometrial receptivity array (ERA) and RNA sequencing endometrial receptivity test (rsERT) provide ways for high-throughput analysis of ER in recent years [Citation11–13]. However, it is still unclear whether rsERT based personalized embryo implantation can offer similar implantation outcomes in patients with RIF in artificial cycle compared to those in natural cycle. This study compared the implantation outcomes of RIF patients in artificial cycle to those in natural cycle, both were treated with rsERT based personalized embryo implantation. We further analyzed the expression levels of key biomarkers involved in pregnancy process in the patients detected as in receptivity status.
Materials and methods
Ethics statement
The study was approved by the Ethics Committee of the Sixth medical center of the Chinese People’s Liberation Army General Hospital. Written informed consent was explained to and signed by all the patients who participated in the current study.
Materials and methods
This study was conducted between July 2019 and December 2021 at the Sixth medical center of the Chinese People’s Liberation Army General Hospital, Beijing, China. A total of 52 patients with RIF were included. These patients were divided into two groups according to their own choice: artificial cycle and natural cycle.
All the patients with artificial cycle were treated with estradiol on the third day of the menstrual cycle followed by progesterone supplementation after 12 days (day 15 of the cycle) and has remained consistent. Endometrial specimens were obtained according to the manufacturer’s instructions from uterine fundus using an endometrial sampler (AiMu Medical Science & Technology Co., Liaoning, China) on LH + 7 (luteinizing hormone surge + 7 days, or ovulation + 5 days) for patients with natural cycle or P + 5 (progesterone treatment + 5 days) for patients with artificial cycle. Briefly, the vulva and cervix were sterilized with iodine before sampling. The tip of the endometrial sampler was placed into the uterine fundus, then the tube core of the sampler was quickly pulled outward to create a negative pressure, and the endometrial tissues were aspirated into the sampler. The collected tissues were immediately placed into RNA-later buffer (Thermo Fisher Scientific, Waltham, MA, USA) and stored at −20 °C for RNA analysis.
The RNA sequencing and corresponding ER analysis were performed by Yikon Genomics Company, Ltd. (Jiangsu, China). Briefly, RNA is extracted from endometrial specimens and reverse transcribed into cDNA. After library construction, the mixed libraries were subjected to the Qubit quantitation assay using HiSeq 2500 platform (Illumina, San Diego, CA, USA). The RNA expression level was calculated using FPKM and ER conditions were analyzed using machine learning method based on established gene library for ER (Yikon Genomics).
The embryo transfer was performed at the optimal WOI based on the ER test. Briefly, after endometrial sampling, the embryo transfer was performed during the following cycle and the time point was personalized, which was determined based on the results of ER test. For example, if a patient in artificial cycle was tested as being in the prereceptive phase (sampling in P + 5) and the results indicated that the transfer should be postponed until P + 6, then the transfer would be performed in P + 6 during the following cycle. The determination of embryo transfer time based on ER test was detailed in previous report [Citation13]. The implantation rate (IR), clinical pregnancy rate (CPR) and live birth rate (LBR) were calculated after implantation.
Statistical analysis
Continuous data were reported as mean ± SD and the statistical analysis was based on unpaired two-tailed t test using GraphPad Prism software (GraphPad software, USA). Categorical data were expressed as counts and percentages and the statistically significance were analyzed using chi-square test (GraphPad software). The differences were considered statistically significant at p < .05.
Results
Characterization of participants
The characteristics of the participants were shown in . There were no significant differences between the patients in natural cycle and those in artificial cycle.
Table 1. Characteristic of the patients.
Receptivity status of patients based on RNA-Seq ER test
The results shown that 44.8% (natural cycle) and 47.8% (artificial cycle) patients were in non-receptive status, which indicated a WOI displacement. Most of them were in prereceptivity status (postponed WOI), with 34.5% in natural cycle and 39.1% in artificial cycle (). This may be due to the local immune dysregulation resulted in repeated implantation [Citation14].
Table 2. Receptivity status of the patients.
Implantation outcomes of patients in artificial cycle and natural cycle
As shown in , the IR of patients in artificial cycle was higher than those in natural cycle (52.2% vs 27.6%), and the difference of patients in prereceptive status was significant. While the CPR is still high for patients in artificial cycle, the difference reduced to 7.2%. However, the LBR for patients in artificial cycle is lower than that in natural cycle. The LBR was higher for artificial cycle patients in the prereceptive and postreceptive status.
Table 3. Implantation outcomes of patients in artificial cycle and natural cycle.
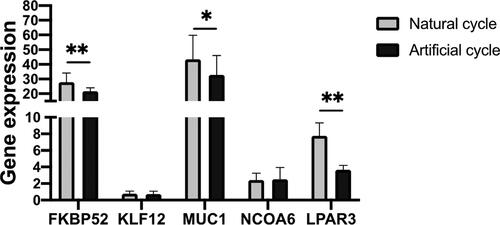
Gene expression of five selected biomarkers of patients in artificial cycle and natural cycle
To understand the relationship between CPR and expression of key biomarkers in WOI, we analyzed the expression level of FKBP52, KLF12, MUC1, NCOA6 and LPAR3 of patients in natural cycle and artificial cycle who detected in receptive status and got clinically pregnant. As shown in , the expressions of FKBP52, MUC1 and LPAR3 were significantly lower in artificial cycle than in natural cycle.
Discussion
Despite the growing understanding of endometrial preparation, there is little consensus on the outcomes of implantation using natural cycle and artificial cycle [Citation15–21]. The current study compared the outcomes of RIF patients in natural cycle and artificial cycle after the personalized embryo implantation.
Before implantation, the ER status were first evaluated and WOI was optimized using a recently reported rsERT since it has been reported that adequate ER is crucial for embryo implantation and approximately 60% of RIF can be attributed to abnormal ER at the point of implantation, which presents as a displacement of the WOI [Citation7,Citation22]. The results showed an approximately 50% of WOI displacement. The outcomes after implantation shown that although there was no significant difference in CPR and LBR, the patients in artificial cycle showed higher IR, and the difference was significant for patients in prereceptive status, which may be due to a more precise control of implantation time, indicating a potentially favorable endometrial preparation method for RIF patients.
To understand the possible relationship between the outcomes and expression of key biomarkers, we further analyzed expression levels of some biomarkers in the patients detected as in receptivity status. The patients in another two statuses were excluded because the replacements of WOI, which means the gene expression levels did not reflect the levels in the optimal WOI. Pregnancy is a complicated process involving embryo implantation, endometrial decidualization, placentation, and fetal delivery [Citation23]. Thus, genes involved in these processed were analyzed including FKBP52, a chaperone of the progesterone receptor considered to be plays a crucial role in this chain of events [Citation24–26], the steroid receptor coactivator family NCOA6 [Citation27], KLF12, reported to be associated with endometrial decidualization [Citation28–30], MUC1, involves in the establishment of receptive [Citation31], and lysophosphatidic acid receptor 3, LPAR3. We found that the expression levels of FKBP52 were lower in patients in artificial cycle than those in natural cycle. Of the note, the patients who got pregnant had lower FKBP52 level than those failed to get pregnant in artificial cycle. Although abnormal down regulation of MUC1 was reported to be associated with poor outcomes [Citation32,Citation33], we found that the expression levels of MUC1 were significantly lower in patients in artificial cycle than in natural cycle and lower in the patients who got pregnant than those failed to get pregnant in artificial cycle. The LPAR3 expression was also lower in patients in artificial cycle than in natural cycle among the patients detected as in receptivity status, which indicated that relatively lower expression of FKBP52, MUC1 and LPAR3 in receptivity status may be related to better implantation outcomes.
Study limitations
In this study, we compared the implantation outcomes of RIF patients in artificial cycle to those in natural cycle after the treatment of rsERT based personalized embryo implantation. Although the IR of patients in artificial cycle was higher than those in natural cycle, the successful implantation can still lead to low LBR. Many factors can influence live birth, including ER status, embryo endocrine, immune, and genetic factors. Our findings may be applicable to providing a potential way to increase the IR, which is a great challenge for RIF patients. However, to coordinate with other factors to achieve a successful live birth may require further investigation.
Conclusion
In conclusion, use of artificial cycle for personalized embryo transfer based on rsERT may yield better pregnancy outcomes for RIF patients. A precise gene expression analysis of FKBP52, MUC1 and LPAR3 for patients detected as in receptivity status based on rsERT provided a potential way to increase the implantation outcomes for RIF patients.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: a review of literature. J Hum Reprod Sci. 2015;8(4):1–4.
- Firouzabadi RD, Davar R, Hojjat F, et al. Effect of sildenafil citrate on endometrial preparation and outcome of frozen-thawed embryo transfer cycles: a randomized clinical trial. Iran J Reprod Med. 2013;11(2):151.
- Craciunas L, Gallos I, Chu J, et al. Conventional and modern markers of endometrial receptivity: a systematic review and meta-analysis. Hum Reprod Update. 2019;25(2):202–223.
- Prapas Y, Prapas N, Jones EE, et al. The window for embryo transfer in oocyte donation cycles depends on the duration of progesterone therapy. Hum Reprod. 1998;13(3):720–723.
- Galliano D, Bellver J, Díaz-García C, et al. ART and uterine pathology: how relevant is the maternal side for implantation? Hum Reprod Update. 2015;21(1):13–38.
- Teh W-T, McBain J, Rogers P. What is the contribution of embryo-endometrial asynchrony to implantation failure? J Assist Reprod Genet. 2016;33(11):1419–1430.
- Sebastian-Leon P, Garrido N, Remohí J, et al. Asynchronous and pathological windows of implantation: two causes of recurrent implantation failure. Hum Reprod. 2018;33(4):626–635.
- Zhu L, Xiao L, Che HS, et al. Uterine peristalsis exerts control over fluid migration after mock embryo transfer. Hum Reprod. 2014;29(2):279–285.
- Zhu L, Che HS, Xiao L, et al. Uterine peristalsis before embryo transfer affects the chance of clinical pregnancy in fresh and frozen-thawed embryo transfer cycles. Hum Reprod. 2014;29(6):1238–1243.
- Díaz-Gimeno P, Ruiz-Alonso M, Blesa D, et al. The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity. Fertil Steril. 2013;99(2):508–517.
- Ruiz-Alonso M, Blesa D, Díaz-Gimeno P, et al. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 2013;100(3):818–824.
- Simón C, Gómez C, Cabanillas S, et al.; ERA-RCT Study Consortium Group. A 5-year multicentre randomized controlled trial comparing personalized, frozen and fresh blastocyst transfer in IVF. Reprod Biomed Online. 2020;41(3):402–415.
- He A, Zou Y, Wan C, et al. The role of transcriptomic biomarkers of endometrial receptivity in personalized embryo transfer for patients with repeated implantation failure. J Transl Med. 2021;19(1):1–13.
- Lédée N, Petitbarat M, Prat-Ellenberg L, et al. Endometrial immune profiling: a method to design personalized care in assisted reproductive medicine. Front Immunol. 2020;11:1032.
- Altmäe S, Tamm-Rosenstein K, Esteban FJ, et al. Endometrial transcriptome analysis indicates superiority of natural over artificial cycles in recurrent implantation failure patients undergoing frozen embryo transfer. Reprod Biomed Online. 2016;32(6):597–613.
- Chang EM, Han JE, Kim YS, et al. Use of the natural cycle and vitrification thawed blastocyst transfer results in better in-vitro fertilization outcomes. J Assist Reprod Genet. 2011;28(4):369–374.
- Kassab A, Sabatini L, Tozer A, et al. The correlation between basal serum follicle-stimulating hormone levels before embryo cryopreservation and the clinical outcome of frozen embryo transfers. Fertil Steril. 2009;92(4):1269–1275.
- Agha-Hosseini M, Hashemi L, Aleyasin A, et al. Natural cycle versus artificial cycle in frozen-thawed embryo transfer: a randomized prospective trial. Turk J Obstet Gynecol. 2018;15(1):12–17.
- Groenewoud ER, Cantineau AEP, Kollen BJ, et al. What is the optimal means of preparing the endometrium in frozen–thawed embryo transfer cycles? A systematic review and meta-analysis. Hum Reprod Update. 2013;19(5):458–470.
- Hancke K, More S, Kreienberg R, et al. Patients undergoing frozen-thawed embryo transfer have similar live birth rates in spontaneous and artificial cycles. J Assist Reprod Genet. 2012;29(5):403–407.
- Mensing L, Dahlberg ES, Bay B, et al. Endometrial preparation methods prior to frozen embryo transfer: a retrospective cohort study comparing true natural cycle, modified natural cycle and artificial cycle. Arch Gynecol Obstet. 2022;306(4):1–8.
- Messaoudi S, Kasmi IEL, Bourdiec A, et al. 15 Years of transcriptomic analysis on endometrial receptivity: what have we learnt? Fertil Res Pract. 2019;5(1):9.
- Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18(12):1754–1767.
- Tranguch S, Cheung-Flynn J, Daikoku T, et al. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci U S A. 2005;102(40):14326–14331.
- Yang Z, Wolf IM, Chen H, et al. FK506-binding protein 52 is essential to uterine reproductive physiology controlled by the progesterone receptor a isoform. Mol Endocrinol. 2006;20(11):2682–2694.
- Zhang S, Lin H, Kong S, et al. Physiological and molecular determinants of embryo implantation. Mol Aspects Med. 2013;34(5):939–980.
- Kawagoe J, Li Q, Mussi P, et al. Nuclear receptor coactivator-6 attenuates uterine estrogen sensitivity to permit embryo implantation. Dev Cell. 2012;23(4):858–865.
- Yan Q, Yan G, Zhang C, et al. miR-21 reverses impaired decidualization through modulation of KLF12 and NR4A1 expression in human endometrial stromal cells. Biol Reprod. 2019;100(5):1395–1405.
- Huang C, Sun H, Wang Z, et al. Increased Krüppel-like factor 12 impairs embryo attachment via downregulation of leukemia inhibitory factor in women with recurrent implantation failure. Cell Death Discov. 2018;4(1):1–13.
- Mei F, Kong C, Wang Y, et al. MiR-133b improves decidualization of endometrial stromal cells by targeting KLF12 in recurrent implantation failure. 2021. https://doi.org/10.21203/rs.3.rs-771502/v1
- Surveyor GA, Gendler SJ, Pemberton L, et al. Expression and steroid hormonal control of Muc-1 in the mouse uterus. Endocrinology. 1995;136(8):3639–3647.
- Xu B, Sun X, Li L, et al. Pinopodes, leukemia inhibitory factor, integrin-β3, and mucin-1 expression in the peri-implantation endometrium of women with unexplained recurrent pregnancy loss. Fertil Steril. 2012;98(2):389–395.
- Shen F, Yan C, Liu M, et al. Decreased expression of mucin-1 in endometriosis endometrium correlated with progesterone receptor B involved in infertility. Arch Gynecol Obstet. 2015;291(2):439–445.