Abstract
Aim
Among the natural polyphenolic compounds, resveratrol (RES) is known for reducing the effects of declining reproductive power through resisting senility, anti-oxidant and anti-inflammatory, while the molecular mechanism of RES in human ovaries is unclear. We aimed to evaluate the most likely mechanisms of RES against apoptosis induced by H2O2 in human ovary granulosa cells.
Methods
Ovarian granulosa cells from infertile women (≤35 years old) were collected. Those patients defined as polycystic ovary syndrome (PCOS), poor ovarian responder (POR) and Endometriosis were excluded. Then they were randomly divided into control group, model group and the treatment group. Cellular apoptosis was analyzed by flow cytometer method. The related protein and mRNA expressions were detected by western blot and RT-PCR.
Results
Apoptosis rates of the treatment group containing RES with concentrations of 1 μM and 10 μM were significantly decreased (p < 0.001). Western blot results demonstrated that the proteins levels of transforming growth factor-β (TGF-β), Bax and Caspase 9 were decreased, and Bcl-2 was increased under RES treatment, while the protein levels of Caspase 8, Caspase 3, growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) expressed no significant difference. The results by RT-PCR of follicle and ovarian development related mRNA factors were consistent with that of western blot assay.
Conclusion
In conclusion, the present study provides the evidence that RES may affects apoptotic factors to protect human ovarian state.
Introduction
As the most important reproductive organ of female, ovary plays a vital role in maintaining reproductive function. Granulosa cells in the follicular microenvironment are in the surrounding area of oocytes and the two-side dialog between them has been widely implicated in the regulation of oocyte growth, maturation and maintenance of microenvironment [Citation1,Citation2]. It has been confirmed that apoptosis of granulosa cells promoted follicular atresia mechanism and reduced fertility potential in mammals [Citation3]. Apoptosis rates were significantly increased by 5% in women with diminished ovarian reserve comparing to women with normal ovarian reserve [Citation4].
Resveratrol, a natural polyphenol phytoalexin, shows anti-aging and prevents ovarian aging effects [Citation5]. The anti-aging mechanisms of RES are associated with its anti-apoptotic and anti-oxidant properties in mammalian reproduction [Citation6]. Oxidative stress was involved in the expression of apoptosis-associated miRNAs that might lead to cellular or molecular damages [Citation7]. In endometriosis granulosa cells, the apoptosis phenotype and endoplasmic reticulum stress of granulosa cells were aggravated [Citation8]. A variety of circulating markers of oxidative stress have been found universally in women with PCOS by 1633 studies [Citation9]. Researches confirmed that RES may have a potential therapeutic effect of improving ovarian function. RES 800 mg/d from the beginning of previous menstruation cycle until the oocyte retrieval day could improve the high-quality oocytes rate and high-quality embryos rate of PCOS patients [Citation10]. However, the effects of apoptosis on human ovaries and response to RES remain unknown.
Apoptosis pathways are mainly divided into death receptor pathway (cell exogenous pathway) and mitochondrial pathway (cell endogenous pathway). In these two pathways, the activation of Caspase-8 and Caspase-9 can induce apoptosis by activating caspase-3 or other caspases [Citation11]. In addition, transforming growth factor β (TGF-β) family plays important roles in many biological processes, including cell growth, differentiation, apoptosis, migration, as well as cancer initiation and progression. Basic studies have found that oxidative stress and apoptosis could be mostly affected via typical Bax/Bcl-2 pathway [Citation12,Citation13]. However, the molecular mechanism of resveratrol affecting on ovarian functional cell apoptosis is still insufficient.
GDF-9 and BMP-15, belonging to TGF-β superfamily, are oocyte-secreted factors and expressed in ovarian oocytes in a developmental manner [Citation14,Citation15]. They are particularly prominent among two-side dialog of the oocyte and its surrounding granulose cells as important regulators of cell proliferation. Exogenous supplement of GDF-9 and BMP-15 in vitro medium for women enduring ovarian dysfunction and infertility can improve the developmental potential of oocytes [Citation16,Citation17]. Could GDF-9 and BMP-15 also be the pathways for resveratrol to affect the reproductive function of oocytes?
Therefore, we hypothesized that resveratrol would serve the potential protective role on human ovarian functions against cells apoptosis. H2O2-induced cells can be used as a model to study molecular mechanisms of RES acting on ovarian microenvironment.
Materials and methods
Cell acquisition and culture
Human granulose cells were collected from infertile patients who underwent IVF treatment, excluding patients with PCOS according to the Rotterdam criteria, POR referring to the Bologna criteria and endometriosis. For all participants, induction protocols included GnRH-a long protocol, GnRH-a antagonist protocol and natural cycle protocol. When 60% follicles diameter ≥18 mm, 5000 ∼ 10000 HCG was injected to induce ovulation. After 36 ∼ 38 h, the oocytes were taken under the guidance of trans-vaginal ultrasound. The cells were added in 5 ml DMEM medium (no.2031106; BI, Inc.) containing serum to terminate digestion, transferred into a 15 ml centrifuge tube and centrifuged at 1000 r/min to prepare single cell suspension. RES powder (cat. no. V900386; sigma, Inc.) was dissolved in 0.1% DMSO in the super clean bench to prepare a 100 mmol/L storage solution. When using, it was diluted 103, 104 and 105 times in the mediums at the concentration of 100 μM, 10 μM and 1 μM. The control group was cultured by DMSO(0.1%) in 5% CO2 incubator at 37 °C for 8 h. The model group was treated with 500 μM H2O2 for 8 h at 37 °C. The treatment group was pretreated with 500 μM H2O2 for 4 h at 37 °C and added by 1 μM, 10 μM and 100 μM RES for another 4 h at 37 °C.
Flow cytometry assay
The granulosa cells were seeded in 24-well plates, 7 × 104 per well, and grouping processing was the same as before. The cells were centrifuged at 1000 rpm for 5 min, and washed twice with PBS at 4 °C. After centrifugation, the supernatant was discarded. 100 μL pretreated 1 × binding buffer was used to resuspend the cells. The cell suspension was transferred to a flow cytometer. 5 μL FITC annexin V and 5 μL PI dyes were added according to the groups. After incubation at room temperature for 15 min, 400 μL FITC annexin V and 5 μL PI dyes were added. Cell apoptosis rate was detected by flow cytometry.
Western blot assay
HGCs (human ovary granulosa cells) suspension with 1 × 105 cells/ml was inoculated into 6-well plates, 2 ml per well. Grouping processing was the same as before with two repeat wells in each group. Cells were lysed using cell lysis buffer RIPA (cat. no. RP-WA0001; Report, Inc.). Total protein concentration was quantified using a BCA protein quantitative Kit (cat. no. RP-WA0201; Report, Inc.). A total of 90 μg protein was used for analysis. Protein electrophoresis was performed using 12% SDS-PAGE and a PVDF membrane that was blocked by 5% nonfat milk at room temperature for 1 h. Western blotting was used to detect the protein expression of cleaved Bcl-2, Bax, Caspase9, Caspase3, TGF-β, GDF-9 and cleaved BMP15 in hGC cells. Primary antibodies included: Anti-cleaved-Bcl-2 (1:1,000; no. AGR55188; Arigo, Inc.), anti-cleaved-Bax (1:1,000;no. ARG66247; Arigo, Inc.), anti-cleaved-Caspase9 (1:1,000; no. ARG54155;Arigo, Inc.), anti-cleaved-Caspase3 (1:1,000; cat. no. ARG66671; Arigo, Inc.), anti-cleaved-TGF-β (1:1,000; cat. no. ARG10002; Arigo, Inc.), anti-cleaved-BMP15 (1:1,000; cat. no. ARG56357; Arigo, Inc.), anti-cleaved-GDF-9 (1:1,000; cat. no. ARG40797; Arigo, Inc.) and anti-β-actin monoclonal (1:10,000; cat. no. AC026; ABclonal Biotech Co., Ltd.). The secondary antibodies used were goat anti-rabbit immunoglobulin G-horseradish peroxidase (1:5,000; cat. no. S1002-100; Report; Seracare). Protein bands were developed using an EZ-ECL kit (cat. no. RP-WA0601; Report;) and analyzed with a Tanon 2500 chemiluminescence imaging system (BIO-RAD Ltd.).
RT-PCR assay
Following cell treatment as described above, total RNA was extracted using TRIzol Reagent (cat. no. 15596026; Life Technologies) according to the manufacturer’s instructions. Total RNA (200 ng) was reverse-transcribed with ReverTra Ace qPCR RT Kit (Toyobo, Japan) according to the instructions. RT-qPCR was performed on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad). The relative quantification of the mRNA levels was performed using the comparative cycle threshold (Ct) method with the formula 2ΔΔCt, and the primer sequences are shown in .
Table 1. Primer sequence and amplification product size.
Statistical analysis
SPSS 22.0 statistical software was used for data processing. The measurement data was expressed in mean numbers, and the statistical analysis was conducted by T-test, with p < 0.05 was defined as the statistical significance.
Results
Effect of RES on apoptosis rate of hGCs under oxidative stress
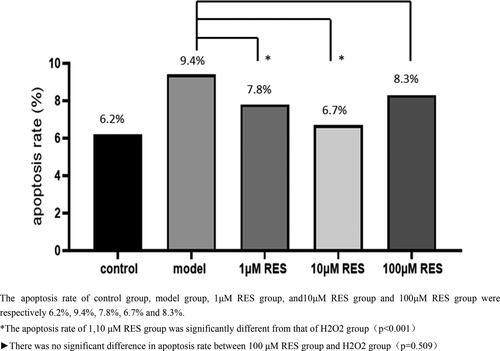
After the human ovarian granulosa cells were treated with RES at concentrations of 1, 10 and 100 μM for 4 h, the flow cytometry results were as shown in . In view of the relatively clear evidence that H2O2 causes oxidative stress response of cells [Citation18], the proportion of apoptotic cells was used to express the degree of oxidative damage of granulosa cells in this article. The scattered spots in Q4 + Q2 area represented the sum of early and late apoptotic cells. The average proportions of control group, model group and RES group (1, 10, 100 μM) were 6.2%, 9.4%, 7.8%, 6.7% and 8.3%, respectively, indicating that H2O2 may cause oxidative damage to granulosa cells. Lower concentrations of RES significantly reduced the apoptosis of human ovarian granulosa cells.
Figure 1. The Effect of RES on apoptosis of human ovarian granulosa cells under oxidative stress by flow cytometry.
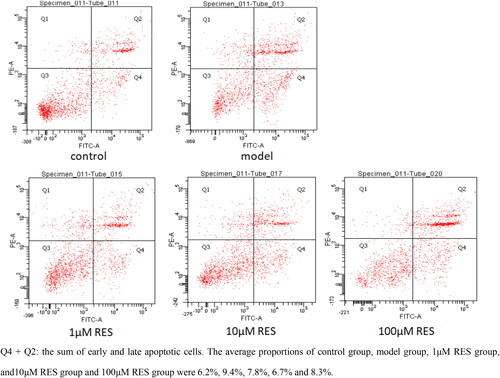
As shown in , flow cytometry was used to detect the apoptosis rate of human ovarian granulosa cells treated with different concentrations of RES for 4 h. It was more intuitive to see that the apoptosis rate of granulosa cells after oxidative stress was the lowest with the effect of 10 μM RES concentration. Moreover, there was no difference between the results of 100 μM RES group and the model group. Therefore, 10 μM can be used as the final concentration to study the mechanism of RES reducing apoptosis of human ovarian granulosa cells.
Expression of apoptosis and proliferation related proteins
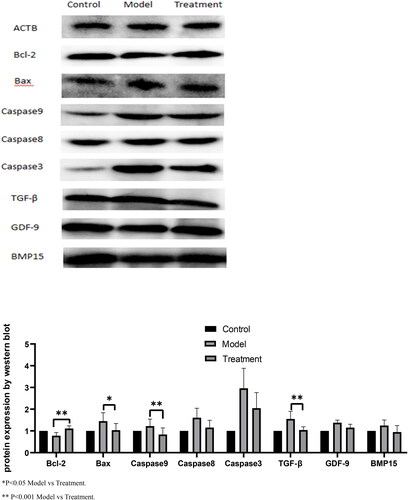
The expression of protein Bcl-2, Bax, caspase9, Caspase3, TGF-β, GDF-9 and BMP-15 were measured by Western blot. The results showed that compared with the model group, the protein levels of Bax, caspase 9 and TGF-β in human ovarian granulosa cells treated with 500 μM H2O2 and 10 μM RES were significantly decreased. Bcl-2 was significantly increased (p < 0.05) in oxidized stressor-treated granulosa cells compared to the controlled level. In addition, no effect was observed on Caspase3, GDF-9 and BMP-15 expression ().
Expression of apoptosis and proliferation related mRNA
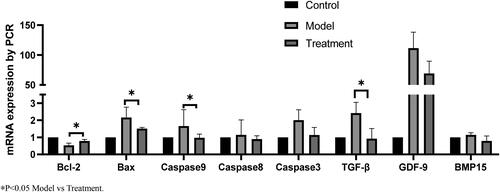
The mRNA expression of TGF-β, Bcl-2, Bax, caspase9, Caspase3, GDF-9 and BMP-15 were measured by RT-PCR. The results showed that compared with the model group, the mRNA levels of Bax, caspase 9 and TGF-β in human ovarian granulosa cells treated with 500 μM H2O2 and 10 μM RES were significantly decreased. Bcl-2 was significantly increased (p < 0.05) in oxidized stressor-treated granulosa cells compared to the controlled level. In addition, no effect was observed on Caspase3, GDF-9 and BMP-15 expression ().
Discussion
Our study showed that the close correlation between RES treatment and decreased apoptosis rate of human granulosa cell under oxidative stress. Lower RES (1 μM, 10 μM) concentrations exhibited significant reduction in apoptosis rate, which was inconsistent with Anna M et al. [Citation19,Citation20] who illustrated that lower concentrations of RES (1–5 μM) elicited a non-significant increase in cell viability. On the other hand, higher RES concentration exhibited no differences in apoptosis rate as compared to H2O2 treated granulosa cells. In further examination, the expression of Bax, caspase 9 and TGF-β in treatment group (10 μM RES) was identified obviously less than model group, which may play important roles in cell oxidative damage and matrix repair to maintain ovarian fertility.
Bcl-2/Bax formed ionic channels in the outer membrane of mitochondria, and regulate outer membrane permeability by controlling their opening. Increasing the permeability, apoptotic inducing factors and apoptotic effectors such as caspase-8, caspase-9, and caspase-3 were activated to initiate apoptosis [Citation21–23]. Caspase-8 and caspase-9 are apoptosis promoters and caspase-3 is a major apoptosis effector. Caspase-8 is the common link of endogenous and exogenous pathways. In the present study, we found significant increases on Bcl-2 after RES treatment, and obvious decreases on Bax, caspase-8 and caspase-9 protein. The downstream of caspase-8 contained regulators of the Bcl-2 family that balance pro-apoptosis and anti-apoptosis [Citation11]. Therefore, Bcl-2/Bax and caspase-9 have a potential to be considered as novel research points for RES to regulate ovarian GCs apoptosis and improve reproductive capacity.
TGF-β has been reported to either induce or suppress programmed cell death in different cell types [Citation24]. TGF-β pathway was considered to be a therapeutic target in early cancer stage, trying to inhibit cell proliferation and to induce cell apoptosis [Citation25]. Bcl-2 protein level decreases upon TGF-β treatment, whereas the bax protein level increases, shifting the intracellular balance between death-promoting and death-inhibiting factors toward death induction [Citation26]. With increasing doses of TGF-β, a substantial number of the cells die by apoptosis, while caspase inhibitors can completely block this apoptotic cell loss [Citation27].
Under certain conditions, TGF-β can also induce cellular senescence, an irreversible form of cell-cycle arrest [Citation28]. Accumulated evidence has indicated a multifaceted association between TGF-β signaling and aging-associated disorders, including muscle atrophy and obesity. The up-regulation of TGF-β ligands contributed to cell degeneration, tissue fibrosis, and metabolic malfunction [Citation28]. In patients with PCOS, up-regulated expression of TGF‑β1 promoted the overgrowth of theca-interstitial cells and apoptosis of granulosa cells, causing excessive accumulation of ECM [Citation29–31]. In patients with endometriosis, a study suggested that the activity of TGF-β1 is increased in endometriotic sites. Does resveratrol play a role in ovaries depending on TGF-β pathway? In fact, our results showed that RES at 10 μM concentration reduced the excessive TGF-β expression of ovarian granulosa cells and prevent excessive apoptosis.
GDF-9 and BMP15, at the laboratory level in mice and bovine embryos, generally promote oocyte maturation under some extracted components (such as curcumin, lycopene) [Citation32,Citation33]. However, in women with normal ovarian function in our study, RES did not significantly affect the expression levels of GDF-9 and BMP15, indicating that the effect of RES may not depend on this way.
Our study established a common oxidative stress model with H2O2 and provided evidence for the effect of RES on cell proliferation. Resveratrol has a biphasic response to change the redox of human cells [Citation20]. Some experts have proved that high RES concentration of 10, 50 μM could promote oxidation, inhibit DNA synthesis and induce apoptosis, and low RES concentration of 1 μM inhibited apoptosis [Citation19]. Our study showed that 1, 10 μM RES significantly inhibited apoptosis. Thus the dose and duration of resveratrol supplementation deserve further clinical trials to avoid its toxic effects.
In summary, our study makes a contribution to identify the availability of RES in people with abnormal ovarian function. It provides a basis for clinical trials to find an appropriate oral dose of RES supplementation on improving ovarian status and the success rate of assisted reproduction.
Availability of data and material
The data that support the findings of this study are available from the corresponding author on reasonable requests.
Ethics approval and consent to participate
All methods were performed in accordance with Declaration of Helsinki. All procedures performed in the study involving human participants were approved by the ethics committee of Fourth Hospital of Shijiazhuang (code 20210033). All the patients signed informed consent forms and agree with treatments on their follicular fluid.
Acknowledgements
The authors would like to thank all participants and The Fourth Hospital of Shijiazhuang who provided spatial and technical support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Su YQ, Sugiura K, Eppig JJ. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med. 2009;27(1):1–6.
- Almeida CP, Ferreira MCF, Silveira CO, et al. Clinical correlation of apoptosis in human granulosa cells: a review. Cell Biol Int. 2018;42(10):1276–1281.
- Matsuda F, Inoue N, Manabe N, et al. Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. J Reprod Dev. 2012;58(1):44–50.
- Fan Y, Chang Y, Wei L, et al. Apoptosis of mural granulosa cells is increased in women with diminished ovarian reserve. J Assist Reprod Genet. 2019;36(6):1225–1235.
- Pyo IS, Yun S, Yoon YE, et al. Mechanisms of aging and the preventive effects of resveratrol on age-related diseases. Molecules. 2020;25(20):4649.
- Pasquariello R, Verdile N, Brevini TAL, et al. The role of resveratrol in mammalian reproduction. Molecules. 2020;25(19):4554.
- Sohel MMH, Akyuz B, Konca Y, et al. Oxidative stress modulates the expression of apoptosis-associated microRNAs in bovine granulosa cells in vitro. Cell Tissue Res. 2019;376(2):295–308.
- Lin X, Dai Y, Tong X, et al. Excessive oxidative stress in cumulus granulosa cells induced cell senescence contributes to endometriosis-associated infertility. Redox Biol. 2020;30:101431.
- Murri M, Luque-Ramirez M, Insenser M, et al. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): a systematic review and meta-analysis. Hum Reprod Update. 2013;19(3):268–288.
- Bahramrezaie M, Amidi F, Aleyasin A, et al. Effects of resveratrol on VEGF & HIF1 genes expression in granulosa cells in the angiogenesis pathway and laboratory parameters of polycystic ovary syndrome: a triple-blind randomized clinical trial. J Assist Reprod Genet. 2019;36(8):1701–1712.
- Roth W, Reed JC. Apoptosis and cancer: when BAX is TRAILing away. Nat Med. 2002;8(3):216–218.
- Kuczera D, Paro de Oliveira HH, Fonseca Guimaraes Fde S, et al. Bax/bcl-2 protein expression ratio and leukocyte function are related to reduction of walker-256 tumor growth after beta-hydroxy-beta-methylbutyrate (HMB) administration in wistar rats. Nutr Cancer. 2012;64(2):286–293.
- Liu S, Zhu Y, Yan S, et al. Phenethyl isothiocyanate induces IPEC-J2 cells cytotoxicity and apoptosis via S-G2/M phase arrest and mitochondria-mediated bax/bcl-2 pathway. Comp Biochem Physiol C Toxicol Pharmacol. 2019;226:108574.
- McPherron AC, Lee SJ. GDF-3 and GDF-9: two new members of the transforming growth factor-beta superfamily containing a novel pattern of cysteines. J Biol Chem. 1993;268(5):3444–3449.
- Dube JL, Wang P, Elvin J, et al. The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol Endocrinol. 1998;12(12):1809–1817.
- Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14(2):159–177.
- Sanfins A, Rodrigues P, Albertini DF. GDF-9 and BMP-15 direct the follicle symphony. J Assist Reprod Genet. 2018;35:1741–1750.
- Storz G, Imlay JA. Oxidative stress. Curr Opin Microbiol. 1999;2:188–194.
- Posadino AM, Giordo R, Cossu A, et al. Flavin oxidase-induced ROS generation modulates PKC biphasic effect of resveratrol on endothelial cell survival. Biomolecules. 2019;9(6):209.
- Posadino AM, Cossu A, Giordo R, et al. Resveratrol alters human endothelial cells redox state and causes mitochondrial-dependent cell death. Food Chem Toxicol. 2015;78:10–16.
- Bernardi P, Broekemeier KM, Pfeiffer DR. Recent progress on regulation of the mitochondrial permeability transition pore; a cyclosporin-sensitive pore in the inner mitochondrial membrane. J Bioenerg Biomembr. 1994;26:509–517.
- Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Ann Rev Immunol. 1998;16:395–419.
- Wu R, Tang S, Wang M, et al. MicroRNA-497 induces apoptosis and suppresses proliferation via the bcl-2/Bax-caspase9-caspase3 pathway and cyclin D2 protein in HUVECs. PLoS One. 2016;11:e0167052.
- Schuster N, Krieglstein K. Mechanisms of TGF-beta-mediated apoptosis. Cell Tissue Res. 2002;307(1):1–14.
- Chen Y, Di C, Zhang X, et al. Transforming growth factor beta signaling pathway: a promising therapeutic target for cancer. J Cell Physiol. 2020;235:1903–1914.
- Motyl T, Grzelkowska K, Zimowska W, et al. Expression of bcl-2 and bax in TGF-beta 1-induced apoptosis of L1210 leukemic cells. Eur J Cell Biol. 1998;75(4):367–374.
- Brown TL, Patil S, Basnett RK, et al. Caspase inhibitor BD-fmk distinguishes transforming growth factor beta-induced apoptosis from growth inhibition. Cell Growth Differ. 1998;9:869–875.
- Tominaga K, Suzuki HI. TGF-beta signaling in cellular senescence and aging-related pathology. Int J Mol Sci. 2019;20(20):5002.
- Shen H, Wang Y. Activation of TGF-beta1/Smad3 signaling pathway inhibits the development of ovarian follicle in polycystic ovary syndrome by promoting apoptosis of granulosa cells. J Cell Physiol. 2019;234:11976–11985.
- Lahav-Baratz S, Kraiem Z, Shiloh H, et al. Decreased expression of tissue inhibitor of matrix metalloproteinases in follicular fluid from women with polycystic ovaries compared with normally ovulating patients undergoing in vitro fertilization. Fertil Steril. 2003;79(3):567–571.
- Ortega I, Wong DH, Villanueva JA, et al. Effects of resveratrol on growth and function of rat ovarian granulosa cells. Fertil Steril. 2012;98:1563–1573.
- Zhang T, Zhou Y, Li L, et al. SIRT1, 2, 3 protect mouse oocytes from postovulatory aging. Aging. 2016;8:685–696.
- Chowdhury MMR, Mesalam A, Khan I, et al. Improved developmental competence in embryos treated with lycopene during in vitro culture system. Mol Reprod Dev. 2018;85:46–61.