Abstract
Objectives
To explore the association of KISS1, LIN28B, vitamin D receptor (VDR), and estrogen receptor α (ERα) gene polymorphisms and the risk of early with fast puberty (EFP) risk, and with hormone levels in EFP cases, in Chinese girls.
Methods
The analysis was based on the data of 141 girls with EFP and 152 girls without EFP. Clinical features were documented, and all SNP genotyping was conducted using SNaPshot method. Statistical analysis was performed to assess the association of the SNPs with EFP risk, and with hormone levels in EFP cases.
Results
There was a significant association between rs7759938-C polymorphism in the LIN28B gene and the risk for EFP in the recessive (TT + CT vs. CC) model (p = 0.040). Remarkably, rs5780218-delA polymorphism in the KISS1 gene and rs2234693-C polymorphism in the ERα gene were significantly associated with peak LH (luteinizing hormone) levels (p = 0.008, 0.045) and peak LH/FSH (follicle-stimulating hormone) ratio (p = 0.007, 0.006). Additionally, on 7 of the 8 variant loci the alleles associated with increased levels of both peak LH levels and peak LH/FSH ratio in EFP cases were also associated with increased CPP risk.
Conclusions
Our findings indicate that rs7759938-C polymorphism in the LIN28B gene might have a protective effect on EFP susceptibility. The most striking findings of this study is that, rs5780218-delA polymorphism in the KISS1 gene and rs2234693-C polymorphism in the ERα gene influenced levels of GnRH-stimulated peak LH and LH/FSH ratio, and in general CPP risk genes might also contributes to the abnormality of hormonal levels in EFP.
Introduction
Puberty, defined as the transition between childhood to adulthood, is characterized by an acceleration of linear growth and development of secondary sexual characteristics [Citation1]. EFP is defined as an onset of puberty beginning at 8–9 years of age and the progression from one pubertal stage to another in <6 months [Citation2]. EFP may lead to shorter final height (FHt) and mental disorders [Citation3]. Thus, there has been increasing attention on EFP, including improving understanding the pathogenesis of EFP.
Reactivation of the hypothalamic-pituitary-gonadal (HPG) axis is considered as the biological determinant of the timing of puberty onset, with HPG reactivation being affected by genetic, environmental, and socioeconomic factors [Citation4]. Of these, genetic factors play an important role, with twin and family studies having shown that genetic factors explain 50–80% of the variation in the timing of puberty onset [Citation5]. Several SNPs in KISS1, LIN28B, VDR, ERα genes, involved in the regulation of the HPG axis, have been shown to be related to the risk of central precocious puberty (CPP) [Citation6–9]. Similar to CPP, which is characterized by onset of puberty before the age of 8 years, early activation of the HPG axis is the main cause of EFP [Citation10]. However, with the exception of the KISS1 gene [Citation10], whether LIN28B, VDR, ERα genes polymorphisms plays a role in EFP among Chinese girls remains unclear. Accordingly, the aim of our study was to explore the relationship between KISS1, LIN28B, ERα, and VDR gene polymorphisms and EFP risk, as well as to assess the association between the selected SNPs and hormone levels, in Chinese girls.
Materials and methods
Statement of ethics
The methods of our study were approved by the ethics committee of Kunming Children’s Hospital. Parents of participants provided informed consent for participant.
Study population
The study population consisted of 152 healthy Chinese Han girls without EFP and 141 Chinese Han girls with EFP, recruited from Kunming Children’s Hospital, China, between 2020 and 2022. According to the literature [Citation2], the inclusion criteria for EFP were as follows: breast development between the age of 8–9 years; progression from one Tanner stage to another in <6 months; and acceleration of linear growth and maturation of bone development. Healthy girls with no secondary sexual characteristics or chronic wasting diseases were selected for the control group.
Participants were physically examined and interviewed by a trained pediatrician to collect clinical information. The following anthropometric data were measured: body mass index (BMI), height, and weight. The following hormone levels were measured by chemiluminescence, using a Diagnostics Roche Cobas e602 analyzer: insulin-like growth factor (IGF-1), testosterone (T), estradiol (E2), LH, and FSH. The GnRH stimulation test was used to assess the pubertal status, with GnRH-stimulation peak LH and FSH levels examined. Bone age (BA) was estimated by the Greulich-Pyle method using left-hand radiographs.
SNP selection and genotyping
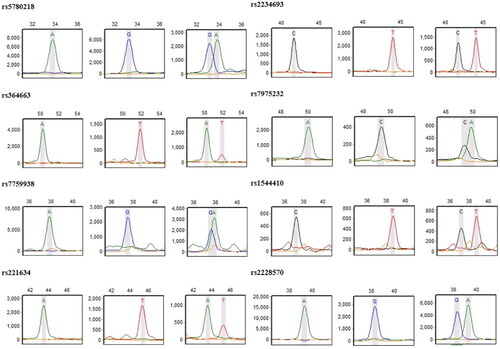
Based on previous studies [Citation6–9], we selected 8 SNPs (rs5780218, rs7759938, rs364663, rs221634, rs2234693, rs7975232, rs1544410, and rs2228570) located in the KISS1, LIN28B, ERα, and VDR genes, which are related to precocious puberty, as candidate loci. Additionally, in the general Chinese population, the minor allele frequency is >0.05.
EDTA anticoagulant whole blood was collected and transferred to a microcentrifuge tube and maintained at −80 °C until used for DNA extraction. DNA was isolated from peripheral blood using a QIAamp DNA Blood Mini Kit (Qiagen, CA, USA), according to the manufacturer’s instructions. Genotyping was conducted using SNaPshot assays. Briefly, DNA samples were amplified by PCR using primers. The PCR amplification conditions were: 95 °C for 4 min, 35 cycles of 94 °C for 20 s, 55 °C for 20 s and 72 °C for 40 s, and a final step at 72 °C for 10 min. After amplification, Shrimp Alkaline Phosphatase (SAP) and Exonuclease I (ExoI) were used to purify PCR products. The purified PCR products were extended with primers designed for single nucleotide extension. The reaction conditions were as follows: 30 cycles of 94 °C for 10 s, 52 °C for 5 s and 60 °C for 30 s. ABI 3730XL sequencing platform (Thermo Fisher Scientific, Inc, USA) was used for sequencing and genotyping samples. The primers sequences for PCR amplification and single nucleotide extension are shown in supplementary (Table S1). Genotyping results were read using GeneMarker software ().
Table 1. Hormonal characteristics in the case group.
Statistical analysis
Data are expressed as the mean ± standard deviation (SD). Tests of normality (Shapiro-Wilk) and equal variance (Levene median) were conducted using SPSS 25.0 software. Genotype frequencies of the SNPs were evaluated for the Hardy–Weinberg equilibrium (HWE) using the chi-squared (χ2) test. Each genotype of selected SNPs was assessed by using logistic regression analysis in additive, dominant, and recessive models. Odd ratios (ORs) were calculated with the associated 95% confidence interval (CI). Linear regression analysis under the additive model was performed to assess the association of the SNPs with hormone levels in EFP cases. All association analysis was adjusted by age and BMI. PLINK (version 1.9) and Haploview4.2 was used to perform genetic analyses. We used Bonferroni correction on P values from SNP-trait association analysis. Additionally, we compared the directions of the effects of SNPs from this study with those obtained from our previous study on CPP [Citation9], and integrated this information into multiple testing correction.
Results
The average age of the EFP group was 9.10 ± 0.46 years compared to an average 8.67 ± 0.74 years in the control group, with a corresponding BMI of 17.45 ± 2.76 and 15.51 ± 1.66, respectively. The mean BA and BA progression (BA-CA) in the EFP group were 10.87 ± 0.65 years and 1.79 ± 0.59 years, respectively. The hormonal features in girls with EPP are presented in . We performed a power analysis of the case-control design of this study using GAS Power Calculator (csg.sph.umich.edu/abecasis/gas_power_calculator/). Under the condition that MAFs of 7 of the 8 SNPs ranging from 0.25 to 0.47, estimated disease prevalence rate of 2%, risk allele effect sizes of ORs 1.3–1.5 based on the effect sizes of the SNPs on CPP from our previous study, under the additive disease model, we expected to achieve a power of 30–60% at 5% significance level.
In the control group, all measured SNPs fit the HWE (p > 0.05). Comparison of the genotype frequencies between the EFP and control group, using additive, dominant and recessive models, are presented in . In the recessive (major allele homozygotes plus heterozygotes vs. minor allele homozygotes) models, the association between rs7759938-C polymorphism in the LIN28B gene and EFP risk was significant. The risk for EFP was lower among girls with a CC genotype of rs7759938 polymorphism (OR, 0.336; 95% CI, 0.118–0.950; p = 0.040). No other gene loci were associated with the risk for EFP.
Table 2. Association between selected SNPs and early and fast puberty risk.
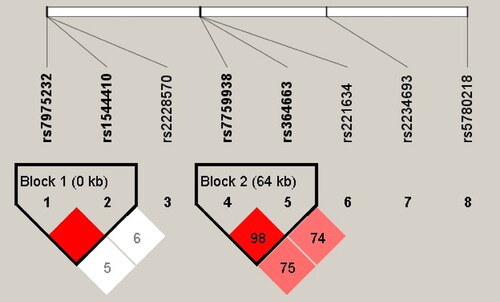
Additionally, a haplotype analysis was conducted for all the genes. Two haplotype blocks were detected (). The frequencies of haplotypes revealed no significant differences between the case and control group (p > 0.05) (Table S2).
Association with hormonal levels were evaluated using linear regression analysis, under the additive model, adjusting for age and BMI. Remarkable findings was in our primary tests on peak LH level and peak LH/FSH ratio, the two indicators used in CPP clinical diagnosis. As shown in , rs5780218-delA polymorphism in the KISS1 gene and rs2234693-C polymorphism in the ERα gene were positively related to peak LH levels and peak LH/FSH ratio with P values of 0.008, 0.045, 0.007, and 0.006, respectively. Three of these 4 P values were less or close to the Bonferroni corrected significant level of 0.0063 (0.05/8). SNP rs1544410-T polymorphism in the VDR gene were negatively associated with peak LH (p = 0.047) and peak FSH (p = 0.027). Additionally, the directions of effects of 7 of the 8 SNP on peak LH levels and peak LH/FSH ratio in EFP cases were in consistent with the directions of their effects on CPP risk (sign test p = 0.003), i.e. SNP alleles associated with increased CPP risk were also associated with increased LH levels and peak LH/FSH ratio in EFP cases. No SNP showed significant association with other hormonal traits, Basal LH, Basal FSH, E2, T and IGF-1.
Table 3. Association of selected SNPs with peak LH, peak FSH, and LH/FSH in case groups and their association with CPP in previous study.
Discussion
Regarding the association of polymorphisms in genes KISS1, LIN28B, ERα, and VDR and the risk for EFP in Chinese girls, we identified a potential association between rs7759938-C polymorphism in the LIN28B gene and the risk for EFP in a recessive model. The most important findings in this study was that SNPs in KISS1 and ERα were associated with peak LH level and peak LH/FSH ratio, and the direction of effects of the SNPs on peak LH level and peak LH/FSH ratio in EFP cases were in consistent with the directions of their effects on CPP risk. These results implicated that some of the CPP risk genes might also contributes to the abnormality of hormonal levels in EFP.
LIN28B, a homologue of LIN28, is a known RNA-binding protein that regulates let-7 microRNA biogenesis, which has an effect on female growth and puberty [Citation11]. Genome-wide association studies (GWAS) have identified the association of polymorphisms in LIN28B with age at menarche (AAM). In their study of the GWAS-identified variants involved in AAM, Delahanty et al. reported a strong association between rs7759938-C polymorphisms in the LIN28B gene and a later age of menarche onset among a Chinese female population [Citation12]. Additionally, Hu et al. [Citation6] reported that a rs7759938-C polymorphism in the LIN28B gene was significantly associated with a lower risk of CPP, particularly in the recessive model. Before puberty, LIN28B may inhibit the secretion of GnRH by stimulating the dynorphin (DYN) gene expression in the hypothalamus; the inhibition effect is attenuated at the onset of puberty [Citation13]. Changes in LIN28B expression are associated with disruption in puberty onset [Citation13]. Particularly, LIN28B contains microRNA regulatory elements in its 3′-untranslated region (UTR), with mutations in this region altering gene function and influencing the timing of puberty [Citation14]. Rs7759938 is located at the 3’UTR may increase LIN28B gene expression [Citation15]. Overexpressing of the LIN28B gene in transgenic mice has been associated with a delayed onset of puberty [Citation16]. Taken together, available evidence suggests that rs7759938-C polymorphism in the LIN28B gene might provide a protective effect against EFP, although future are needed to confirm the role of the LIN28B gene in the pathogenesis of EFP.
During pubertal onset, increased secretion of gonadotropin-releasing hormone (GnRH) stimulates the secretion of gonadotropin. such as LH and FSH, which subsequently result in gonadal steroid production, including E2 and T. Interestingly, we found that KISS1, ERα, and VDR gene polymorphisms influenced hormonal levels of LH and FSH in girls with EFP. Our findings further revealed that rs5780218-delA in KISS1 and rs2234693-C in ERα were positively associated with increased GnRH-stimulated peak LH levels in the case groups. Kisspeptin, encoded by KISS1, plays a critical role in regulating the release of GnRH, LH, and FSH [Citation17]. Kisspeptin administration in healthy women increases in LH pulse secretion [Citation18], while deleterious variations in KISS1 have been associated with precocious puberty [Citation19]. Furthermore, rs5780218 polymorphism of the KISS1 gene has been associated with an increased the risk of CPP among girls [Citation9,Citation10]. Rs5780218 is located at the promoter region of the KISSI gene, influencing its transcription [Citation20]. Estrogen is thought to be an important modulator of puberty onset. Estrogen binding to ERα expressed in hypothalamic KISS1 neurons induce the activation of KISS1 neurons [Citation21]. Therefore, the effect of estrogen on KISS1 expression is mainly influenced by ERα [Citation22]. The C allele of rs2234693 in the ERα gene, increasing ERα gene expression, may produce a potential functional binding site for specific transcription factors, which would enhance estrogenic activity [Citation23,Citation24]. Moreover, KISS1 neurons, activated by estrogens in the anteroventral periventricular area (AVPV) are crucial for surge of LH secretion [Citation21]. Accordingly, it is plausible that KISS1 and ERα polymorphism by affecting KISS1 expression increases GnRH-stimulated peak LH levels, inducing earlier onset and progression of puberty.
In conclusion, our results indicated that rs7759938 polymorphism of the LIN28B gene might exhibit a protective effect against EFP. In addition, our study is the first, to our knowledge, to show that rs5780218-delA in KISS1, rs2234693-C in ERα polymorphisms influence GnRH-stimulated peak LH and LH/FSH ratio, and in general CPP risk genes might also contributes to the abnormality of hormonal levels in Chinese girls with EFP. Studies with a greater number of patients should be conducted to further verify this conclusion.
Supplemental Material
Download Zip (236.8 KB)Acknowledgment
We thank all the volunteers for their participation in this study.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bradley SH, Lawrence N, Steele C, et al. Precocious puberty. BMJ. 2020;368:1.
- Fu J, Zhang J, Chen R, et al. Long-term outcomes of treatments for central precocious puberty or early and fast puberty in Chinese girls. J Clin Endocrinol Metab. 2020;105:dgz027.
- Lazar L, Kauli R, Pertzelan A, et al. Gonadotropin-suppressive therapy in girls with early and fast puberty affects the pace of puberty but not total pubertal growth or final height. J Clin Endocrinol Metab. 2002;87(5):2090–6.
- Fanis P, Skordis N, Toumba M, et al. Central precocious puberty caused by novel mutations in the promoter and 5’-UTR region of the imprinted MKRN3 gene. Front Endocrinol. 2019;10:677.
- Leka-Emiri S, Chrousos GP, Kanaka-Gantenbein C. The mystery of puberty initiation: genetics and epigenetics of idiopathic Central precocious puberty (ICPP). J Endocrinol Invest. 2017;40(8):789–802.
- Hu Z, Chen R, Cai C. Association of genetic polymorphisms around the LIN28B gene and idiopathic Central precocious puberty risks among Chinese girls. Pediatr Res. 2016;80(4):521–525.
- Chen YC, Chen LM, Lin HH, et al. Association study of LIN28B in girls with precocious puberty. J Pediatr Endocrinol Metab. 2017;30:663–667.
- Luo Y, Liu Q, Lei X, et al. Association of estrogen receptor gene polymorphisms with human precocious puberty: a systematic review and meta-analysis. Gynecol Endocrinol. 2015;31(7):516–521.
- Li Y, Tao N, Chen M, et al. Gene polymorphisms associated with Central precocious puberty and hormone levels in Chinese girls. Int J Endocrinol. 2022;2022:9450663.
- Li D, Wu Y, Cheng J, et al. Association of polymorphisms in the kisspeptin/GPR54 pathway genes with risk of early puberty in Chinese girls. J Clin Endocrinol Metab. 2020;105:dgz229.
- Corre C, Shinoda G, Zhu H, et al. Sex-specific regulation of weight and puberty by the Lin28/let-7 axis. J Endocrinol. 2016;228(3):179–191.
- Delahanty RJ, Beeghly-Fadiel A, Long JR, et al. Evaluation of GWAS-identified genetic variants for age at menarche among Chinese women. Hum Reprod. 2013;28(4):1135–1143.
- Cao G, Gao Z, Jiang Y, et al. Lin28 gene and mammalian puberty. Mol Reprod Dev. 2020;87(5):525–533.
- Tommiska J, Sorensen K, Aksglaede L, et al. LIN28B, LIN28A, KISS1, and KISS1R in idiopathic Central precocious puberty. BMC Res Notes. 2011;4:363.
- Ponomarenko I, Reshetnikov E, Polonikov A, et al. Candidate genes for age at menarche are associated with uterine leiomyoma. Front Genet. 2020;11:512940.
- Zhu H, Shah S, Shyh-Chang N, et al. Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Nat Genet. 2010;42(7):626–630.
- Moustafa A. Effect of omega-3 or omega-6 dietary supplementation on testicular steroidogenesis, adipokine network, cytokines, and oxidative stress in adult male rats. Oxid Med Cell Longev. 2021;2021:5570331.
- Jayasena CN, Comninos AN, Veldhuis JD, et al. A single injection of kisspeptin-54 temporarily increases luteinizing hormone pulsatility in healthy women. Clin Endocrinol (Oxf). 2013;79(4):558–563.
- Teles MG, Silveira LF, Tusset C, et al. New genetic factors implicated in human GnRH-dependent precocious puberty: the role of kisspeptin system. Mol Cell Endocrinol. 2011;346(1–2):84–90.
- Amorim P, Grande IPP, Batista RL, et al. Association between KISS1 rs5780218 promoter polymorphism and onset of growth hormone secreting pituitary adenoma. Ann Endocrinol (Paris). 2019;80(2):96–100.
- Garcia-Galiano D, Pinilla L, Tena-Sempere M. Sex steroids and the control of the KiSS1 system: developmental roles and major regulatory actions. J Neuroendocrinol. 2012;24(1):22–33.
- Navarro VM, Castellano JM, Fernandez-Fernandez R, et al. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145(10):4565–4574.
- Herrington DM, Howard TD, Hawkins GA, et al. Estrogen-receptor polymorphisms and effects of estrogen replacement on high-density lipoprotein cholesterol in women with coronary disease. N Engl J Med. 2002;346(13):967–974.
- Kumagai H, Miyamoto-Mikami E, Hirata K, et al. ESR1 rs2234693 polymorphism is associated with muscle injury and muscle stiffness. Med Sci Sports Exerc. 2019;51(1):19–26.