Abstract
Objective: To investigate whether hypersensitive C-reactive protein (Hs-CRP), homocysteine, fibrinogen, and omentin-1 could predict gestational diabetes mellitus (GDM) risk. Methods: Case–control study was conducted at Hengshui People’s Hospital. The GDM group included data about 150 patients aged between 22 and 35 years in 24–28 weeks. An equivalent comparative control group without GDM was composed of the same pool of patients. Body mass index (BMI), total cholesterol (TC), triglyceride, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), oral glucose tolerance test (OGTT) 0–2h, hs-CRP, homocysteine, fibrinogen, and omentin-1 levels were studied in the serum samples of research groups. Univariate logistic regression analysis was used to explore the risk factors of GDM. The area under the curve (AUC) was calculated by the receiver operating characteristic curve (ROC) to analyze the predictive values. Results: Hs-CRP, homocysteine, and fibrinogen in GDM group were significantly higher than those in non-GDM group. Omentin-1 were significantly lower than those in non-GDM group. Logistic regression showed that hs-CRP, homocysteine, fibrinogen, and omentin-1 were risk factors for GDM. The AUC of the established GDM risk prediction model was 0.977, and the sensitivity and specificity were 92.10% and 98.70%, respectively; which were greater than that of hs-CRP, homocysteine, fibrinogen, and omentin-1 alone. Conclusions: Hs-CRP, homocysteine, fibrinogen, and omentin-1 in pregnancy have important clinical value for the prediction of GDM. We used these laboratory indications to establish a GDM risk prediction model that allows for early detection and treatment of GDM, lowering the morbidity of maternal and infant complications.
Introduction
Gestational diabetes mellitus (GDM) is a metabolic condition in which women without diabetes develop hyperglycemia during pregnancy. The prevalence of GDM varies by country and area, ranging from 1% to 30% of pregnancies, with the greatest rates in Africa, Asia, and India [Citation1]. GDM is on the rise all throughout the world, according to epidemiological studies [Citation1–3]. Hyperglycemia during pregnancy is linked to a variety of complications, including obesity, cardiovascular disease, pre-eclampsia, and even stillbirth [Citation1]. GDM has a significant impact on maternal and fetal health, hence screening predictive and diagnostic biomarkers for GDM in the early stages of pregnancy is essential [Citation4].
Various biomarkers have been discovered in metabolic studies that may be relevant in early screening for GDM [Citation5]. Recent research suggests that hs-CRP can be utilized to predict GDM [Citation6]. It is known that it is directly engaged in insulin receptor signaling transduction [Citation7].
The clotting function and lipid metabolism of pregnant women change dramatically as the pregnancy progresses. During a full-term pregnancy, the production of coagulation factors VII, VIII, IX, X, XII, and fibrinogen increases rapidly. Physiological hypercoagulability enhances blood coagulation ability, reducing postpartum hemorrhage risk [Citation8]. Fibrinogen is the largest chain protein in plasma. Whether this increase is natural or pathological, few studies have been conducted to determine if it may be utilized as a possible clinical signal to predict the risk of later GDM. Omentin-1 is a protein generated mostly from human adipose tissue and placental tissue that has attracted a lot of research due to its ability to reduce insulin resistance [Citation9]. There has been a link between elevated homocysteine levels during pregnancy and spontaneous abortion, intrauterine growth restriction, placental infarction, and neural tube defects. Homocysteine has been linked to early pregnancy losses and poor pregnancy outcomes, according to some studies [Citation10].
However, the results of these studies were variable, and there is a need for further research in the different settings. This study intended to establish a case–control study to collect general data such as pregnant women’s ages, pre-pregnancy BMI, as well as early pregnancy coagulation function and glycolipid metabolism indicators. Then, the current study was conducted to investigate the correlation between hs-CRP, homocysteine, fibrinogen and Omentin-1 and glycolipid metabolism indicators and to assess the performance of hs-CRP, homocysteine, fibrinogen, and omentin-1 in predicting GDM in the first trimester.
Methods
Objective
This case–control study was conducted at Hengshui People’s Hospital, Hengshui, China, between January 2020 and January 2021. In this study, single-fetal primiparas aged between 22 and 35 were checked in the outpatient department of Obstetrics and Gynecology. Exclusion criteria were obesity, fetal deformity, medications that affect insulin and glucose levels, hypertension or concomitant systemic disease, pre-gestational known diabetes (Types 1–2), as well as family history.
Fasting blood samples were taken throughout the first trimester of pregnancy in the outpatient department in the morning. In the second trimester (24–28 weeks), 300 patients were performed 75 g OGTT. GDM was diagnosed on the basis of the following WHO criteria: fasting ≥92 mg/dL (5.1 mmol/L), at 1st hour ≥180 mg/dL (10.0 mmol/L), and at 2nd hour ≥153 mg/dL (8.5 mmol/L) [Citation11]. Group GDM (n = 150) consisted of non-obese patients with GDM, while the control group (n = 150) consisted of non-obese pregnant women with normal glucose tolerance who were of similar age and gestational age. Body mass index (BMI) was calculated from maternal recall of weight prior to pregnancy and the height measured during the first visit before 8th week of gestation. This study was approved by the Ethics Committee of Halixun International Peace Hospital. Written informed consent was obtained from all participants.
Biochemical assays
The blood samples were centrifuged at 4,000 × g for 5 min after allowing the blood to clot for 30 min at room temperature, while maintained in a vertical position. Serum and plasma samples were stored at −80 °C until analysis. TC, triglyceride, HDL-C, and LDL-C were measured using one automatic biochemical analyzer (Hitachi 912, Roche Diagnostics GmbH, Germany). The omentin-1 concentration was measured by enzyme-linked immunosorbent assay technique (Human Omentin-1 Elisa, BioVendor R&D Products, Czech Republic). Plasma fibrinogen levels were determined by thrombin time assay kit, using automated Blood Coagulation Analyzer. Homocysteine was detected by enzyme cycling method using automatic biochemical analyzer. Hs-CRP level was determind by immunoturbidimetry.
Statistical analyses
Data were analyzed with SPSS 21.0 software (SPSS Inc., Chicago, IL). Data distribution was assessed using Shapiro–Wilk test. Values are given as means (standard deviation [SD]) or medians (25th, 75th percentiles). Continuous variables were compared between two groups were performed with t-tests or Mann–Whitney U test. Correlations between quantitative variables were verified using the Pearson’s correlation coefficient. Univariate logistic regression analysis was used to explore the risk factors of GDM. The diagnostic value of variables levels was further evaluated by constructing a ROC curve using sensitivity (true positive rate) and 1-specificity (false positive rate). Herein, p < 0.05 was considered as a statistically significant difference.
Results
Characteristics of pregnant women between GDM group and non-GDM group
summarized the clinical, demographic, and laboratory characteristics of the participants. The GDM group’s BMI was considerably greater than those of the non-GDM Group. There was no significant difference in the age and gestational week between these two groups. The GDM group’s TC, triglyceride, HDL-C, LDL-C, OGTT0h, OTGG1h, OTGG2h, hs-CRP, homocysteine, and fibrinogen levels were greater than those of the non-GDM Group. While the omentin-1 of the GDM group was much lower than that of the non-GDM group, and the difference was statistically significant (p < 0.05) ().
Table 1. Demographic characteristics of the patients and controls groups.
Associations between homocysteine, hs-CRP, fibrinogen and omentin-1 and metabolic outcomes
Next, we analyzed whether hs-CRP, homocysteine, fibrinogen, and omentin-1 levels were in any relationship to metabolic disturbances within the GDM group. We found that hs-CRP, homocysteine, fibrinogen concentrations increased together with levels of both TC, TG, LDL-C, OTGG0h, OTGG1h, and OTGG2h. Hs-CRP, homocysteine, and fibrinogen concentrations decreased together with the level of HDL-C. Serum omentin-1 levels were negatively correlated with TG, LDL-C, OTGG0h, OTGG1h, and OTGG2h levels ().
Table 2. Correlation between hypersensitive C-reactive protein, homocysteine, fibrinogen, omentin-1 and body mass index, total cholesterol, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, oral glucose tolerance test 0–2h.
Multivariate analysis of factors affecting the incidence of GDM
The results identified that hs-CRP (OR = 7.54, 95% CI: 3.523–16.136), homocysteine (OR = 1.241, 95% CI: 1.123–1.372), fibrinogen (OR = 8.562, 95% CI: 3.769-19.448), and omentin-1 (OR = 0.968, 95% CI: 0.955–0.982) were risk factors for GDM ().
Table 3. Logistic regression analysis of influencing factors in gestational diabetes mellitus.
The diagnostic value of laboratory indicators and the established GDM risk prediction model for GDM
The ROC curves for each indication and combination test were created using the GraphPad Prism program, as illustrated in . Hs-CRP, homocysteine, fibrinogen, and omentin-1 were the single markers with the high diagnostic value. When the threshold was 2.28 mg/L, the AUC of hs-CRP was 0.89, and the sensitivity and specificity were 77.30% and 94.70%, respectively. When the threshold was 15.70 μmol/L, the AUC of homocysteine was 0.78, and the sensitivity and specificity were 62.70% and 94.10%, respectively. When the threshold was 2.80, the AUC of fibrinogen was 0.87, and the sensitivity and specificity were 86.70% and 85.14%, respectively. When the threshold was 244.14 mg/L, the AUC of omentin-1 was 0.836, and the sensitivity and specificity were 99.30% and 96.70%, respectively.
Figure 1. Diagnostic efficacy analysis of pregnancy-associated hypersensitive C-reactive protein, homocysteine, fibrinogen, omentin-1 and combined detection for gestational diabetes mellitus (GDM). The receiver operating characteristic curve analysis demonstrated that the sensitivity, specificity and the area under the curve (AUC) of hs-CRP level in the diagnosis of GDM were 77.30%, 94.70%, and 0.89, respectively. The sensitivity and specificity of homocysteine level in diagnosing GDM were 62.70%, 94.10%, the AUC was 0.78. The sensitivity, specificity, and AUC of fibrinogen in diagnosing GDM were 64.71%, 70.59%, and 0.721, respectively. The sensitivity and specificity of omentin-1 level in diagnosing GDM were 99.30% and 96.70%, the AUC was 0.836. When the threshold was 0.54, the AUC of the combined detection was 0.977. The combined detection’s sensitivity and specificity were 92.10% and 98.70%, respectively.
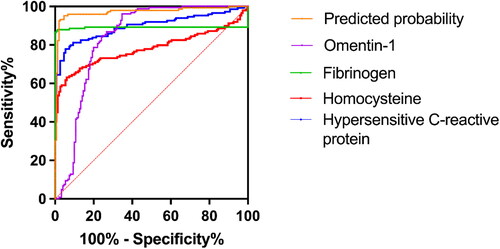
Discrimination and calibration were used to evaluate the model, with discrimination being measured by calculating the area under the ROC curve result for the projected probability. When the threshold was 0.54, the AUC of the combined detection was 0.977. The combined detection’s sensitivity and specificity were 92.10% and 98.70%, respectively. The AUC value of the model was 0.977 (95% CI: 0.959–0.995), indicating that the prediction model had a good degree of discrimination ().
Discussion
GDM is a global problem that takes its toll with each passing year [Citation12]. The fetus’ demand for nutrition rises in the second trimester as the gestational weeks grow, resulting in a reduction in the pregnant woman’s insulin sensitivity and an increase in blood glucose levels, leading to gestational diabetes mellitus and poor pregnancy outcomes. GDM patients have a broken endocrine/antagonistic insulin balancing system, as well as evident aberrant glucose and lipid metabolism and insulin resistance, as compared to normal pregnancy [Citation13].
This research demonstrated that GMD women had significantly higher levels of BMI, TC, TG, LDL-C, OGTT0h, OGTT1h, and OGTT2h in the early stages of pregnancy. HDL-C levels in patients with GDM were lower than in the control group. Diabetes can lead to insulin resistance due to disorders of glucose and lipid metabolism [Citation14]. The two complement each other, promote each other, and accelerate the decline of insulin function. Long-term effects of hyperglycemia and insulin resistance can result in premature birth, premature rupture of membranes, miscarriage, etc., and seriously put mothers and babies at risk. It is possible for insulin resistance to be exacerbated when there are problems with glucose and lipid metabolism. The two work together to complement, enhance, and hasten the loss of insulin activity. Long-term hyperglycemia and insulin resistance can result in preterm delivery, early rupture of membranes, miscarriage, and other complications, putting mothers and newborns in danger.
The exact cause of GDM is not fully understood, but it has been lined to genetics, insulin resistance, metabolic diseases, and infection. The onset of gestational diabetes should be monitored regularly. Serological markers are the current research hotspots, and they are critical for early illness detection, prevention, and therapy.
Omentin-1 is an adipokine expressed mainly in the small and large intestines. It is also found in visceral adipose tissue, the placenta, the heart, the lungs, and the ovaries [Citation15]. It exhibits anti-inflammatory properties, via lowering the C-reactive protein (CRP) and TNF- levels [Citation16]. Its dysfunction disrupts glucose homeostasis and induces the development of insulin resistance, thus, contributing to the development of diabetes. Omentin-1 is hypothesized to improve glucose absorption in adipocytes and insulin sensitivity. Omentin-1 is lower in individuals with obesity and diabetes [Citation17]. Our study showed that patients with GDM had significantly lower omentin-1 levels than the individuals in the control group. Recent research has shown that omentin-1 levels was not only related to blood glucose levels, but also related to lipid metabolism [Citation18]. In obese individuals with T2DM, weight reduction has been shown to boost levels of this adipokines [Citation11].
Hs-CRP is a metabolic disease-associated acute phase response protein. An elevated serum hs-CRP level has been found in patients with diabetes, hyperlipidemia, and cardiovascular diseases [Citation19]. Insulin resistance triggers an oxidative stress response, promoting hs-CRP production, and triggering an inflammatory response, further promoting insulin resistance. Both contribute to the onset and progression of GDM through continuous stimulation [Citation20]. Our study showed that patients with GDM had significantly higher hs-CRP levels than those in the control group.
Fibrinogen is an acute phase response protein that increases significantly in response to tissue injury and inflammation. This increases promotes the production of microthrombosis in the glomerulus because it is a precursor of fibrin. Significantly higher fibrinogen levels in the GDM group were consistent with those in previous reports [Citation21]. Fibrinogen levels were higher in patients with GDM than those in the control group. Because fibrinolysis activity is reduced in pregnant women, the amount of fibrinogen increases, thereby resulting in a hypercoagulable condition and increasing the risk of thrombosis.
Homocysteine, produced in the cell endothelium, can alter the production and transformation of folic acid and vitamin B12, reduce blood vessel wall flexibility, and increase the concentration of active oxides [Citation22]. The dietary requirements of patients with gestational diabetes increase in the second trimester of pregnancy. The body is undernourished and in a state of hyperglycemia, resulting in folic acid loss and an increase in the level of homocysteine level [Citation23]. In a meta-analysis by Gong et al. serum homocysteine concentrations were higher in women with GDM than those in the controls. More consistent evidence was found in women in their second trimester and those older than 30 years. The blood concentrations of homocysteine decrease throughout pregnancy due to the gestational reduction in albumin, and folic acid administration [Citation24]. In the present study, we found significant differences in those results between the GDM and control groups.
In this study, a Pearson correlation analysis showed that hs-CRP, homocysteine, and fibrinogen levels were positively correlated with blood sugar and blood lipids levels in patients with gestational diabetes. Omentin-1 levels were negatively associated with triglyceride, LDL-C, OTGG0h, OTGG1h, and OTGG2h levels. Patients with gestational diabetes have abnormally high blood sugar and lipids levels and disrupted body metabolism. The levels of serum hs-CRP and homocysteine increase simultaneously, favorably associated with blood sugar and lipid levels. The prompt determination of the presence and progression of gestational diabetes allows proactive intervention to avoid the onset of preeclampsia.
This study showed that hs-CRP, homocysteine, fibrinogen, and omentin-1 have predictive values in patients with GDM. The AUCs of hs-CRP, homocysteine, fibrinogen, and omentin-1 in the diagnosis of GDM were 2, 3, 4, and 5, respectively, according to the ROC curve analysis. Both have better diagnostic values in diagnosing GDM. The AUC of the combined diagnostic of these four indicators was 2, 3, 4, and 5, which was higher than the AUC of each individual biomarker. Furthermore, the combined analysis of all four biomarkers showed greater sensitivity, specificity, positive predictive value, and negative predictive value than that of the individual biomarkers. Therefore, the combined determination of the four biomarkers in the serum of pregnant women can increase the reliability of GDM screening.
This study had some limitations. Including the waist-to-hip ratio of the volunteers before pregnancy may be beneficial. Since this was a retrospective clinical study, and the relationship between homocysteine, CRP, fibrinogen and omentin within the control group and lipidemia status or BMI could not be explored. Therefore, there is a need to increase the sample size to increase the sensitivity of the prediction model.
Institutional review board statement
This study was reviewed and approved by the Ethics Committee of Halixun International Peace Hospital.
Informed consent statement
All patients in our study provided informed consent.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- McIntyre HD, Catalano P, Zhang C, et al. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5:1.
- Chiefari E, Arcidiacono B, Foti D, et al. Gestational diabetes mellitus: an updated overview. J Endocrinol Invest. 2017;40(9):899–5.
- Johns EC, Denison FC, Norman JE, et al. Gestational diabetes mellitus: mechanisms, treatment, and complications. Trends Endocrinol Metab. 2018;29(11):743–754.
- Artzi NS, Shilo S, Hadar E, et al. Prediction of gestational diabetes based on nationwide electronic health records. Nat Med. 2020;26(1):71–76.
- Naser W, Adam I, Rayis DA, et al. Serum magnesium and high-sensitivity C-reactive protein as a predictor for gestational diabetes mellitus in Sudanese pregnant women. BMC Pregnancy Childbirth. 2019;19:301.
- Nabouli MR, Lassoued L, Bakri Z, et al. Modification of total magnesium level in pregnant Saudi women developing gestational diabetes mellitus. Diabetes Metab Syndr. 2016;10(4):183–185.
- Gröber U, Schmidt J, Kisters K. Magnesium in prevention and therapy. Nutrients. 2015;7(9):8199–8226.
- James AH, Rhee E, Thames B, et al. Characterization of antithrombin levels in pregnancy. Thromb Res. 2014;134(3):648–651.
- Scheja L, Heeren J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat Rev Endocrinol. 2019;15(9):507–524.
- Radzicka S, Ziolkowska K, Zaborowski MP, et al. Serum homocysteine and vitamin B12 levels in women with gestational diabetes mellitus. Ginekol Pol. 2019;90(7):381–387.
- Mierzyński R, Dłuski D, Nowakowski Ł, et al. Adiponectin and omentin levels as predictive biomarkers of preterm birth in patients with gestational diabetes mellitus. Biomed Res Int. 2018;18:7154216.
- Szmuilowicz ED, Josefson JL, Metzger BE. Gestational diabetes mellitus. Endocrinol Metab Clin North Am. 2019;48(3):479–493.
- Liu PJ, Liu Y, Ma L, et al. The predictive ability of two triglyceride-associated indices for gestational diabetes mellitus and large for gestational age infant among Chinese pregnancies: a preliminary cohort study. Diabetes Metab Syndr Obes. 2020;13:2025–2035.
- Pazhohan A, Rezaee Moradali M, Pazhohan N. Association of first-trimester maternal lipid profiles and triglyceride-glucose index with the risk of gestational diabetes mellitus and large for gestational age newborn. J Matern Fetal Neonatal Med. 2019;32:1167–1175.
- Peña-Cano MI, Valencia-Ortega J, Morales-Ávila E, et al. Omentin-1 and its relationship with inflammatory factors in maternal plasma and visceral adipose tissue of women with gestational diabetes mellitus. J Endocrinol Invest. 2022;45(2):453–462.
- Sun J, Ren J, Zuo C, et al. Circulating apelin, chemerin and omentin levels in patients with gestational diabetes mellitus: a systematic review and meta-analysis. Lipids Health Dis. 2020;19:26.
- Francis EC, Li M, Hinkle SN, et al. Adipokines in early and mid-pregnancy and subsequent risk of gestational diabetes: a longitudinal study in a multiracial cohort. BMJ Open Diabetes Res Care. 2020;8:e001333.
- de Gennaro G, Palla G, Battini L, et al. The role of adipokines in the pathogenesis of gestational diabetes mellitus. Gynecol Endocrinol. 2019;35(9):737–751.
- Zhou Y, Qi C, Li S, et al. Diabetic nephropathy can be treated with calcium dobesilate by alleviating the chronic inflammatory state and improving endothelial cell function. Cell Physiol Biochem. 2018;51:1119–1133.
- Ridker PM. High-sensitivity C-reactive protein and cardiovascular risk: rationale for screening and primary prevention. Am J Cardiol. 2003;92:17K–22K.
- Baboolall U, Zha Y, Gong X, et al. Variations of plasma D-dimer level at various points of normal pregnancy and its trends in complicated pregnancies: a retrospective observational cohort study. Medicine. 2019;98:0000000000015903.
- Deng M, Zhou J, Tang Z, et al. The correlation between plasma total homocysteine level and gestational diabetes mellitus in a Chinese Han population. Sci Rep. 2020;10:18679.
- Lai JS, Pang WW, Cai S, et al. High folate and low vitamin B12 status during pregnancy is associated with gestational diabetes mellitus. Clin Nutr. 2018;37(3):940–947.
- Gong T, Wang J, Yang M, et al. Serum homocysteine level and gestational diabetes mellitus: a meta-analysis. J Diabetes Investig. 2016;7:622–628.
