Abstract
Background: Over the last decade, an emerging role of novel cytokines in the pathogenesis of gestational diabetes mellitus (GDM) has been proposed. The present study was implemented to provide a more accurate estimate of the effect size of the association between leptin, tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) and the risk of GDM.Methods: Online databases were looked up to January 2023 using the search string: (leptin OR TNF-α OR IL-6) AND “gestational diabetes.” Observational studies investigating the association of selected cytokines and GDM risk were included. Odds ratios and their 95% confidence intervals (CIs) were extracted and random-effects models were used to estimate the pooled effect.Results: Twenty-four studies were included in the meta-analysis. A significant association was found between higher circulating leptin and the risk of GDM and the pooled estimate was 1.16 (95%CI: 1.07, 1.27). Higher circulating levels of IL-6 and TNF-α were associated with increased risk of GDM, and the pooled estimates were 1.35 (95%CI: 1.05, 1.73) and 1.28 (95%CI: 1.01, 1.62), respectively.Conclusions: The studied cytokines could be implicated in the GDM pathogenesis and used as potential biomarkers for assessing the GDM risk. Additional longitudinal studies with large sample sizes are needed for a further evaluation of these findings.
Introduction
As the most common complication of pregnancy, gestational diabetes mellitus (GDM), has important maternal and neonatal health consequences [Citation1]. Insulin resistance seems to play a vital role in the pathogenesis of GDM, occurring in the second trimester of pregnancy and progressively increasing to levels similar to those detected in type 2 diabetes mellitus until delivery [Citation2]. The increase in insulin resistance is attributed to increased maternal adiposity and placental hormones [Citation3]. In this regard, several studies have focused on some potential mediators of insulin resistance including adipose tissue-derived cytokines (adipokines) such as leptin, tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) [Citation4]. These inflammatory cytokines can be also produced by the placenta [Citation2].
Recently, there is growing evidence regarding the association between inflammatory cytokines and the risk of GDM. Previous research has revealed that higher leptin concentrations in early gestation increase the risk of GDM being diagnosed later in pregnancy [Citation5]. Increased leptin levels, however, were independently and inversely associated with the risk of GDM in comparison with the controls [Citation6]. Higher levels of TNF-α and IL-6 in women diagnosed with GDM have been associated with both increased and decreased risk of GDM in the case-control studies [Citation7–9]. Concerning conflicting results, in the current study a systematic review and meta-analysis were carried out looking at available literature regarding the association of maternal circulating concentrations of selected cytokines and the risk of GDM. Therefore, this study aimed to provide a more accurate estimate of the effect size and to examine whether these cytokines can be used as the risk factors of GDM.
Materials and methods
The present work complied with the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines [Citation10].
Search strategy
Online databases (PubMed, Scopus, Web of Science, Embase, and ProQuest) were used and carefully searched up to January 2023. A full search strategy was applied and the following search terms were combined and adapted to each database using Boolean operators; (“gestational diabetes” OR “insulin resistance” OR “pregnancy, diabetes” OR “glucose intolerance”) AND (leptin OR “tumor necrosis factor-alpha” OR interleukin-6 OR adipokine OR inflammation). No restriction pertained.
Eligibility criteria
After screening the titles and abstracts, two independent reviewers (EH and ZM) evaluated the potentially eligible studies by reading the full texts. Studies were included if they were: (1) peer-reviewed original articles; (2) observational studies in humans; (3) focused on the association of maternal circulating levels of at least one of the selected cytokines including leptin, TNF-α, and IL-6 with the risk of GDM. Studies were excluded if they were: (1) irrelevant to the original research questions; (2) reviews or letters; (3) did not provide the required data for our analysis; (4) the desired cytokines were not investigated in association with GDM risk; (5) maternal blood was not the object for measuring maternal cytokine levels or the blood sample was collected pre-pregnancy.
Quality assessment
The quality of included studies was checked out by two independent reviewers (EH and ZM) using the Newcastle-Ottawa Scale [Citation11], for different study designs. The scale ranged from “0 to 9” (0–10 for cross-sectional studies). Scoring three domains of the scale including “Selection,” “Comparability,” and “Exposure/Outcome,” a study was considered to be of “good quality,” “fair quality,” or “poor quality” [Citation12]. The quality of evidence was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method [Citation13].
Data extraction
Data were obtained according to below items: publication (first author’s last name, year, and country of publication), study design, number of women with GDM and normal glucose tolerant women, participants’ age, examined cytokine (s) and method of measurement, time of blood sample collection, pre-pregnancy body mass index (BMI), GDM diagnostic time and criteria, effect estimates and 95% confidence intervals (CIs), and confounding variables adjusted in the multivariable model. Disagreements were solved by discussion.
Statistical analysis
Stata version 11 (StataCorp, College Station, TX, USA) was used for meta-analysis. The multivariable-adjusted odds ratios (ORs) were combined to find the association between maternal cytokine (s) levels and the risk of GDM across the studies using random-effects models, unless otherwise stated. We produced forest plots using the logarithm of the OR with its standard error. Statistical heterogeneity between the studies was examined with the Q test and I2 statistics. The I2 > 50% was considered as the indication of heterogeneity [Citation14]. Subgroup analyses were performed to assess the sources of heterogeneity using the following issues: study design (case-control vs. cohort or cross-sectional studies); region (Asia, Europe, United States, Africa, and Australia); GDM diagnostic criteria (The International Association of Diabetes and Pregnancy Study Groups (IADPSG), Carpenter & Coustan, National Diabetes Data Group, and other criteria; ); time of exposure measurement (first vs. the second trimester of pregnancy); and BMI (<25 kg/m2 vs. ≥25 kg/m2). Sensitivity analysis was accomplished by removing one study at a time to determine the extent to which a specific study might have affected the result. We used funnel plots, the Begg’s-adjusted rank correlation test, Egger’s regression test, and trim-and-fill method to assess publication bias [Citation35,Citation36]. p < .05 was used as the cutoff for statistical significance.
Table 1. Characteristics of the included studies.
Results
Study characteristics
Through a comprehensive search of databases, the numbers of 8912 references were identified of which 4480 remained after the elimination of duplicates (). Title and abstract screening were performed and 96 potentially eligible references were fully reviewed. Considering the predefined inclusion criteria, 24 eligible studies were included in the systematic review [Citation5–9,Citation15–33]. The characteristics of the included studies are presented in . In all studies, OR was used as the effect estimate, of which six studies were prospective cohort [Citation15,Citation17,Citation20,Citation22,Citation26,Citation27], eight were case-control [Citation7–9,Citation21,Citation29,Citation31–33], four were nested case-control [Citation5,Citation16,Citation24,Citation25], and six were cross-sectional studies [Citation6,Citation18,Citation19,Citation23,Citation28,Citation30]. Thirteen studies were conducted in Asia [Citation6–9,Citation19,Citation21,Citation23,Citation26,Citation28–32], five in Europe [Citation16,Citation17,Citation22,Citation25,Citation27], four in the United States of America (USA) and Canada [Citation15,Citation18,Citation24,Citation33], one in Africa [Citation5], and one in Australia [Citation20]. The quality assessment and the scores of the individual studies are presented in .
Table 2. Quality assessment of the included studies.
Maternal circulating leptin and the risk of GDM
A total of 12 effect estimates from 11 independent studies were combined to pool the effect of maternal leptin levels on the risk of GDM () [Citation5,Citation15–17,Citation19,Citation21,Citation22,Citation24,Citation25,Citation27,Citation31]. The range of individual ORs varied from 0.95 to 3.45. Six out of these studies reported a significant positive association between maternal leptin levels and the risk of GDM [Citation5,Citation17,Citation19,Citation21,Citation24,Citation31]. Using the random-effects model, the pooled estimate was 1.16 (95%CI: 1.07, 1.27; I2 = 88.4% (95%CI: 81.6, 92.7); P-heterogeneity <.001) as demonstrated in . In the case-control studies and using IADPSG criteria for GDM diagnosis, the results of subgroup analysis indicated that a one-unit increase in leptin level was associated with a higher risk of GDM ().
Figure 2. Forest plot of the association between maternal leptin levels and risk of GDM by study design. OR: odds ratio; CI: confidence interval; GW: gestational week; square, study-specific OR estimate; horizontal line, 95% CI; diamond, pooled OR estimate and its 95% CI.
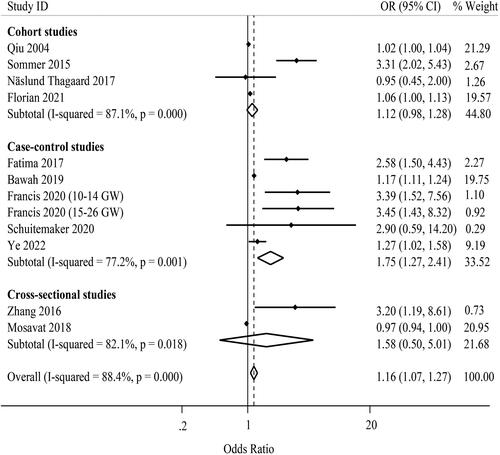
Table 3. Pooled estimates (odds ratios and 95% confidence intervals) of the association between maternal circulating leptin, IL-6, and TNF-α and GDM risk by study design, geographic region, GDM diagnostic criteria, time of exposure measurement, and BMI.
The result of Begg’s test indicated the absence of publication bias (p = .681). However, there was the possibility of publication bias based on the funnel plot asymmetry (Supporting Information Figure S1) and the result of Egger’s test (p = .004). Trim-and-fill analysis attenuated the pooled estimate and it was not significant (OR: 1.01; 95%CI: 1.00, 1.21). Upon performing sensitivity analysis, the pooled estimate calculated for the effect of leptin levels on the risk of GDM was robust (Supporting Information Figures S2 and S3).
Maternal circulating IL-6 and the risk of GDM
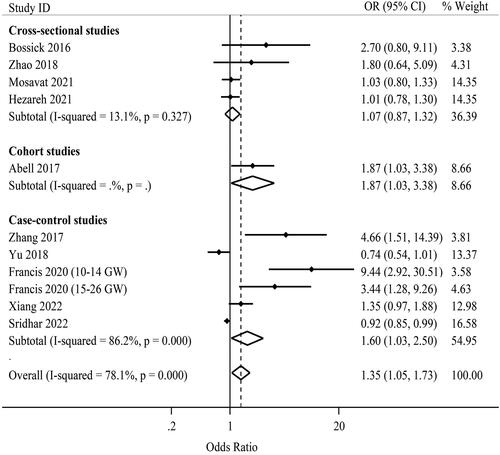
Eleven effect estimates from 10 independent studies were combined to pool the effect of maternal IL-6 levels on the risk of GDM () [Citation8,Citation9,Citation18,Citation20,Citation23,Citation24,Citation28,Citation30,Citation32,Citation33]. The range of individual ORs varied from 0.74 to 9.44. Three of these studies found a significant positive association between maternal IL-6 levels and the risk of GDM [Citation9,Citation20,Citation24]. Using the random-effects model, the pooled estimate was 1.35 (95%CI: 1.05, 1.73; I2 = 78.1% (95%CI: 61.3, 87.7); P-heterogeneity <.001) as presented in . In the case-control studies, using Carpenter and Coustan criteria for GDM diagnosis, and considering BMI ≥ 25 kg/m2, the results of subgroup analysis indicated that increased IL-6 levels were associated with a higher risk of GDM ().
The result of Begg’s test uncovered the presence of publication bias (p = .010). Besides, there was the possibility of publication bias based on the funnel plot asymmetry (Supporting Information Figure S4) and the result of Egger’s test (p = .002). Trim-and-fill analysis attenuated the pooled estimate and it was not significant (OR: 1.08; 95%CI: 0.83, 1.41). Upon performing the sensitivity analysis, the pooled estimate for the effect of IL-6 levels on the risk of GDM was sensitive to Zhang et al. [Citation9] and Francis et al. [Citation24], so that by omitting these studies the result was not significant (Supporting Information Figures S5 and S6).
Maternal circulating TNF-α and the risk of GDM
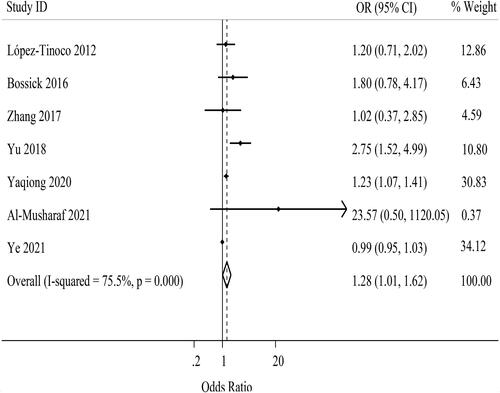
A total of seven effect estimates from seven independent studies were combined to pool the effect of maternal TNF-α levels on the risk of GDM () [Citation7–9,Citation16,Citation18,Citation26,Citation29]. Among the included studies, one was cross-sectional [Citation18], one was cohort [Citation26], and five were case-control [Citation7–9,Citation16,Citation29] studies. The range of individual ORs varied from 0.99 to 23.57. Two of these studies discovered a significant positive association between maternal TNF-α levels and the risk of GDM [Citation7,Citation8]. Using the random-effects model, the pooled estimate was 1.28 (95%CI: 1.01, 1.62; I2 = 75.5% (95%CI: 48.1, 88.4); P-heterogeneity <.001) as illustrated in . Due to the insufficient number of studies examining the association of TNF-α levels with the risk of GDM, subgroup analysis was only performed using BMI as grouping variable and there was no difference between two groups ().
The result of Begg’s test indicated the absence of publication bias (p = .652). Nevertheless, the funnel plot (Supporting Information Figure S7) and the result of Egger’s test (p = .039) showed the possibility of publication bias. Trim-and-fill analysis had no effect on the pooled estimate but it was not significant anymore (OR: 1.27; 95%CI: 1.00, 1.61). Upon performing the sensitivity analysis, the pooled estimate for the effect of TNF-α levels on the risk of GDM was sensitive to 5 studies [Citation7,Citation8,Citation16,Citation18,Citation26], so by omitting these studies the result was not significant (Supporting Information Figures S8 and S9).
The quality of evidence as assessed by the GRADE criteria is presented in . There was very low certainty in the pooled estimate of the GDM risk. The initial rating for observational studies was subsequently downgraded, based upon concerns about each of the five domains: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The evidence rating was downgraded due to the between-study variability (I2> 75%) and the indirectness, due to the low generalizability of each study population to our population of interest. There was also an indication of possible publication bias which further downgraded the rating due to the visual inspection of publication bias in the funnel plot and the results of Begg’s test and Egger’s test and it is most likely to be due to small studies not being published for their negative results.
Table 4. Quality of evidence included in the systematic review and meta-analysis of maternal circulating leptin, IL-6, and TNF-α in association with GDM risk, based on the GRADE approach.
Discussion
In the current systematic review and meta-analysis, it was highlighted that higher maternal levels of leptin, IL-6, and TNF-α are associated with an increased risk of GDM; while, the highest effect was attributed to elevated IL-6 levels.
Pregnancy status was found to elevate serum leptin concentrations [Citation37]. The alterations in leptin have been noticed in early pregnancy (14 weeks of gestation) and continued to increase along with advancing pregnancy [Citation38]. Higher leptin levels were found in women with GDM compared to non-GDM controls [Citation21,Citation39]. Yet, maternal leptin levels did not alter [Citation37] or even in some cases decreased [Citation40] in the women with GDM compared to the controls in other studies. It should be noted that in previous studies, the association between cytokines and the risk of GDM was evaluated based on the mean difference in maternal serum concentrations of cytokines in GDM and non-GDM women, and no actual risk assessment such as “OR” or relative risk was evaluated [Citation39,Citation41]. Considering the growing incidence of GDM and its associated comorbidities, there has been a need to risk assessment models using a combination of clinical and biochemical data [Citation4]. Using the risk assessment models, studies have shown that higher leptin levels in early pregnancy were considered a predictor for GDM, independent of the maternal BMI and other risk factors [Citation5,Citation15]. Increased leptin levels, however, were independently and inversely associated with the risk of GDM in comparison with the controls [Citation6]. In a large cohort study, no association was revealed between higher leptin levels and the risk of GDM [Citation22] while, higher leptin concentrations were associated with a decreased risk of GDM in severely obese pregnant women [Citation22]. Regarding conflicting results of the effect of maternal leptin levels on the risk of GDM and the significance of the risk assessment, we found it necessary to obtain a single summary estimate of the effect. To the best of our knowledge, this is the first study that estimated the pooled effect of the selected cytokines on the GDM risk using odds ratio from individual studies.
In early and mid-pregnancy, higher maternal circulating levels of TNF-α and IL-6 were observed in the women with GDM compared to non-GDM controls and were associated with the increased risk of GDM [Citation24,Citation42]. In a prospective cohort study, higher concentrations of IL-6 measured at 12–15 weeks of gestation significantly increased the risk of GDM [Citation20]. Nevertheless, the risk of GDM was not changed and even decreased along with increasing IL-6 concentrations in the women with GDM compared to control groups of other studies [Citation6,Citation8]. In two case-control studies, a higher concentration of TNF-α was associated with a greater risk of GDM after adjusting for confounding variables [Citation7,Citation8]. In contrast, no association was discerned between elevated TNF-α levels and the risk of late-onset GDM [Citation9,Citation16,Citation18].
In our meta-analysis, a high inconsistency was found among selected studies regarding the association between maternal circulating levels of the selected cytokines and the risk of GDM. Considering different designs and the potential risk of bias in observational studies, we performed meta-regression analyses to explore the potential sources of heterogeneity. The inconsistency remained after performing subgroup analyses and neither the study design nor other factors such as geographical region, GDM diagnostic criteria, and time of exposure measurement were recognized to be a source of heterogeneity. Yet, in cross-sectional studies, using Carpenter and Coustan criteria, and considering BMI ≥ 25 kg/m2, the subgroup analysis indicated a reduction in heterogeneity when examining the association of IL-6 with the GDM risk. This would make these variables including design, GDM diagnostic criteria, and BMI to be discussable as a source of heterogeneity. The high inconsistency that remained within each design implies that several factors might differentiate the studies. To exemplify, in the subgroup analysis of cohort studies examining leptin as exposure, a different assay method was used for leptin by Sommer et al. [Citation17] which was able to eliminate the heterogeneity observed in this subgroup when the study was excluded from the analysis. Additional factors such as small sample sizes, population characteristics, and variables used as confounders could also contribute to high inconsistency in our results. These factors were considered limiting factors in the included studies. The nature of case-control and cross-sectional studies could be regarded as another limitation underlined in the literature as cohort studies provide the most appropriate cause-effect relationship between two variables among observational studies. In our investigation, a single time-point measure of cytokines rather than multiple measurements across pregnancy was performed in some studies [Citation27]. Multiple measurements of the cytokines would be required to elucidate how the occurrence of changes in them during pregnancy can affect the association between these markers and the risk of GDM in a more comprehensive way [Citation27]. Eventually, additional limiting factors argued in the literature included the possibility of remaining confounding variables [Citation24] and the possibility of selection or misclassification bias [Citation4,Citation20].
In the subgroup analysis, we found a significant higher association of IL-6 with the risk of GDM in overweight/obese pregnant women. Increased maternal adiposity enhances the low-degree inflammation present in normal pregnancy and induces the secretion of pro-inflammatory cytokines such as TNF-α and IL-6 [Citation43]. Higher level of these cytokines can interrupt insulin signaling and may serve to reinforce the preexisting low-degree inflammation in pregnancy and cause progression to GDM [Citation43]. Nevertheless, the role of hormones secreted by the placenta should also be considered in the enhanced insulin resistance [Citation4].
Our study has some limitations as well. First, high inconsistency among studies could not be reasonably explained after performing the subgroup analysis. Therefore, the reliability of the results might be negatively influenced. Second, published studies focusing on the association between selected cytokines and the risk of GDM are rare at present. This restricted the power of our analyses, and therefore, the results should be interpreted in the context of limitations in available data. The conclusions should be also drawn with caution taking a series of factors such as actual clinical significance, uncontrolled bias, and study quality into account.
In conclusion, the findings of our meta-analysis represented that higher circulating levels of leptin, IL-6, and TNF-α increase the risk of GDM compared to non-GDM controls. The results showed that the studied cytokines can be implicated in GDM pathogenesis and used as potential biomarkers for evaluating the GDM risk. Yet, the number of studies examining the association between these cytokines and the risk of GDM is limited and the current evidence requires more clarification. In this respect, additional longitudinal studies with large sample sizes are necessary for a further evaluation of these findings.
Authors’ contributions
EH, performed the search and title and abstract screening, reviewed the full-text articles, extracted and analyzed data, and prepared the manuscript. ZM, contributed to performing the search, reviewing the full-text articles and extracting of data. ASA, contributed to performing the search and data analyses. RA and GM, interpreted the results and revised the manuscript. All authors complied with the final version submitted for publication.
Supplemental Material
Download MS Word (38.5 KB)Disclosure statement
The authors have no conflicts of interest.
Additional information
Funding
References
- McIntyre HD, Catalano P, Zhang C, et al. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5(1):1–13.
- Miehle K, Stepan H, Fasshauer M. Leptin, adiponectin and other adipokines in gestational diabetes mellitus and pre-eclampsia. Clin Endocrinol. 2012;76(1):2–11.
- Al-Badri MR, Zantout MS, Azar ST. The role of adipokines in gestational diabetes mellitus. Ther Adv Endocrinol. 2015;6(3):103–108.
- Abell SK, Courten BD, Boyle JA, et al. Inflammatory and other biomarkers: role in pathophysiology and prediction of gestational diabetes mellitus. IJMS. 2015;16(12):13442–13473.
- Bawah AT, Seini MM, Abaka-Yawason A, et al. Leptin, resistin and visfatin as useful predictors of gestational diabetes mellitus. Lipids Health Dis. 2019;18(1):221.
- Mosavat M, Omar SZ, Tan PC, et al. Leptin and soluble leptin receptor in association with gestational diabetes: a prospective case–control study. Arch Gynecol Obstet. 2018;297(3):797–803.
- Yaqiong L, Guohua W, Fuyan Y, et al. Study on the levels of 25(OH)D, inflammation markers and glucose and fat metabolism indexes in pregnant women of han nationality in Jiangsu province with gestational diabetes mellitus. Medicine. 2020;99(35):e21654.
- Yu H, Liu Z, Dong S. Changes in intestinal flora, TNF-α, L-17, and IL-6 levels in patients with gestational diabetes mellitus. Eur J Inflamm. 2018;16:205873921879355.
- Zhang J, Chi H, Xiao H, et al. Interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α) single nucleotide polymorphisms (SNPs). Inflammation and Metabolism in Gestational Diabetes Mellitus in Inner Mongolia. Med Sci Monit. 2017;23:4149–4157.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34.
- Wells GA, Shea B, O’connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Ottawa, Ontario: The Ottawa Health Research Institute.
- Shih T, Lee K, Grogan T, et al. Infliximab in hidradenitis suppurativa: a systematic review and meta-analysis. Dermatol Ther. 2022;35(9):e15691.
- Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394.
- Higgins J. Cochrane handbook for systematic reviews of interventions. Version 5.1. 0. The Cochrane Collaboration [updated 2011 Mar]. Available from: cochrane-handbook.org. 2011.
- Qiu C, Williams MA, Vadachkoria S, et al. Increased maternal plasma leptin in early pregnancy and risk of gestational diabetes mellitus. Obstet Gynecol. 2004;103(3):519–525.
- López-Tinoco C, Roca M, Fernández-Deudero A, et al. Cytokine profile, metabolic syndrome and cardiovascular disease risk in women with late-onset gestational diabetes mellitus. Cytokine. 2012;58(1):14–19.
- Sommer C, Jenum AK, Waage CW, et al. Ethnic differences in BMI, subcutaneous fat, and serum leptin levels during and after pregnancy and risk of gestational diabetes. Eur J Endocrinol. 2015;172(6):649–656.
- Bossick AS, Peters RM, Burmeister C, et al. Antenatal inflammation and gestational diabetes mellitus risk among pregnant African-American women. J Reprod Immunol. 2016;115:1–5.
- Zhang Y, Hao-hang Z, Jia-hui L, et al. Changes in serum adipocyte fatty acid-binding protein in women with gestational diabetes mellitus and normal pregnant women during mid- and late pregnancy. J Diabetes Investig. 2016;7(5):797–804.
- Abell SK, Shorakae S, Harrison CL, et al. The association between dysregulated adipocytokines in early pregnancy and development of gestational diabetes. Diabetes Metab Res Rev. 2017;33(8):e2926.
- Fatima SS, Alam F, Chaudhry B, et al. Elevated levels of chemerin, leptin, and interleukin-18 in gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2017;30(9):1023–1028.
- Näslund Thagaard I, Krebs L, Jens-Christian H, et al. Adiponectin and leptin as first trimester markers for gestational diabetes mellitus: a cohort study. Clin Chem Lab Med. 2017;55(11):1805–1812.
- Zhao XL, Liu JL, Shen LL, et al. Correlation between inflammatory markers (hs-CRP, TNF-alpha, IL-1 beta, IL-6, IL-18), glucose intolerance, and gestational diabetes mellitus in pregnant women. Int J Clin Exp Med. 2018;11(8):8310–8316.
- Francis EC, Li MY, Hinkle SN, et al. Adipokines in early and mid-pregnancy and subsequent risk of gestational diabetes: a longitudinal study in a multiracial cohort. BMJ Open Diabetes Res Care. 2020;8(1):e001333.
- Schuitemaker JHN, Beernink RHJ, Franx A, et al. First trimester secreted Frizzled-Related protein 4 and other adipokine serum concentrations in women developing gestational diabetes mellitus. PLoS One. 2020;15(11):e0242423.
- Al-Musharaf S, Sabico S, Hussain SD, et al. Inflammatory and adipokine status from early to midpregnancy in Arab women and its associations with gestational diabetes mellitus. Dis Markers. 2021;2021:1–8.
- Florian AR, Cruciat G, Pop RM, et al. Predictive role of altered leptin, adiponectin and 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid secretion in gestational diabetes mellitus. Exp Ther Med. 2021;21(5):520.
- Mosavat M, Mirsanjari M, Lwaleed BA, et al. Adipocyte-specific fatty acid-binding protein (AFABP) and chemerin in association with gestational diabetes: a case-control study. J Diabetes Res. 2021;2021:1–7.
- Ye W, Chen L, Yang Y, et al. Formyl peptide receptor-2 is upregulated in the blood and placenta of patients with gestational diabetes mellitus. J Obstet Gynaecol. 2021;47(10):3471–3479.
- Hezareh F, Moghaddam-Banaem L, Shahali S. The relationship between maternal serum interleukin-6 and CRP levels at first trimester of pregnancy and gestational diabetes occurrence. Iran J Obstet Gynecol Infertil. 2021;24(5):59–68.
- Ye Y, Wu P, Wang Y, et al. Adiponectin, leptin, and leptin/adiponectin ratio with risk of gestational diabetes mellitus: a prospective nested case-control study among Chinese women. Diabetes Res Clin Pract. 2022;191:110039.
- Xiang L-L, Chen C, Wang Q-Y, et al. Impact of inflammatory factors, hemoglobin A1c, and platelet parameters in gestational diabetes mellitus. Arch Gynecol Obstet. 2023;307:439–446.
- Sridhar VS, Liu H, Lovblom LE, et al. Associations among biomarkers of inflammation, tubular injury and lipid metabolism with gestational diabetes mellitus status, microalbuminuria and retinopathy in the microalbuminuria and retinopathy in gestational diabetes study. Can J Diabetes. 2023;47:43–50.
- Diabetes Canada Clinical Practice Guidelines Expert Committee. Diabetes Canada 2018 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes. 2018;42:S1–S325.
- Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634.
- Sutton AJ, Duval SJ, Tweedie R, et al. Empirical assessment of effect of publication bias on meta-analyses. BMJ. 2000;320(7249):1574–1577.
- Ebert T, Gebhardt C, Scholz M, et al. Adipocytokines are not associated with gestational diabetes mellitus but with pregnancy status. Cytokine. 2020;131:155088.
- Guelfi KJ, Ong MJ, Li S, et al. Maternal circulating adipokine profile and insulin resistance in women at high risk of developing gestational diabetes mellitus. Metabolism. 2017;75:54–60.
- Xu J, Yan Hong Z, Chen YP, et al. Maternal circulating concentrations of tumor necrosis factor-alpha, leptin, and adiponectin in gestational diabetes mellitus: a systematic review and meta-analysis. Sci World J. 2014;2014:1–12.
- McLachlan KA, O’Neal D, Jenkins A, et al. Do adiponectin, TNFalpha, leptin and CRP relate to insulin resistance in pregnancy? Studies in women with and without gestational diabetes, during and after pregnancy. Diabetes Metab Res Rev. 2006;22(2):131–138.
- MdM R-R, Ramos-García P, López-Tinoco C, et al. Significance of serum-plasma leptin profile during pregnancy in gestational diabetes mellitus: a systematic review and meta-analysis. J Clin Med. 2022;11(9):2433.
- Khosrowbeygi A, Rezvanfar MR, Ahmadvand H. Tumor necrosis factor- α, adiponectin and their ratio in gestational diabetes mellitus. Caspian J Intern Med. 2018;9(1):71–79.
- Brink HS, van der Lely AJ, van der Linden J. The potential role of biomarkers in predicting gestational diabetes. Endocr Connect. 2016;5(5):26–34.