Abstract
Objective
To determine the incidence and severity of ovarian hyperstimulation syndrome (OHSS) in high responders (25–35 follicles with a diameter of ≥12 mm on day of triggering) who received a gonadotropin-releasing hormone (GnRH) agonist to trigger final follicular maturation.
Methods
We used individual data from women who participated in four different clinical trials and were high responders to ovarian stimulation in a GnRH antagonist protocol in this retrospective combined analysis. All women were evaluated for signs and symptoms of OHSS using identical criteria based on Golan’s system (1989).
Results
High responders (n = 77) were of different ethnicities. There were no differences in baseline characteristics between women with or without signs and symptoms of OHSS. Mean ± standard deviation baseline data were: age, 32.3 ± 3.5 years; anti-Müllerian hormone, 42.4 ± 20.7 pmol/L; antral follicle count, 21.5 ± 9.2. Before triggering, duration of stimulation was 9.5 ± 1.6 days and the mean number of follicles with a diameter of ≥12 mm and ≥17 mm was 26.5 ± 4.4 and 8.8 ± 4.7, respectively. Mean serum estradiol (17,159 pmol/l) and progesterone (5.1 nmol/l) levels were high at 36 h after triggering. Overall, 17/77 high responders (22%) developed signs and symptoms of mild OHSS which lasted 6–21 days. The most frequently prescribed medication was cabergoline to prevent worsening of OHSS. No severe OHSS occurred and no OHSS cases were reported as serious adverse events.
Conclusions
High responders receiving GnRH agonist for triggering should be informed that they may experience signs and symptoms of mild OHSS.
Trial registration numbers:
Introduction
Ovarian hyperstimulation syndrome (OHSS), usually a complication of fertility treatment, includes abdominal discomfort and distension, enlarged ovaries, ascites and other, possibly more severe complications of enhanced ovarian vascular permeability [Citation1, Citation2]. The risk of OHSS significantly increases in women with more than 15 oocytes without improving live birth rates in fresh cycles [Citation3] and increases exponentially in women with 18–20 oocytes [Citation4, Citation5].
Due to its half life, human chorionic gonadotropin (hCG) has a sustained luteotrophic effect contributing to OHSS by inducing granulosa-lutein cells to produce vascular endothelial growth factor (VEGF) and increasing ovarian vascular permeability [Citation6]. A single high-dose of hCG applied to induce ovulation or to trigger final follicular maturation may cause early onset OHSS. The higher the ovulatory serum hCG levels, the higher the risk of developing OHSS, especially when ≥25 follicles are observed on the day of triggering [Citation7]. Thus, lowering or withholding the dose of hCG for triggering, may prevent early OHSS [Citation2, Citation8]. However, endogenous hCG produced by the trophoblast in early pregnancy may accelerate early OHSS or induce late-onset OHSS. When the ovarian response is too high, most IVF clinics freeze all embryos to eliminate the risk of late-onset OHSS [Citation9, Citation10].
Triggering of final follicular maturation with a gonadotropin-releasing hormone (GnRH) agonist (GnRH-a) in combination with a freeze-all strategy was first introduced with the GnRH antagonist protocol [Citation11] and is safer for high responders than a long agonist protocol [Citation12]. Triggering with a GnRH-a reduces the OHSS risk by shortening the duration of luteal stimulation, lowering steroid levels by inducing luteolysis [Citation13] and reducing the VEGF secretion [Citation14]. GnRH-a triggering induces an initial flare-up of luteinizing hormone (LH) that lasts for 24–36 h, completing oocyte maturation [Citation15] but reduces the risk of OHSS due to its a rapid luteolysis. GnRH-a triggering is currently the preferred treatment for high responders.
Initially, the combination of GnRH antagonist protocol with GnRH-a triggering, followed by freezing all embryos was thought to completely avoid OHSS [Citation16]. Nonetheless, cases of severe OHSS following GnRH-a triggering have been reported [Citation17]. Other factors, such as endogenous secretion of hCG due to early (ectopic) pregnancy following intercourse [Citation18, Citation19], may contribute to OHSS.
This analysis evaluates the incidence and severity of OHSS in high responders following triggering of final follicular maturation with a GnRH-a and to evaluate OHSS signs and symptoms.
Materials and methods
Trial designs
Women included in this evaluation participated in one of four similarly designed randomized, controlled, assessor-blind, parallel groups, multicenter, non-inferiority trials comparing the efficacy and safety of individualized fixed-dose follitropin delta with either follitropin alfa or follitropin beta in a conventional adjustable dosing regimen [Citation20–23]. Each trial was approved by an Independent Ethics Committee at each center and conducted in accordance with the Declaration of Helsinki, the International Council for Harmonization Guidelines for Good Clinical Practice, and applicable regulatory requirements. All participants provided written, informed consent.
Trial population
High responders with 25–35 follicles with diameter ≥12 mm at day of triggering who received a GnRH-a trigger for final follicular maturation and a ‘freeze all’ approach were included in this analysis. There was no upper limit for serum anti-Müllerian hormone (AMH) level at screening, which was assessed using the Elecsys® AMH Plus immunoassay from Roche Diagnostics (Rotkreuz, Switzerland).
Trial procedures
In each trial, women were randomized to receive either follitropin delta (Rekovelle®, Ferring Pharmaceuticals, Denmark) or follitropin alfa (Gonal-f®, Merck, The Netherlands)/follitropin beta (Follistim®, Merck & Co., Inc, USA/Puregon®, N.V. Organon, The Netherlands), as previously reported [Citation20–23]. As soon as ≥3 follicles (with a diameter ≥17 mm) were observed, final follicular maturation was triggered. Patients with 25–35 follicles with diameter ≥12 mm could receive a GnRH-a (subcutaneous triptorelin 0.2 mg in Spain, United Kingdom, Czech Republic, Russia, Italy, Brazil, Denmark and China, or intranasal buserelin 600 µg/nafarelin 800 µg in Japan). Blood samples were collected throughout the trial for analysis of AMH, follicle-stimulating hormone (FSH), LH, estradiol (E2), inhibin-B, inhibin-A and progesterone at central laboratories. Oocyte retrieval took place 36 h (± 2 h) after triggering of final follicular maturation.
OHSS classification
All cases of OHSS were reported as adverse events of special interest. Each case of OHSS was graded and categorized according to Golan’s classification system () [Citation24, Citation25]. Evaluation was repeated if severity of OHSS worsened. The highest grade or severity at any OHSS evaluation was used in this analysis. Early OHSS was defined as OHSS with onset ≤9 days after triggering, and late OHSS was with onset >9 days after triggering of final follicular maturation.
Table 1. Classification of mild, moderate and severe OHSS according to Golan’s system [Citation24].
Statistical analysis
This is a retrospective analysis of all women who received a single dose of GnRH-a to induce triggering of final follicular maturation in one of the four comparative trials for follitropin delta (i.e. high responders). Two women enrolled in both ESTHER-1 and ESTHER-2 received GnRH-a triggering in both trials and were treated as different subjects here. Data evaluation was based on individual patient data and combined summary statistics of these individual data. Baseline characteristics, stimulation characteristics and outcomes were summarized for all subjects; subjects with OHSS and subjects without OHSS using arithmetic mean and standard deviation (mean ± SD) or percentages. No formal statistical analyses were performed, but the association between early OHSS and E2/follicle at end of stimulation was explored using a logistic regression model with the ratio E2/follicles ≥12 mm as continuous covariate.
Results
Baseline characteristics of high responders receiving GnRH agonist for triggering
A total of 77 women who received an agonist trigger were included in this analysis (triptorelin 0.2 mg: n = 70; buserelin 600 µg: n = 6; nafarelin 800 µ: n = 1). There were no clinically relevant differences in baseline characteristics between women with (n = 17) or without OHSS (n = 60; ). Mean age was 32.3 years; bodyweight, 58.4 kg; BMI, 21.9 kg/m2; baseline AMH, 42.4 pmol/L and mean antral follicle count (AFC), 21.5. The main cause of infertility was a male factor.
Table 2. Baseline characteristics of all women who received a GnRH agonist trigger.
Ovarian stimulation outcomes in high responders triggered with a GnRH agonist
The mean duration of stimulation was 9.5 days with no differences for women with or without OHSS (). With respect to the mean number of follicles of ≥12 mm or ≥17 mm in diameter, there were no differences between the subroups with or without OHSS. The variability in serum hormone concentrations was large. There was a trend for a higher mean E2 production per follicle ≥12 mm for women in the OHSS subgroup. Further analysis did not reveal any association between the number of follicles at the end of stimulation and the probability of OHSS (data not shown), but there was a positive correlation between serum E2 per follicle ≥12 mm and the probability of OHSS, although not statistically significant (). On average, 23 oocytes were collected (range: 2–44 oocytes). The women with OHSS had a mean of one oocyte more than those without OHSS.
Figure 1. Probability of OHSS vs E2 per follicle with diameter ≥ 12 mm at end of ovarian stimulation E2, estradiol, OHSS, ovarian stimulation.
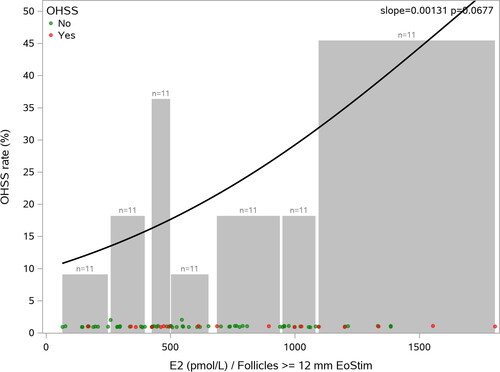
Table 3. Ovarian stimulations in high responders triggered with a GnRH agonist.
Early OHSS following GnRH agonist triggering
A total of 17/77 high responders (22%) developed early OHSS (). The number of follicles ≥12 mm at the end of stimulation ranged from 15 to 30 and serum E2 between 4,200 and >36,000 pmol/L. In 5/17 women (29%), the GnRH-a was given prematurely, thus by the end of stimulation, 25 follicles ≥12 mm had not developed; however, 4 of these 5 women had very high serum E2 (>20,000 pmol/L).
Table 4. Characteristics and symptoms of each woman with early OHSS following GnRH agonist triggering.
No severe OHSS occurred and none of the OHSS events were reported as serious adverse events. In total, 16 (21%) women reported abdominal distension or discomfort and 14 (18%) women had enlarged ovaries. Ten (13%) women developed small volumes of ascites (1.2–16 cm2) observed on ultrasound. According to the Golan’s classification, OHSS cases with ultrasound evidence of ascites should be considered moderate OHSS, but in view of the absence of any other signs and symptoms, most clinicians would consider small volumes of ascites as not clinically relevant. The duration of the OHSS signs and symptoms was between 6 and 21 days, and cabergoline was the most frequently prescribed medication to prevent escalation of signs and symptoms. One patient with serum AMH of 119 pmol/L at screening and 41 oocytes also received human albumin and low molecular weight heparin, and her signs and symptoms were limited to abdominal distension/discomfort and enlarged ovaries.
Discussion
This is the first combined analysis of high responders who had ≥25 follicles with a diameter of ≥12 mm on day of triggering and received a single dose of GnRH-a to trigger final follicular maturation. Nearly all (90%) of the high responders received triptorelin 0.2 mg for triggering of final follicular maturation. Only 22% developed OHSS with mild signs and symptoms, indicating that triggering high responders with a GnRH-a helps avoid the occurrence of severe OHSS. However, none of the high responders in this analysis had an excessive ovarian response of more than 30 follicles of ≥12 mm in diameter.
A few high responders received the GnRH-a for triggering without reaching the predefined criteria of at least 25 follicles ≥12 mm, which may mean the cutoff was too high. Following hCG administration, the risk of OHSS considerably increases for women with 15 or more oocytes [Citation3]; however, there is no consensus on the number or size of follicles, with or without serum E2 levels, that should be reached, before using a GnRH agonist to trigger final follicular maturation. Previous retrospective analyses indicated that 19 or more follicles ≥11 mm on the day of hCG leads to an increased risk for moderate to severe OHSS, whereas serum E2 levels on the day of hCG administration performed less well in predicting OHSS [Citation5]. Interestingly, in our evaluation, patients who did develop signs and symptoms of mild OHSS showed some association between their E2 levels on the day of triggering and the risk of OHSS, whereas the association between number and size of follicles and the risk of OHSS had disappeared.
In clinical practice, different GnRH-a doses and routes of administration are used routinely for triggering high responders, oocyte donors and (social) freezing programs as most treatment approaches have been established empirically [Citation26]. Unfortunately, few dose-range studies of GnRH-a have been published. Two comparative dose studies showed that a single subcutaneous dose of leuprolide 1 and 2 mg [Citation27] or triptorelin 0.2, 0.3 and 0.4 mg [Citation28] are similarly effective in terms of number of mature oocytes and high-quality embryos. In our analysis, none of the high responders had a suboptimal response to GnRH-a triggering as the number of retrieved oocytes correlated with the number of follicles ≥12 mm in diameter prior to triggering. Since the majority of patients received triptorelin 0.2 mg, this agonist and dose may be favored over other GnRH-a and/or doses that resulted in inadequate responses [Citation29]. Moreover, its application is congruent with the ESHRE Ovarian Stimulation Guideline, which states that for high responders triptorelin 0.1–0.4 mg may be used [Citation10].
The mechanism of action and the ability of GnRH-a to induce rapid luteolysis and thus reduce the risk of OHSS have been well-described [Citation30]; however, there are limited studies actually reporting on the incidence of OHSS following triggering in high responders using the same GnRH-a compound, dose and route of administration. The outcome of our analysis is reassuring – when applying a GnRH antagonist protocol, a single dose of triptorelin 0.2 mg can prevent the risk of severe OHSS, at least if up to 30 follicles ≥12 mm have developed on day of triggering. Nonetheless, as not all patients appear to follow the same luteolysis patterns after GnRH-a [Citation31], prospectively monitoring luteal progesterone (and estradiol) levels during the luteal phase and in relation to the onset of OHSS signs and symptoms would improve our understanding of the risk of OHSS after GnRH-a in high responders. This would be a good approach to use in a future mechanism of action study.
It is noted that several women who did develop mild signs and symptoms of OHSS also received dopamine agonist treatment (cabergoline) to prevent escalation of OHSS signs and symptoms, which has previously been reported to be effective [Citation32]; however, whether this treatment affected the present observation is unclear as the preventive effect of dopamine agonists on the development of moderate or severe OHSS remains uncertain [Citation33]. In addition, two women were treated with hexaethyl starch (HES) to prevent or treat OHSS. It should be noted that the application of HES is not advised because there is evidence that HES causes increased mortality in critically ill and septic patients compared with patients receiving crystalloids [Citation34]. One patient with OHSS signs and symptoms was treated with albumin, which has not been proven to have clinical benefit [Citation35].
Finally, the GnRH-a trigger plays an important role beyond OHSS prevention [Citation36], including improved oocyte yield and maturation, possibly because it also induces an FSH midcycle surge [Citation37]. This hypothesis has been confirmed in a prospective controlled trial in which patients received the hCG trigger bolus with or without a bolus of 450 IU FSH [Citation38].
In conclusion, in this combined analysis of individual patient data of 77 high responders who received GnRH-a triggering, only 17 patients (22%) developed mild symptoms of OHSS, which were limited to abdominal distension/discomfort, enlarged ovaries, nausea and/or small volumes of ascites. Patients should still be informed about these adverse events when receiving GnRH-a triggering.
Authors’ contributions
M.F.S. and J.G.V. were investigators in two trials included in this analysis. All authors reviewed the outcomes of the analysis and provided interpretation of the data. B.M. drafted the manuscript and all authors approved the final version.
Acknowledgements
The authors would like to thank Ankur Chakraborty and Marie Göthberg for their contributions to this combined data analysis, Celia Parkyn and Philippe Pinton for their editorial contribution, and all investigators and their staff for their contributions to the previous follitropin delta trials, which made it possible to pool the outcomes of all patients who received GnRH agonist for triggering final follicular maturation.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Disclosure statement
B.M., G.D. and P.H. are Ferring Pharmaceuticals employees. M.F.S. has received institutional clinical trial fees from Ferring Pharmaceuticals. F.H., and J.A.G-V do not have any potential financial or commercial conflicts of interests.
Additional information
Funding
References
- Delvigne A, Rozenberg S. Review of clinical course and treatment of ovarian hyperstimulation syndrome (OHSS). Hum Reprod Update. 2003; Jan-Feb9(1):1–6.
- Pellicer N, Galliano D, Pellicer A. Chapter 22 - Ovarian hyperstimulation syndrome. In: leung PCK, Adashi EY, editors. The ovary. (Third Edition): Cambridge, MA: Academic Press; 2019. p. 345–362.
- Steward RG, Lan L, Shah AA, et al. Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: an analysis of 256,381 in vitro fertilization cycles. Fertil Steril. 2014; Apr101(4):967–973.
- Papanikolaou EG, Pozzobon C, Kolibianakis EM, et al. Incidence and prediction of ovarian hyperstimulation syndrome in women undergoing gonadotropin-releasing hormone antagonist in vitro fertilization cycles. Fertil Steril. 2006; Jan85(1):112–120.
- Griesinger G, Verweij PJ, Gates D, et al. Prediction of ovarian hyperstimulation syndrome in patients treated with corifollitropin alfa or rFSH in a GnRH antagonist protocol. PLoS One. 2016;11(3):e0149615.
- Soares SR, Gomez R, Simon C, et al. Targeting the vascular endothelial growth factor system to prevent ovarian hyperstimulation syndrome. Hum Reprod Update. 2008; Jul-Aug14(4):321–333.
- Shapiro BS, Daneshmand ST, Garner FC, et al. Effects of the ovulatory serum concentration of human chorionic gonadotropin on the incidence of ovarian hyperstimulation syndrome and success rates for in vitro fertilization. Fertil Steril. 2005; Jul84(1):93–98.
- Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Hum Reprod Update. 2002; Nov-Dec8(6):559–577.
- D’Angelo A, Amso N. Embryo freezing for preventing ovarian hyperstimulation syndrome. Cochrane Database Syst Rev. 2007; Jul 18(3):CD002806.
- , Bosch E, Broer S, The ESHRE Guideline Group On Ovarian Stimulation, et al. ESHRE guideline: ovarian stimulation for IVF/ICSI(dagger). Hum Reprod Open. 2020;2020(2):hoaa009.
- Itskovitz-Eldor J, Kol S, Mannaerts B. Use of a single bolus of GnRH agonist triptorelin to trigger ovulation after GnRH antagonist ganirelix treatment in women undergoing ovarian stimulation for assisted reproduction, with special reference to the prevention of ovarian hyperstimulation syndrome: preliminary report: short communication. Hum Reprod. 2000; Sep15(9):1965–1968.
- Youssef MA, Van der Veen F, Al-Inany HG, et al. Gonadotropin-releasing hormone agonist versus HCG for oocyte triggering in antagonist-assisted reproductive technology. Cochrane Database Syst Rev. 2014; Oct 31(10):CD008046.
- Kol S. Luteolysis induced by a gonadotropin-releasing hormone agonist is the key to prevention of ovarian hyperstimulation syndrome. Fertil Steril. 2004; Jan81(1):1–5.
- Miller I, Chuderland D, Ron-El R, et al. GnRH agonist triggering modulates PEDF to VEGF ratio inversely to hCG in granulosa cells. J Clin Endocrinol Metab. 2015; Nov100(11):E1428–36.
- Fauser BC, de Jong D, Olivennes F, et al. Endocrine profiles after triggering of final oocyte maturation with GnRH agonist after cotreatment with the GnRH antagonist ganirelix during ovarian hyperstimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002; Feb87(2):709–715.
- Devroey P, Polyzos NP, Blockeel C. An OHSS-Free clinic by segmentation of IVF treatment. Hum Reprod. 2011; Oct26(10):2593–2597.
- Ling LP, Phoon JW, Lau MS, et al. GnRH agonist trigger and ovarian hyperstimulation syndrome: relook at ‘freeze-all strategy. Reprod Biomed Online. 2014; Sep29(3):392–394.
- Gurbuz AS, Gode F, Ozcimen N, et al. Gonadotrophin-releasing hormone agonist trigger and freeze-all strategy does not prevent severe ovarian hyperstimulation syndrome: a report of three cases. Reprod Biomed Online. 2014; Nov29(5):541–544.
- Orvieto R, Vanni VS. Ovarian hyperstimulation syndrome following GnRH agonist trigger-think ectopic. J Assist Reprod Genet. 2017; Sep34(9):1161–1165.
- Nyboe Andersen A, Nelson SM, Fauser BC, et al. Individualized versus conventional ovarian stimulation for in vitro fertilization: a multicenter, randomized, controlled, assessor-blinded, phase 3 noninferiority trial. Fertil Steril. 2017; Feb107(2):387–396 e4.
- Bosch E, Havelock J, Martin FS, et al. Follitropin Delta in repeated ovarian stimulation for IVF: a controlled, assessor-blind phase 3 safety trial. Reprod Biomed Online. 2019; Feb38(2):195–205.
- Ishihara O, Arce JC, Japanese Follitropin Delta Phase 3 Trial G Individualized follitropin Delta dosing reduces OHSS risk in japanese IVF/ICSI patients: a randomized controlled trial. Reprod Biomed Online. 2021 May;42(5):909–918.
- Qiao J, Zhang Y, Liang X, et al. A randomised controlled trial to clinically validate follitropin Delta in its individualised dosing regimen for ovarian stimulation in asian IVF/ICSI patients. Hum Reprod. 2021; Aug 1836(9):2452–2462.
- Golan A, Ron-el R, Herman A, et al. Ovarian hyperstimulation syndrome: an update review. Obstet Gynecol Surv. 1989; Jun44(6):430–440.
- Fernandez-Sanchez M, Visnova H, Yuzpe A, et al. Individualization of the starting dose of follitropin Delta reduces the overall OHSS risk and/or the need for additional preventive interventions: cumulative data over three stimulation cycles. Reprod Biomed Online. 2019; Apr38(4):528–537.
- Garcia-Velasco JA. Agonist trigger: what is the best approach? Agonist trigger with vitrification of oocytes or embryos. Fertil Steril. 2012; Mar97(3):527–528.
- Pabuccu EG, Pabuccu R, Caglar GS, et al. Different gonadotropin releasing hormone agonist doses for the final oocyte maturation in high-responder patients undergoing in vitro fertilization/intra-cytoplasmic sperm injection. J Hum Reprod Sci. 2015; Jan-Mar8(1):25–29.
- Vuong TN, Ho MT, Ha TD, et al. Gonadotropin-releasing hormone agonist trigger in oocyte donors co-treated with a gonadotropin-releasing hormone antagonist: a dose-finding study. Fertil Steril. 2016; Feb105(2):356–363.
- Popovic-Todorovic B, Santos-Ribeiro S, Drakopoulos P, et al. Predicting suboptimal oocyte yield following GnRH agonist trigger by measuring serum LH at the start of ovarian stimulation. Hum Reprod. 2019; Oct 234(10):2027–2035.
- Fatemi HM, Garcia-Velasco J. Avoiding ovarian hyperstimulation syndrome with the use of gonadotropin-releasing hormone agonist trigger. Fertil Steril. 2015; Apr103(4):870–873.
- Lawrenz B, Ruiz F, Engelmann N, et al. Individual luteolysis post GnRH-agonist-trigger in GnRH-antagonist protocols. Gynecol Endocrinol. 2017; Apr33(4):261–264.
- Busso C, Fernandez-Sanchez M, Garcia-Velasco JA, et al. The non-ergot derived dopamine agonist quinagolide in prevention of early ovarian hyperstimulation syndrome in IVF patients: a randomized, double-blind, placebo-controlled trial. Hum Reprod. 2010; Apr25(4):995–1004.
- Tang H, Mourad SM, Wang A, et al. Dopamine agonists for preventing ovarian hyperstimulation syndrome. Cochrane Database Syst Rev. 2021; Apr 144(4):CD008605.
- Royal College of Obstetricians & Gynecologists. The management of ovarian hyperstimulation syndrome. Green-top Guideline No. 5. 2016.
- Pfeifer S, Butts S, Dumesic D, et al. Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertility and Sterility. 2016;106(7):1634–1647.
- Castillo JC, Haahr T, Martinez-Moya M, et al. Gonadotropin-releasing hormone agonist for ovulation trigger - OHSS prevention and use of modified luteal phase support for fresh embryo transfer. Ups J Med Sci. 2020; May125(2):131–137.
- Kol S, Humaidan P. LH (as HCG) and FSH surges for final oocyte maturation: sometimes it takes two to tango? Reprod Biomed Online. 2010; Nov21(5):590–592.
- Lamb JD, Shen S, McCulloch C, et al. Follicle-stimulating hormone administered at the time of human chorionic gonadotropin trigger improves oocyte developmental competence in in vitro fertilization cycles: a randomized, double-blind, placebo-controlled trial. Fertil Steril. 2011; Apr95(5):1655–1660.
