Abstract
No-daily hormonal contraception includes short-acting reversible contraceptives (SARC), which contain estrogen and progestin (vaginal ring and transdermal patch), and long-acting reversible contraceptives (LARC), which contain only progestin (levonorgestrel-releasing intrauterine device and etonogestrel subdermal implant). No-daily hormonal contraceptives are reversible, avoid oral daily intake and have high contraceptive efficacy. They offer advantages over the traditional oral route, increasing user compliance, and reducing forgetfulness. Furthermore, they have several non-contraceptive benefits. This review aims to highlight the strengths of choices other than the traditional ‘pill’, with the goal of implementing contraceptive counseling, which should be personalized and tailored to each woman. Different subsets of patients may use no-daily contraception at different stages of their lives, with the option of either LARC or SARC. Specific contexts for its use are adolescence, perimenopause, obese women, eating disorders or intestinal malabsorption, breastfeeding, and post voluntary termination of pregnancy. Non-daily contraceptives can be an attractive alternative to the daily contraceptive pill, with benefits that are relevant to each woman desiring contraception, especially in unique and specific settings where customization of the contraceptive method is essential.
Introduction
The use of modern contraceptive methods in women of reproductive age is extremely heterogeneous around the world, ranging from 3.7% in Albania to 81.6% in Finland. Overall, 45.2% of contraceptive users rely on permanent or long-acting methods [i.e. female and male sterilization, intrauterine devices (IUD), subdermal implants], 46.1% on short-acting methods [such as male condoms, oral contraceptive pills (OC), injectables, and other modern methods), and 8.7% on traditional methods (withdrawal, rhythm methods, and others] [Citation1].
Adherence to a specific method of contraception, defined as the proportion of women or cycles with self reported correct use of the assigned device, is the result of all those elements contributing to its selection and is the key factor of effectiveness in real life [Citation2]. Moreover, it correlates strictly to acceptability in terms of side effects and appreciation of possible extra-contraceptive benefits for general and reproductive health, as well as for quality of life.
No-daily hormonal contraception today represents a step forward in favoring adherence and long-acting reversible contraception (LARC), which only contain progestins (levonorgestrel-releasing intrauterine device (LNG-IUD) and etonogestrel (ETN) subdermal implant), seems to be the most reasonable choice for this purpose [Citation3]. However, it contains only progestins and is not suitable for all women that prefer having control of their used methods or do not accept the eventual unpredictable bleeding profile. Moreover, some women prefer or need to assume estrogens in combination [Citation4] and they can achieve this goal by using short-acting reversible contraception (SARC) including weekly patches or monthly vaginal rings [Citation2]. No-daily methods have been on the market for several years, but the value of selecting one method or another in women’s lives has not yet been fully elucidated.
This narrative review brings together the evidence on the most widely available no-daily contraceptive technologies, broadening the horizon of their use in clinical practice. Our scope is to highlight the strengths of choices other than the traditional ‘pill’, with the ultimate goal of implementing contraceptive counseling, which should be personalized and tailored to each individual woman.
Materials and methods
Search strategy and selection criteria
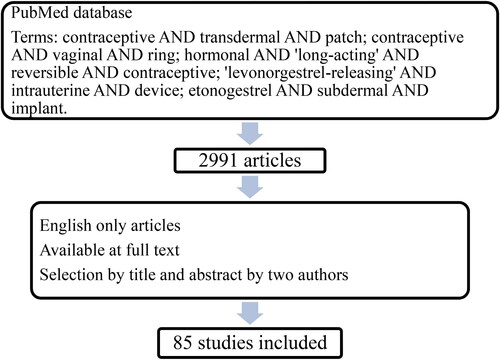
We searched PubMed using the following keywords: contraceptive AND transdermal AND patch; contraceptive AND vaginal AND ring; hormonal AND ‘long-acting’ AND reversible AND contraceptive; ‘levonorgestrel-releasing’ AND intrauterine AND device; etonogestrel AND subdermal AND implant. The search was conducted on November 18, 2022.
Only the publications written in English were considered and a selection was made by title and abstract; original papers selected for inclusion were independently reviewed by two of us (VB and SA) (). When there were conflicts in the selection of the studies, the impact factor of the journal was evaluated, and priority was given to higher quality, more recent, and more prestigious journals.
Discussion
No-daily hormonal contraceptives include methods that do not involve taking a contraceptive preparation daily; nevertheless guarantee a return to fertility when discontinued. This category of contraceptives includes no-daily SARC, containing estrogens and progestin (vaginal ring and transdermal patch), and LARC, which are no-daily by definition and contain only progestins (levonorgestrel-releasing intrauterine system (LNG-IUD) and etonogestrel (ETN) subdermal implant) (Table1). Medroxyprogesterone acetate (MPA) intramuscular or subdermal injections use as a contraceptive is off-label in many countries, while it is used for the palliative therapy of metastatic and inoperable endometrial cancer and advanced stage hormone-dependent breast cancer [Citation5]. Other types of injectables and implants, such as 5 years LNG implants, are less common and registered in a few countries. Therefore, those methods will not be considered in this review.
No-daily hormonal contraceptives have high contraceptive efficacy [Citation6], and they are reversible. They offer advantages over the traditional oral route, avoiding the need for daily pill intake of combined OC (COC), increasing user compliance, and reducing forgetfulness [Citation2]. Furthermore, they have non-contraceptive benefits, such as reducing dysmenorrhea, menstrual bleeding, and premenstrual syndrome [Citation7, Citation8].
No-daily short-acting reversible contraceptives (SARC)
Among SARC, no daily formulations include the vaginal ring and the transdermal patch. They act by inhibiting ovulation and by modulating the composition of cervical mucus [Citation9]. Their contraceptive effectiveness and failure rates are similar to those of COC [Citation6].
These methods allow monthly menstrual flow, a rapid return to fertility after discontinuation, and good acceptability due to their non-invasiveness ( and ).
Table 1. No daily hormonal contraceptives. SARC: short-acting reversible contraceptives; LARC: long-acting reversible contraceptives; LNG-IUD: levonorgestrel-releasing intrauterine system; ETN etonogestrel; EE: ethinylestradiol; LNG: levonorgestrel.
Table 2. Pros and cons of no-daily SARC. SARC: short-acting reversible contraceptives; EE: ethinylestradiol.
Table 3. Pros and cons of LARC hormonal contraceptives.
Bypassing the gastrointestinal tract, the ring and the patch allow avoiding the first-pass effect and, therefore, drug bioavailability is higher. This allows good efficacy even in women with gastrointestinal malabsorption [Citation10]. Increased bioavailability, along with the ability to provide a sustained drug release rate, allows using lower doses. The decreased dosage could improve cycle control while reducing side effects. However, the presence of ethinylestradiol (EE) makes these formulations unsuitable during breastfeeding or in patients with contraindications to estrogens [Citation11].
Vaginal ring
The vaginal route is an ideal method of drug administration, and the advantages of this method are well-established [Citation12]. The ring matrix releases on average daily doses of 15 μg of EE and 120 μg of ETN over 3 weeks [Citation13–15]. According to a Cochrane review, ring users appear more satisfied with their method than COC users and contraceptive effectiveness is not different for the vaginal ring in comparison to COC [Citation2]. It appears that vaginal ring users are less likely to discontinue (overall or due to adverse events) than COC users [Citation16,Citation17].
Fewer adverse effects may be related to steady-state hormone levels, in contrast to the hormonal peaks achieved with COC [Citation2]. Results from a study that compared the pharmacokinetics of EE released from three hormonal contraceptive methods using the vaginal, transdermal, and oral route of administration, show that for vaginal ring users, exposure to EE is on average 3.4 times lower than for those who use the transdermal patch and approximately twice as low as those who use the COCs. Of the three contraceptive methods, exposure to EE is the lowest for the ring group, and subjects using vaginal rings have the least variation in EE serum levels [Citation11].
The rate of breakthrough bleeding and spotting is lower than with COC. Nausea and acne appear less likely among ring users compared to the COC group [Citation2].
According to a randomized study by Stewart et al. [Citation18], vaginal ring users appear less likely than COC users to report increased body weight, headaches, negative mood impact, or sex drive.
Data suggest that biofilm formation on the vaginal ring does not alter the vaginal microbiome or impact mucosal host defense; on the contrary, vaginal ring-releasing hormones may be important for the protection of the vaginal microbiota [Citation19]. As part of a prospective comparative study in asymptomatic women starting contraception, De Seta et al. reported that women who use the vaginal ring show a significant increase in the number of lactobacilli in the vaginal flora compared to both baseline and COC users [Citation20]. This is most likely attributed to the action of the EE on vaginal flora composition [Citation21,Citation22].
EE enhances procoagulant factors, such as factor VIIa and fibrinogen, and decreases the activity of anticoagulation mechanisms. The acquired hypercoagulability seems to be independent of the route, but directly dependent on the dose of EE [Citation23]. Although EE is present in the vaginal ring, the daily doses of EE (15 μg) are lower than in most pills.
In a randomized study by Duijkers et al. [Citation24] vaginal ring users show a lower mean area under the curve for insulin compared to the COC group with LNG. Similarly, in a prospective randomized study of young, healthy, lean women in need of hormonal contraception, vaginal ring use does not impair insulin sensitivity as compared with COC use [Citation25].
However, two other studies [Citation26,Citation27] showed no significant differences in the carbohydrate metabolism measures for the ring users. The evidence regarding the action on insulin sensitivity is therefore not yet conclusive.
Transdermal patch
The transdermal patch is a weekly combined contraceptive method designed to deliver 20 μg of EE and 150 μg of norelgestromin daily. It does not differ significantly in contraceptive effectiveness compared to COC [Citation2].
In the study that compared the pharmacokinetics of EE released from three hormonal contraceptive methods, the patch was found to produce EE serum levels higher than those expected with a COC containing 30 mcg of EE. The transdermal patch maintains a steadier level of serum estrogen than COC, however, the area under the curve is higher for patch users [Citation10]. For this reason, the US Food and Drug Administration (FDA) warns that women using the patch may be exposed to more estrogen on average than women taking a pill with EE 35 μg [Citation28].
Exposure to EE following transdermal patch application has been compared for different sites; absorption is approximately 20% less when the patch is worn on the abdomen compared with the arm, buttock, or torso based on serum concentrations, although mean serum concentrations are still within reference ranges [Citation29].
Breakthrough bleeding and spotting are less common within the patch group than in the COC group, and patch users appear more likely to be very satisfied with their method than COC users [Citation30].
Although patch users show better adherence per cycle than COC users, more patch users discontinue early than COC users [Citation2]. Patch users are more likely to discontinue due to adverse events since they report breast discomfort or pain, nausea, vomiting, and dysmenorrhea more often compared to COC.
A systematic review [Citation31] identified conflicting evidence from 7 observational studies that compared the venous thromboembolic (VTE) risk associated with the use of the transdermal patch to that with the use of COC containing LNG or norgestimate. One retrospective cohort study [Citation32] and one case-control study [Citation33] report significant, 2-fold greater VTE risk among transdermal patch users compared to COC users [Citation34].
Long-acting reversible hormonal contraceptives (LARC)
LARC are contraceptive methods approved for consecutive use for 3 to 5 years. They include the Levonorgestrel-releasing intrauterine system (LNG-IUD), and the ETN subdermal implant.
Many studies have demonstrated that LARC methods are more effective than SARC [Citation3,Citation33] since there is no difference between typical and perfect use [Citation5]. These contraceptive methods do not require any user action after insertion and the user cannnot alter the method’s efficacy [Citation3]. For this reason, LARC are defined as ‘forgettable contraceptives’ [Citation35], being suitable for the categories of patients for which behavior-related variables may affect compliance (disabled people, adolescents, or those who tend to forget) [Citation3,Citation36,Citation37].
These devices also have high continuation rates: in the CHOICE Project continuation rates for participants who chose LARC methods were higher than for those who chose SARC contraceptives [Citation38]. The effectiveness of LARC methods is comparable to that of female sterilization and is independent of age, parity, or body mass index (BMI) [Citation3], but allows return to fertility.
Since hormonal LARC exclusively release progestin substances, they do not increase the risk of VTE and can be used in most of the patients for which estrogens are contraindicated [Citation39].
Multiple factors, including the high upfront cost, are responsible for the low use of LARC [Citation3]. However, durability of the contraceptive method amortizes costs. LARC appears to become cost-neutral within 3 years of initiation when compared with SARC contraception [Citation40]. Moreover, LARCs, having a very good effectiveness, can be considered cost-effective since they avoid pregnancies and all direct and indirect costs associated to them.
Abnormal uterine bleeding is the main cause of the early discontinuation of LARC. Counseling and anticipatory guidance are important and can help prevent early removals [Citation3].
Intrauterine contraception (IUC)
Complications with IUC are uncommon and include expulsion (2–10% during the first year) [Citation5], method failure, and uterine perforation (a rare event, occurring in 1.4 per 1,000 LNG-IUD insertions) [Citation41].
The use of the IUC does not increase the absolute risk of ectopic pregnancy, since IUC effectively prevents pregnancy; however, if pregnancy does occur with an IUC in place, it is more likely to be ectopic [Citation42].
Studies that examined women who were diagnosed with pelvic inflammatory disease (PID) after IUC insertion found mixed results. The study with the largest sample size found a greater incidence of PID in the first 20 days after insertion. IUC usage represents an unacceptable health risk in women with puerperal sepsis, immediate post-septic abortion, uterine fibrosis with distortion of the uterine cavity, and persistently elevated β- Human Chorionic Gonadotropin (hCG) levels or malignant disease [Citation42]. The same is true also if it is initiated in women presenting unexplained vaginal bleeding, cervical cancer awaiting treatment, endometrial cancer, current PID, and current purulent cervicitis or chlamydial infection or gonorrhea [Citation43].
Levonorgestrel releasing intrauterine system (LNG-IUD)
Different LNG-IUD are available containing various doses of LNG. LNG- IUD FDA-approved up to 8 years of consecutive use can contain 52 mg of LNG (releasing 20 mcg per day) or 19.5 mg (13 mcg/day). LNG- IUD containing 13.5 mg of LNG (8 mcg/day) is FDA-approved only for 3 years of consecutive use [Citation41].
The mechanism of action of LNG-IUD is primarily the thickening of cervical mucus, impaired sperm penetration [Citation3], and massive decidual changes in the endometrium [Citation44]. Bleeding patterns are similar when comparing different dosages of LNG-IUD, with a marked reduction in the number of bleeding/spotting days after the initial 3 months of use and continuing to decline thereafter [Citation45]. LNG-IUD is more effective in reducing heavy menstrual bleeding than COC [Citation46]. The LNG-IUD may control abnormal uterine bleeding as well as uterine volume in adenomyosis and fibroids, in these subsets of patients LNG-IUD can determine the decrease of uterine volume [Citation47]. In women with idiopathic heavy menstrual bleeding, the LNG-IUD reduces menstrual blood loss more effectively and has a higher likelihood of treatment success than oral medroxyprogesterone acetate [Citation48].
The 19.5 mg and the 13.5 mg LNG-IUD are considered low-dose LNG-IUD. They have smaller T-frames (28 × 30 mm vs 32 × 32 mm) and smaller hormone reservoirs, allowing them to be placed using a smaller diameter placement tube [Citation49]. In a randomized, open-label, three-arm, phase II study by Gemzell-Danielsson et al. [Citation45] studying the efficacy, bleeding profile, and safety of two low-dose and one high-dose LNG-IUD, it appears that 98.5% of successful placements were achieved at the first attempt. The placement was rated as ‘‘easy’’ for 94% of subjects in the low-dose group compared with 86.2% of subjects in the high-dose group. In a participant-blinded randomized trial on 318 adolescents, the pain level was significantly higher after the levonorgestrel 52-mg IUD placement, than the levonorgestrel 19.5-mg IUD, and the levonorgestrel 19.5-mg IUD placemat was easier when compared with the levonorgestrel 52-mg IUD [Citation50].
Most women who use a low-dose LNG-IUD continue to ovulate [Citation42].
Although the LNG-IUD releases only a small amount of steroid, some women may experience hormone-related effects, such as headaches, nausea, breast tenderness, mood changes, and ovarian cyst formation. The drug-related adverse event that occurs more frequently with 52 mg LNG-IUD is ovarian cyst (> 3 cm) formation, caused by persistent ovarian follicles and generally resolving spontaneously [Citation45,Citation51]. The 52-mg LNG-IUD is used to treat menstrual-related disorders such as menorrhagia and dysmenorrhea, and atypical endometrial hyperplasia [Citation52].
LNG-IUD appears to be a safe and effective contraceptive method for obese women. The system is not associated with an increased risk of VTE and exerts only minimal effects on plasma lipids and glucose metabolism. An advantage might also be the protection of the endometrium in obese women [Citation51].
Contraceptive implants
Progestogen-releasing contraceptive implants are placed subdermally. The ETN subdermal implant is radio-opaque and is easily visualized on X-rays [Citation42]. It consists of an ethylene-vinyl acetate copolymer core that contains 68 mg of ETN surrounded by an ethylene-vinyl acetate copolymer skin and it is approved for use for up to 3 years [Citation53].
The primary mechanism of action of the ETN subdermal implant is suppression of ovulation, additional contraceptive efficacy may be conferred by thickening cervical mucus [Citation42].
The ETN subdermal implant is the most effective method of reversible contraception, with a typical-use pregnancy rate of 0.05% [Citation5]. Fertility returns rapidly after discontinuation, usually after 3–4 weeks from removal [Citation42,Citation54]. A non-contraceptive benefit of the ETN subdermal implant is a significant decrease in dysmenorrhea [Citation55].
After ETN subdermal implant insertion, changes in menstrual bleeding patterns are common and include amenorrhea or infrequent, frequent, or prolonged bleeding [Citation42].
In the event of cycles with luteal activity, there is a consistent percentage of luteinized unruptured follicles among implant users [Citation56].
Complications related to its insertion (1%), such as pain, slight bleeding, hematoma formation, deep or incorrect insertion, unrecognized non-insertion, and removal (1.7%) are uncommon. All healthcare providers who perform implant insertions and removals must receive training. Other complications include gastrointestinal disorders, cephalea, mastodynia, and acne (10–14%) [Citation42].
The limited evidence available is reassuring regarding bone mineral density, a surrogate marker for fracture risk [Citation57].
In the long term, the ETN subdermal implant does not appear to be associated with an increased risk of thrombotic stroke and myocardial infarction [Citation58], but according to WHO medical eligibility criteria, it is contraindicated in women with acute deep VTE, a personal history of severe liver disease, or breast cancer [Citation59].
No daily-hormonal contraception in specific settings
It is relevant to underline that no-daily contraceptives can be attractive alternatives to COC, especially in unique and specific settings where the personalization of the contraceptive method is essential. This benefit is critical for example in adolescents, perimenopausal patients, obese women, patients with intestinal malabsorption or eating disorders, breastfeeding women, and post-voluntary termination of pregnancy ().
Table 4. Recommendations and use of no-daily contraception in specific settings LARC: long-acting reversible contraceptives; LNG-IUD: levonorgestrel-releasing intrauterine system; ETN etonogestrel; EE: ethinylestradiol; IUC: Intrauterine contraception.
Adolescents
In the absence of medical contraindications, all currently available contraceptives are safe and effective for use in adolescents [Citation60]. Non-contraceptive side effects of hormonal contraception, such as improvement in acne, hirsutism, and dysmenorrhea may also play a key role in the decision-making process [Citation61].
Regarding skin patches, adolescents cite cost concerns, skin irritation, and detachment or loosening of the adhesive patch as the most common reasons for discontinuation [Citation62]. For adolescents, comfort using a vaginal product such as tampons, as well as positive feelings and knowledge of reproductive anatomy, is associated with an increased willingness to try a contraceptive vaginal ring [Citation63].
Since adolescents and young women (less than 21 years old) who use SARC have significantly higher contraceptive failure rates than older women [Citation64], LARC appears to be a viable alternative in this category of patients since they require no action on the part of the adolescent after placement, resulting in typical use rates that closely approximate perfect use [Citation65]. Both the American College of Obstetrics and Gynecology (ACOG) and the American Academy of Pediatrics (AAP) recommend LARC use for adolescents, to decrease the rates of unintended adolescent pregnancy and abortion [Citation66]. Adolescents are often nulliparous, evidence suggests that complications such as uterine perforation, ectopic pregnancy, and pelvic inflammatory disease are uncommon in all users, including adolescents and nulliparous women [Citation42]. Analysis of CHOICE study data suggest expulsion rates may be higher in adolescents than in older women, and lower in nulliparous than in parous women. A Cochrane review [Citation67] showed that naproxen 300 mg in two separate doses may decrease pain in the first hours after insertion in nulliparous women. Lidocaine 4% topical gel may lessen pain during IUC insertion and shortly thereafter in nulliparous women, while lidocaine and prilocaine cream, and 1% paracervical block may be effective but they have not been specifically studied for nulliparous women. The wait time between application and intervention for these medications to act ranges from three to seven minutes.
Perimenopause
No method is contraindicated based on age only; however, above 40 years of age, COC is considered MEC category 2 [Citation43], underlining the need for special prescriptive care in this category of patients. In this age group, careful assessment of cardiovascular, metabolic, medical history and lifestyle risks is mandatory. Although in most of these patients, low-dose estrogen can be used.
Contraception with progestin alone may be a viable alternative in smokers and in those individuals having high BMI, diabetes associated with vascular complications, or in the presence of migraine. However, progestin-only contraception poorly controls vasomotor symptoms.
In perimenopausal women with no contraindications, the LNG-IUD with additional estrogen when indicated appears effective for perimenopausal symptoms and long-term benefits associated with the contraceptive effect [Citation4, Citation68]. Vaginal ring can also be a good option for nonsmoking perimenopausal women [Citation69].
Obesity
In obese women, the baseline risk for VTE is a 2–4-fold increase in comparison to normal-weight women, and it increases with age [Citation51]. A Cochrane Review concluded that there is no general evidence of an association between BMI and decreased efficacy with combined hormonal contraception (CHC) [Citation70], but CHC further increases the risk for VTE in obese women. Therefore, these contraceptive methods should only be used if no other acceptable contraceptives are available or acceptable, or if the benefits still outweigh the risks [Citation71].
It appears that in women using the transdermal patch, a weight of > 90 kg appears to be associated with an increased rate of pregnancy [Citation34,Citation71].
There are currently little data on the efficacy of the contraceptive vaginal ring in obese women. In a prospective study on 20 normal weight (BMI 19–24.9) and 20 obese women (BMI 30–39.9) using EE and ETN contraceptive vaginal ring, it appears that contraceptive vaginal ring effectiveness is similar in women with a BMI up to 39.9. The lower serum EE levels in obese women may explain the greater reported bleeding or spotting days [Citation72].
For the ETN subdermal implant, pregnancy rates are similarly low in obese, overweight, and normal-weight [Citation73], though ETN plasma levels in obese women are lower [Citation51]. Thus, even if epidemiologic and clinical data at present do not indicate a decreased efficacy in obese women caution is recommended. As ETN plasma levels decline over time an earlier replacement of the ETN subdermal implant after 24 months may be considered in women with BMI greater than 30 [Citation51]. The ETN subdermal implant has a little and clinically nonrelevant impact on fasting glucose and insulin in obese women [Citation74].
The LNG-IUD is a safe and effective contraceptive method for obese women without contraindications. With the LNG-IUD, LNG plasma levels are lower in obese women in comparison to non-obese, but because of the local effects of this system in the uterine cavity, efficacy should not be compromised. The system is not associated with an increased risk of VTE and exerts only minimal effects on plasma lipids and glucose metabolism. An advantage might also be the strong protection of the endometrium in these women [Citation51].
Eating disorders
No-daily hormonal contraceptive methods avoid the oral route, this being very useful in case of eating disorders, where vomiting and diarrhea may cause the failure of COC [Citation75].
Patients with a history of an eating disorder may have little subcutaneous tissue, which could theoretically increase the risk of deep ETN subdermal implant insertion [Citation76]. Moreover, the implant may be slightly more visible if patients have very thin arms [Citation76].
Intestinal malabsorption
Since no-daily hormonal contraceptive methods avoid the oral route, they can be safely used in case of intestinal malabsorption. Gastrointestinal disorders, such as chronic diarrhea, gastroenteritis, inflammatory bowel disease, and celiac disease, speed up transit or alter absorption (ileostomy or jejunal bypass) and may cause the failure of COC [Citation75]. Also, women who had bariatric surgery should be advised that the effectiveness of COC could be reduced [Citation34], due to the risk of malabsorption [Citation77].
Breastfeeding
For nursing mothers, more than 6 weeks after delivery [Citation43] and postpartum nonbreastfeeding women more than 21 days postpartum, LARC contraceptive methods are categorized as MEC category 1, whereas CHC falls into MEC category 3 or 2 [43,40].
ETN subdermal implant does not interfere with breastfeeding and can be inserted immediately after delivery [Citation43].
Risks for IUC-related events including expulsion, pain, infection, and removals appear similar or lower for breastfeeding women compared with non-breastfeeding women. Uterine perforation is rare; the risk appears 6- to 10-fold higher among breastfeeding compared with non-breastfeeding women [Citation78]. In a prospective cohort study, the significantly increased risk of perforation among breastfeeding women was shown when IUC insertion occurred within 36 weeks (9 months) postpartum but not thereafter [Citation41].
After-voluntary termination of pregnancy
Women who have experienced a voluntary termination of pregnancy are at high risk of repeating unintended pregnancies. Ovulation may resume as early as 10 days after the abortion [Citation79]. LARC is a good option for adolescents, and women who have a higher risk of forgetfulness or in cases of repeated voluntary termination of pregnancy, to reduce the risk of error and failure [Citation65, Citation80].
The IUC and the ETN subdermal implant can be inserted concurrently with the surgical procedure or at the time of the follow-up visit. For individuals undergoing medical abortion with the combination of mifepristone and misoprostol regimen or the misoprostol-only regimen, IUC can be inserted following complete abortion has been established [Citation81]. Women who choose to have an IUC insertion immediately after abortion have higher rates of use compared with those who choose to insert the IUD after a time interval from the voluntary termination of pregnancy [Citation82], and lower rates of repeated abortion than those who choose a non-IUC contraceptive method [Citation83].
The ETN subdermal implant can be inserted at the time of mifepristone administration since ETN released from the ETN subdermal implant does not interfere with the action of mifepristone [Citation81, Citation84]. Unintended pregnancy within six months after abortion appear lower with immediate insertion of the subdermal implant compared with delayed insertion (RR 0.25, 95% CI 0.08 to 0.77). There may be no difference between immediate and delayed insertion on rates of abnormal bleeding at one month after abortion [Citation85].
The option of starting hormonal contraception should be given immediately after the first pill of the medical abortion regimen for individuals undergoing medical abortion who desire hormonal contraception, including contraceptive patch, contraceptive ring, and ETN subdermal implant [Citation81].
Conclusions
In conclusion, no-daily hormonal contraception is a viable non-oral option for a wide range of women, due to its high effectiveness and many different extra-contraceptive benefits. These benefits are relevant to any woman desiring contraception. In addition, several subsets of patients may use no-daily contraception in different stages of their lives, with the possibility of choosing both LARC and SARC. Specific settings for its use are adolescence, perimenopause, obese women, eating disorders or intestinal malabsorption, breastfeeding, and post-voluntary termination of pregnancy. Furthermore, no-daily hormonal contraception limits or avoids the risk of forgetfulness. Each woman should be offered a method depending on her personal history and reproductive life phase using tailored contraceptive counseling based on the biopsychosocial model.
Further research perspectives in this area could address the acceptance of no daily SARC and LARC, especially in the young and adolescent population in regions where these methods have been introduced with more difficulty and delay, such as in southern Europe, and the reasons for this. It might be of interest to evaluate the most effective methods to reduce the main side effects of these contraceptive methods, such as spotting in subdermal implant carriers. In addition, the prevention of bone damage in individuals with eating disorders is a major issue that needs further investigation.
Disclosure statement
Prof Rossella E. Nappi had past financial relationships (lecturer, member of advisory boards and/or consultant) with Boehringer Ingelheim, Ely Lilly, Endoceutics, Merck Sharpe & Dohme, Palatin Technologies, Pfizer Inc, Procter & Gamble Co, TEVA Women’s Health Inc and Zambon SpA. At present, she has ongoing relationship with Astellas, Bayer HealthCare AG, Exceltis, Fidia, Gedeon Richter, HRA Pharma, Novo Nordisk, Organon & Co, Shionogi Limited and Theramex, conflicts of interest are outside the submitted work. Other authors report no conflicts of interest in this work.
Additional information
Funding
References
- United Nations. World family planning 2020. United Nations, 2020. Available from: https://www.un.org/development/desa/pd/news/world-family-planning-2020-highlights.
- Lopez LM, Grimes DA, Gallo MF, et al. Skin patch and vaginal ring versus combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2013;2013(4): 1. PMID: 23633314; PMCID: PMC7154336.
- Bahamondes L, Fernandes A, Monteiro I, et al. Long-acting reversible contraceptive (LARCs) methods. Best Pract Res Clin Obstet Gynaecol. 2020;66:28–8. Epub 2019 Dec 20. PMID: 32014434.
- Santoro N, Teal S, Gavito C, et al. Use of a levonorgestrel-containing intrauterine system with supplemental estrogen improves symptoms in perimenopausal women: a pilot study. Menopause. 2015;22(12):1301–1307. PMID: 26575111.
- Jain J, Jakimiuk AJ, Bode FR, et al. Contraceptive efficacy and safety of DMPA-SC. Contraception. 2004;70(4):269–275. PMID: 15451329.
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397–404. Epub 2011 Mar 12. PMID: 21477680; PMCID: PMC3638209.
- Iacovides S, Avidon I, Baker FC. What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update. 2015;21(6):762–778. Epub 2015 Sep 7. PMID: 26346058.
- Nappi RE, Sances G, Allais G, et al. Effects of an estrogen-free, desogestrel-containing oral contraceptive in women with migraine with aura: a prospective diary-based pilot study. Contraception. 2011;83(3):223–228. Epub 2010 Sep 20. PMID: 21310283.
- Darney P. Safety and efficacy of a triphasic oral contraceptive containing desogestrel: results of three multicenter trials. Contraception. 1993;48(4):323–337. PMID: 8222660.
- Rice C, Killick S, Hickling D, et al. Ovarian activity and vaginal bleeding patterns with a desogestrel-only preparation at three different doses. Hum Reprod. 1996;11(4):737–740. PMID: 8671319.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, et al. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72(3):168–174. PMID: 16102549.
- Roumen FJ, Mishell DR.Jr. The contraceptive vaginal ring, NuvaRing(®), a decade after its introduction. Eur J Contracept Reprod Health Care. 2012;17(6):415–427. Epub 2012 Oct 31. PMID: 23113828.
- Mishell DR, Jr, Lumkin ME. Contraceptive effect of varying dosages of progestogen in silastic vaginal rings. Fertil Steril. 1970;21(2):99–103. PMID: 5414562.
- Alexander NJ, Baker E, Kaptein M, et al. Why consider vaginal drug administration? Fertil Steril. 2004;82(1):1–12. PMID: 15236978.
- Wieder DR, Pattimakiel L. Examining the efficacy, safety, and patient acceptability of the combined contraceptive vaginal ring (NuvaRing). Int J Womens Health. 2010;2:401–409. PMID: 21151688; PMCID: PMC2990910.
- Sabatini R, Cagiano R. Comparison profiles of cycle control, side effects and sexual satisfaction of three hormonal contraceptives. Contraception. 2006;74(3):220–223. Epub 2006 Jun 21. PMID: 16904415.
- Gilliam ML, Neustadt A, Kozloski M, et al. Adherence and acceptability of the contraceptive ring compared with the pill among students: a randomized controlled trial. Obstet Gynecol. 2010;115(3):503–510. PMID: 20177280.
- Stewart FH, Brown BA, Raine TR, et al. Adolescent and young women’s experience with the vaginal ring and oral contraceptive pills. J Pediatr Adolesc Gynecol. 2007;20(6):345–351. PMID: 18082856; PMCID: PMC3163239.
- Carson L, Merkatz R, Martinelli E, et al. The vaginal microbiota, bacterial biofilms and polymeric drug-releasing vaginal rings. Pharmaceutics. 2021;13(5):751. PMID: 34069590; PMCID: PMC8161251.
- De Seta F, Restaino S, De Santo D, et al. Effects of hormonal contraception on vaginal flora. Contraception. 2012;86(5):526–529. Epub 2012 Apr 20. PMID: 22520642.
- De Seta F, Restaino S, Banco R, et al. Effects of estroprogestins containing natural estrogen on vaginal flora. Gynecol Endocrinol. 2014;30(11):830–835. Epub 2014 Jul 4. PMID: 24993504.
- Veres S, Miller L, Burington B. A comparison between the vaginal ring and oral contraceptives. Obstet Gynecol. 2004;104(3):555–563.
- Cagnacci A. Hormonal contraception: venous and arterial disease. Eur J Contracept Reprod Health Care. 2017;22(3):191–199. Epub 2017 Mar 29. PMID: 28351175.
- Duijkers I, Killick S, Bigrigg A, et al. A comparative study on the effects of a contraceptive vaginal ring NuvaRing and an oral contraceptive on carbohydrate metabolism and adrenal and thyroid function. Eur J Contracept Reprod Health Care. 2004;9(3):131–140. PMID: 15697102.
- Cagnacci A, Ferrari S, Tirelli A, et al. Route of administration of contraceptives containing desogestrel/etonorgestrel and insulin sensitivity: a prospective randomized study. Contraception. 2009;80(1):34–39. Epub 2009 Mar 17. PMID: 19501213.
- Cagnacci A, Ferrari S, Tirelli A, et al. Insulin sensitivity and lipid metabolism with oral contraceptives containing chlormadinone acetate or desogestrel: a randomized trial. Contraception. 2009;79(2):111–116.
- Elkind-Hirsch KE, Darensbourg C, Ogden B, et al. Contraceptive vaginal ring use for women has less adverse metabolic effects than an oral contraceptive. Contraception. 2007;76(5):348–356.
- US Food and Drug Administration. Ortho Evra (norelgestromin/ethinyl estradiol) Information. Available from: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/ortho-evra-norelgestrominethinyl-estradiol-information (accessed 05 Feb 2022).
- Abrams LS, Skee DM, Natarajan J, et al. Pharmacokinetics of a contraceptive patch (evra/ortho evra) containing norelgestromin and ethinyloestradiol at four application sites. Br J Clin Pharmacol. 2002;53(2):141–146. PMID: 11851637; PMCID: PMC1874289.
- Urdl W, Apter D, Alperstein A, et al. Contraceptive efficacy, compliance and beyond: factors related to satisfaction with once-weekly transdermal compared with oral contraception. Eur J Obstet Gynecol Reprod Biol. 2005;121(2):202–210.
- Tepper NK, Dragoman MV, Gaffield ME, et al. Nonoral combined hormonal contraceptives and thromboembolism: a systematic review. Contraception. 2017;95(2):130–139.
- Lidegaard O, Nielsen LH, Skovlund CW, et al. Venous thrombosis in users of non-oral hormonal contraception: follow-up study, Denmark 2001-10. BMJ. 2012;344:e2990.
- Dore DD, Norman H, Loughlin J, et al. Extended case-control study results on thromboembolic outcomes among transdermal contraceptive users. Contraception. 2010;81(5):408–413.
- FSRH Guideline Combined Hormonal Contraception, 2019.
- Grimes DA. Forgettable contraception. Contraception. 2009;80(6):497–499.
- Hillard PJ. Menstrual suppression with the levonorgestrel intrauterine system in girls with developmental delay. J PediatrAdolesc Gynecol. 2012;25:308–313.
- Kirkham YA, Allen L, Kives S, et al. Trends in menstrual concerns and suppression in adolescents with developmental disabilities. J Adolesc Health. 2013;53(3):407–412.
- Secura GM, Allsworth JE, Madden T, et al. The contraceptive CHOICE project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203(2):115.e1–115.e7. Auge1-7. Epub 2010 Jun 11. PMID: 20541171; PMCID: PMC2910826.
- The United States Medical Eligibility Criteria for Contraceptive Use, 2016. (US MEC); Available from: https://www.cdc.gov/reproductivehealth/contraception/mmwr/mec/appendixk.html#personal.
- Trussell J, Hassan F, Lowin J, et al. Achieving cost-neutrality with long-acting reversible contraceptive methods. Contraception. 2015;91(1):49–56.
- Heinemann K, Reed S, Moehner S, et al. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91(4):274–279.
- Committee on Practice Bulletins-Gynecology, Long-Acting Reversible Contraception Work Group. Practice bulletin no. 186: long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130(5):e251–e269. PMID: 29064972.
- Medical eligibility criteria for contraceptive use. 5th ed. Geneva: World Health Organization; 2015. PMID: 26447268.
- Ortiz ME, Croxatto HB. Copper-T intrauterine device and levonorgestrel intrauterine system: biological bases of their mechanism of action. Contraception. 2007;75(6 Suppl):S16–S30. Epub 2007 Mar 29. PMID: 17531610.
- Gemzell-Danielsson K, Schellschmidt I, Apter D. A randomized, phase II study describing the efficacy, bleeding profile, and safety of two low-dose levonorgestrel-releasing intrauterine contraceptive systems and mirena. Fertil Steril. 2012;97(3):616–622. e1-3. Epub 2012 Jan 4. PMID: 22222193.
- Lethaby A, Hussain M, Rishworth JR, et al. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2020;6(6):CD002126. Update in: cochrane Database Syst Rev. 2020 Jun 12;6:CD002126. PMID: 25924648.
- Magalhaes J, Ferreira-Filho ES, Soares-Junior JM, et al. Uterine volume, menstrual patterns, and contraceptive outcomes in users of the levonorgestrel-releasing intrauterine system: a cohort study with a five-year follow-up. Eur J Obstet Gynecol Reprod Biol. 2022;276:56–62. Epub 2022 Jul 2. PMID: 35809459.
- Chelmow D. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116(6):1455–1456. PMID: 21099621.
- Gemzell-Danielsson K, Apter D, Hauck B, et al. The effect of age, parity and body mass index on the efficacy, safety, placement and user satisfaction associated with two Low-Dose levonorgestrel intrauterine contraceptive systems: subgroup analyses of data from a phase III trial. PLoS One. 2015;10(9):e0135309. PMID: 26378938; PMCID: PMC4574776.
- Anjos FCQS, Marcelino AC, Espejo-Arce X, et al. Pain and ease of insertion of three different intrauterine devices in Brazilian adolescents: a participant-blinded randomized trial. Contraception. 2023;122:109997. Epub ahead of print. PMID: 36841463.
- Merki-Feld GS, Skouby S, Serfaty D, et al. European society of contraception statement on contraception in obese women. Eur J Contracept Reprod Health Care. 2015;20(1):19–28. Epub 2014 Nov 7. PMID: 25380138.
- Burke AE. The state of hormonal contraception today: benefits and risks of hormonal contraceptives: progestin-only contraceptives. Am J Obstet Gynecol. 2011;205(4 Suppl):S14–S7. Epub 2011 Apr 29. PMID: 21961819.
- Le J, Tsourounis C. Implanon: a critical review. Ann Pharmacother. 2001;35(3):329–336.
- Darney P, Patel A, Rosen K, et al. Safety and efficacy of a single-rod etonogestrel implant (implanon): results from 11 international clinical trials. Fertil Steril. 2009;91(5):1646–1653.
- Mansour D, Korver T, Marintcheva-Petrova M, et al. The effects of implanon on menstrual bleeding patterns. Eur J Contracept Reprod Health Care. 2008;13(Suppl 1):13–28. Jun PMID: 18330814.
- Alvarez F, Brache V, Faundes A, et al. Ultrasonographic and endocrine evaluation of ovarian function among norplant implants users with regular menses. Contraception. 1996;54(5):275–279. PMID: 8934060.
- Modesto W, Dal Ava N, Monteiro I, et al. Body composition and bone mineral density in users of the etonogestrel-releasing contraceptive implant. Arch Gynecol Obstet. 2015;292(6):1387–1391. Dec Epub 2015 Jun 19. PMID: 26088190.
- Lidegaard Ø, Løkkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366(24):2257–2266. PMID: 22693997.
- Medical Eligibility Criteria for Implants|Family Planning. Available from: https://www.fphandbook.org/medical-eligibilitycriteria-implants. (accessed on 8 November 2020)
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(3):1–103.
- Bahamondes L, Valeria Bahamondes M, Shulman LP. Non-contraceptive benefits of hormonal and intrauterine reversible contraceptive methods. Hum Reprod Update. 2015;21(5):640–651. Epub 2015 Jun 1. PMID: 26037216.
- Logsdon S, Richards J, Omar HA. Long-term evaluation of the use of the transdermal contraceptive patch in adolescents. ScientificWorldJournal. 2004;4:512–516.
- Carey AS, Chiappetta L, Tremont K, et al. The contraceptive vaginal ring: female adolescents’ knowledge, attitudes and plans for use. Contraception. 2007;76(6):444–450.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366(21):1998–2007.
- Committee on Adolescent Health Care Long-Acting Reversible Contraception Working Group, The American College of Obstetricians and Gynecologists. Committee opinion no. 539: adolescents and long-acting reversible contraception: implants and intrauterine devices. ObstetGynecol. 2012;120(04):983–988.
- Raidoo S, Pearlman Shapiro M, Kaneshiro B. Contraception in adolescents. Semin Reprod Med. 2022;40(01/02):089–097. Epub ahead of print. PMID: 34500476.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;2015(7):CD007373. PMID: 26222246; PMCID: PMC9580985
- Guerin J, Engelmann A, Mattamana M, et al. Use of hormonal contraceptives in perimenopause: a systematic review. Pharmacotherapy. 2022;42(2):154–164.
- Ballagh SA. Vaginal rings for menopausal symptom relief. Drugs Aging. 2004;21(12):757–766. PMID: 15382956.
- Lopez LM, Grimes DA, Chen M, et al. Hormonal contraceptives for contraception in overweight or obese women. Cochrane Database Syst Rev. 2013;4:CD008452.
- Zieman M, Guillebaud J, Weisberg E, et al. Contraceptive efficacy and cycle control with the ortho evra/evra transdermal system: the analysis of pooled data. Fertil Steril. 2002;77(2 Suppl 2):S13–S18.
- Westhoff CL, Torgal AH, Mayeda ER, et al. Pharmacokinetics and ovarian suppression during use of a contraceptive vaginal ring in normal-weight and obese women. Am J Obstet Gynecol. 2012;207(1):39.e1–39.e6. Epub 2012 Apr 28. Erratum in: am J Obstet Gynecol. 2013 Apr;208(4):326. PMID: 22727346; PMCID: PMC3403702.
- Xu H, Wade JA, Peipert JF, et al. Contraceptive failure rates of etonogestrel subdermal implants in overweight and obese women. Obstet Gynecol. 2012;120(1):21–26.
- Vicente L, Mendonca D, Dingle M, et al. Etonogestrel implant in women with diabetes mellitus. Eur J Contracept Reprod Health Care. 2008;13(4):387–395.
- Hanker JP. Gastrointestinal disease and oral contraception. Am J Obstet Gynecol. 1990;163(6 Pt 2):2204–2207.
- FSRH CEU Statement: Contraception for women with eating disorders. 15th June 2018 (updated 10th May 2021).
- Paulen ME, Zapata LB, Cansino C, et al. Contraceptive use among women with a history of bariatric surgery: a systematic review. Contraception. 2010;82(1):86–94. Epub 2010 Mar 29. PMID: 20682146.
- Berry-Bibee EN, Tepper NK, Jatlaoui TC, et al. The safety of intrauterine devices in breastfeeding women: a systematic review. Contraception. 2016;94(6):725–738. Epub 2016 Jul 13. PMID: 27421765.
- Lahteenmaki P, Luukkainen T. Return of ovarian function after abortion. Clin Endocrinol (Oxf). 1978;8(2):123–132.
- National Institute for Health and Care Excellence (NICE). Long acting reversible contraception (update) (NICE Clinical Guideline 30), 2014. Available from: www.rcog.org.uk.
- Medical management of abortion. Geneva: World Health Organization; 2018. PMID: 30702834.
- Fox MC, Oat-Judge J, Severson K, et al. Immediate placement of intrauterine devices after first and second trimester pregnancy termination. Contraception. 2011;83(1):34–40.
- Goodman S, Hendlish SK, Reeves MF, et al. Impact of immediate postabortal insertion of intrauterine contraception on repeat abortion. Contraception. 2008;78(2):143–148.
- Roe AH, Bartz D. Contraception after surgical and medical abortion: a review. Obstet Gynecol Surv. 2017;72(8):487–493.
- Sothornwit J, Eamudomkarn N, Lumbiganon P, et al. Immediate versus delayed postabortal insertion of contraceptive implant. Cochrane Database Syst Rev. 2022;5(5):CD013565. PMID: 35583092; PMCID: PMC9115762.