?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Polycystic ovary syndrome (PCOS) is a common endocrine disorder that usually begins during adolescence. Patients may have severe metabolic disorders.
Objective
To investigate the levels of visfatin and apelin in adolescent girls with PCOS and to explore the importance of visfatin and apelin in glucose and lipid metabolism.
Methods
A total of 88 girls (aged 12–20 years) were prospectively and consecutively recruited during two years for the PCOS group (n = 44) and the control group (n = 44). Serum visfatin, apelin and other metabolic parameters were measured. Receiver operator characteristics (ROC) curve analysis was performed to reveal the diagnostic potential.
Results
Visfatin, apelin and indicators of glucose and lipid-metabolism were not different for PCOS patients compared to control. However, insulin resistance (IR) in the PCOS-group was more frequent (p < 0.05). Visfatin in non-IR patients was higher than in IR-patients in the PCOS-group (p < 0.05). However, there was no difference in apelin levels between IR and non-IR patients in the PCOS-group (p > 0.05). ROC-curve analyses demonstrated that the optimal value of visfatin for predicting IR in PCOS-patients was 7.14 ng/mL, with 78.1% sensitivity and 68.7% specificity. In the PCOS-group, visfatin was positively correlated with high density lipoprotein cholesterol (HDL-C), and negatively correlated with HOMA-IR, apolipoprotein B (Apo-B), cholesterol (CHO), low density lipoprotein cholesterol (LDL-C) and CHO/HDL-C ratio (p < 0.05). Apelin had no correlation with all indices (p > 0.05).
Conclusions
Higher visfatin levels may prevent IR in adolescent PCOS patients, showing a positive predictive value for IR and also reflecting a beneficial effect on lipids. It is a possible protective factor at certain stages of metabolic syndrome.
Introduction
The polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders. Prevalence of PCOS increases up to 30% in adolescents [Citation1] and has been associated with various reproductive and metabolic abnormalities. The etiology is not yet clear, the pathogenesis is complex and related to environmental factors (especially nutrition), and the disease can begin in early adolescence [Citation2]. Previous studies have shown that the proportion of metabolic phenotypes in adolescent PCOS patients is generally increased. In one study, the odds ratio (OR) of PCOS patients developing the metabolic syndrome was 4.26 compared with the healthy adolescent control group [Citation3]. Insulin resistance (IR) is one of the most important factors for metabolic abnormalities in patients with PCOS [Citation4]. Adipose tissue is important for energy reserve and is closely related to PCOS. Adipocytokines can participate in glucose and lipid metabolism and are therefore being increasingly investigated in studies related to PCOS.
Visfatin is a protein cytokine that is highly expressed in the adipose tissue and closely related to glucose and lipid metabolism [Citation5]. Visfatin affects glucose homeostasis by acting on islet B cells and regulating genes related to oxidative stress, inflammatory response and circadian rhythm [Citation6]. Apelin, a polypeptide with multiple biological activities produced by adipocytes, mainly increases the uptake of glucose by adipose tissue and skeletal muscle. In addition, it can also play an insulin-like hypoglycemic role [Citation7]. At present, there is great controversy regarding visfatin and apelin level in PCOS. They can not only play an insulin-like hypoglycemic role, but can also promote inflammation, which further can aggravate IR [Citation5]. It is therefore unclear whether visfatin and apelin play a beneficial or pathogenic role in the regulation of glucose and lipid metabolism in PCOS patients.
Adolescence is a critical period in the pathophysiology of PCOS. Therefore, the purpose of this study was: (1) to investigate visfatin and apelin levels in adolescent girls with PCOS in comparison to age-matched girls without PCOS, and (2) to explore the function of visfatin and apelin in glucose and lipid metabolism in adolescent PCOS patients.
Methods
Subjects
We prospectively enrolled 88 girls aged 12–20 years consecutively attending the Department of Gynecological Endocrinology, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, China, between July 2018 and January 2020. The study included 44 girls in the PCOS group and 44 otherwise healthy age-matched girls in the control group. As diagnostic criteria for PCOS, we used the guidelines issued by the ‘Endocrinology Group and Guidelines Expert Group of the Obstetrics and Gynecology Branch of the Chinese Medical Association’, which are modified Rotterdam’s diagnostic criteria, based on research considering genetic and other differences of Chinese women compared to women in the Western world [Citation8]. Briefly, the inclusion criteria for our study were: (1) more than 2 years after menarche; (2) oligomenorrhoea for at least 2 years; (3) hyperandrogenism; (4) polycystic ovaries based on ultrasound. The requirements for the control-group were girls who were in need of physical examination, e.g. due to dysmenorrhea, with menarche for more than 2 years, regular menstruation (menstrual cycle 21–35 days), no signs of hyperandrogenism, no acanthosis nigricans, and normal basic endocrine parameters. Excluded from the study were girls with systemic or psychiatric disease, and/or taking any medications that can impact glucose or lipid metabolism (e.g. contraceptive pills, metformin, anti-epileptics, antipsychotics, statins, or fish oil).
All patients gave their informed consent. Minors had their informed consent forms signed by guardians instead. The study was approved by the ethics committee of Beijing Obstetrics and Gynecology Hospital, Capital Medical University (Protocol number 2018-KY-011-01).
Clinical and laboratory assessments
Age, menstrual characteristic pattern, height and weight were recorded. Blood samples were collected after an overnight fasting of at least 8 h on days 2–4 of the menstrual cycle. Serum was separated by centrifugation and stored at −20 °C. Fasting blood glucose (FBG), fasting insulin (FINS), triglyceride (TG), cholesterol (CHO), high density lipoprotein-cholesterol (HDL-C), low density lipoprotein-cholesterol (LDL-C), apolipoprotein A1 (Apo-A1), apolipoprotein B (Apo-B) were measured by Synchron LX-20 (Beckman Coulter, USA). CHO/HDL-C and TG/HDL-C ratios were calculated. Visfatin and apelin levels were measured by competitive enzyme-linked immunosorbent assay (Phoenix, USA). Total testosterone (TT) and free testosterone (FT) were measured by liquid chromatography-tandem mass spectrometry based methods (Sciex, USA; Nexera, Japan). The homeostasis model assessment insulin resistance (HOMA-IR) index was calculated by multiplying FBG (mmol/L) by FINS (mIU/L) and dividing the product by 22.5.
Diagnostic and inclusion criteria
Referring to the large sample study of adolescent IR in China [Citation9], patients with a HOMA-IR value greater than 3 were diagnosed with IR.
Study design, sample size calculation and statistical analysis
The main outcome of this study was to determine the differences in visfatin and apelin levels between the adolescent PCOS group and the control group. For this aim we used a ‘Case-control study’ design. We used the following formula to calculate the minimal sample size for this type of study:
i.e. the minimal sample size should be 13, referring to previous similar research with
=1.48% and
=1.42 [Citation10]. In order to prevent data gaps, we finally choose to include 44 patients in each group. The statistical significance level was set at 5% (α = 0.05) using a two-sided test Zα = 1.96, 1 − β = 0.8.
SPSS 22.0 package program was used for statistical analysis. The quantitative variables that were normally distributed are presented as mean ± standard deviations (SD), and values not normally distributed are presented as percentiles 25 and 75 (p25–p75). The mean differences between the case and control-groups were compared using t-test. The Mann-Whitney U test was used for continuous variables that did not show normal distribution. Visfatin and apelin levels were analyzed using Receiver Operating Characteristics (ROC) curve, and sensitivity and specificity were calculated for the diagnosis of IR in PCOS.
Results
Characteristics
There were no significant differences regarding age, visfatin and apelin levels and indicators of glucose and lipid metabolism between PCOS patients and the control group. However, the PCOS group had significantly higher BMI and a higher proportion of patients with IR (p = 0.002 and p = 0.024, respectively). Likewise, in the PCOS group, TT and FT were both significantly higher (p < 0.001) ().
Table 1. Characteristics of studied patients: PCOS and controls.
Adipose factors comparing IR and non-IR patients
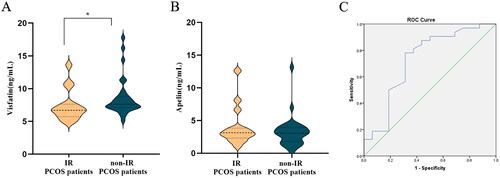
In the PCOS group, those without IR displayed significantly higher visfatin levels when compared to those with IR (p < 0.05). However, there was no significant difference in the level of apelin between IR and non-IR patients in the PCOS group (p > 0.05) (A and 1B). In contrast, in the control group, there was no difference in the levels of visfatin and apelin between IR and non-IR patients (p > 0.05).
Diagnostic value of visfatin
ROC-curve analysis was used to evaluate the predictive ability of visfatin to diagnose IR in adolescent PCOS patients. Since, ROC-area under the curve is 0.729, visfatin indeed has a diagnostic value. The optimal value of visfatin for predicting IR in PCOS patients in our study is 7.14 ng/mL, with 78.1% sensitivity and 68.7% specificity (). Youden Index is 0.469.
Relationship between visfatin and apelin levels and indices of glucose and lipid metabolism
In the PCOS group, visfatin was significantly positively correlated with HDL-C, and negatively correlated with HOMA-IR, Apo-B, CHO, LDL-C, and CHO/HDL-C ratio values (p < 0.05). Apelin had no correlation with all indices (p > 0.05). In the control group, visfatin had no correlation with any index (p > 0.05). Apelin was significantly positively correlated with HDL-C levels (p < 0.05) ().
Table 2. Relationship between visfatin and apelin levels and indices of glucose and lipid metabolism.
Discussion
This study compared various metabolic indicators in Chinese adolescent PCOS patients and healthy adolescent girls. It was found that visfatin and apelin levels did not differ between PCOS group and controls. We also found that visfatin may be to some extent a predictive factor for IR, with higher levels reflecting a beneficial effect and lipids; hence it may be a protective factor in the development of the metabolic syndrome in adolescent PCOS patients.
In previous studies, scholars tried to explore the relationship between visfatin and PCOS, but the results of various studies were contradictory. Some researchers have concluded that the level of visfatin in patients with PCOS is significantly higher than in the controls [Citation11,Citation12]. However, other studies have also found that visfatin levels in PCOS patients were not different from controls [Citation13–15]. Regarding adolescent PCOS patients, fewer reports have been published, and these are inconsistent [Citation16,Citation17]. In our study, there was no significant difference in visfatin between adolescent PCOS patients and controls.
In terms of visfatin, it can reduce blood glucose levels and increase insulin sensitivity, which is important in the context of diabetes [Citation18]. On the other hand, visfatin can also promote inflammation and immune regulation. High concentrations of visfatin can cause immune cells to gather, causing chronic inflammation of fat cells, thus affecting the metabolic balance [Citation5]. We found a significant negative correlation between visfatin and HOMA-IR. Visfatin levels in the non-IR PCOS group was significantly higher than in the IR PCOS group. Our study was aimed at adolescent PCOS patients, who are in the early stage of metabolic abnormalities, so visfatin may reflect a protective effect in terms of IR. In addition, through ROC curve analysis we found that visfatin can predict IR. The best cutoff value for predicting IR was 7.14 ng/mL, with a sensitivity of 78.1% and specificity of 68.7%. Adolescent girls may have a physiological IR, but we found that the difference of visfatin between IR- and non-IR patients only exists in PCOS patients; which suggests that visfatin may be an effective predictor of whether IR occurs in adolescent PCOS patients.
Among PCOS patients it was observed that visfatin had a significant positive correlation with HDL-C, and a significant negative correlation with Apo-B, CHO, LDL-C. These correlations were not found in the control-group. Wang et al. [Citation19] also found that visfatin was positively correlated with HDL-C and negatively correlated with LDL-C and TG. Visfatin is involved in the biosynthesis of nicotinamide adenine dinucleotide (NAD). The change in HDL-C is significantly related to the concentration of NAD in cells. The beneficial state of high HDL-C and low CHO levels in the circulation may be related to the increase in NAD levels. Therefore, it may be suggested that visfatin levels reflect the beneficial lipid mass spectrum in the circulation. Similarly, Smith et al. [Citation20] reported that non-fasting visfatin levels in Asian Indians were positively correlated with HDL-C and Apo-A1. In addition, in our study, there was a significant negative correlation between visfatin and CHO/HDL-C ratio in adolescent PCOS patients. At present, the role of visfatin in atherosclerosis is still controversial. The mainstream theory is that visfatin plays different roles inside and outside the cell, promoting atherosclerosis in the cell, and protecting atherosclerosis outside [Citation21]. Our study focused on adolescent PCOS patients, who are still at the beginning of the disease. Their visfatin levels are negatively correlated with the lipid-ratio, indicating that visfatin may be a protective factor for the occurrence of cardiovascular and cerebrovascular diseases.
In terms of apelin, current research conclusions differ greatly regarding its relationship to PCOS. Some studies have found significantly increased apelin levels in PCOS patients [Citation22,Citation23]. Contrary to this, Chang et al. [Citation24] and Altinkaya et al. [Citation25] have found apelin levels in PCOS patients were lower when compared to controls. However, Benk et al. [Citation26] and Olszanecka Glinianowicz et al. [Citation27] found no differences in apelin levels between PCOS patients and controls. In our study, there was no difference in apelin levels between adolescent PCOS patients and controls. Recently, it has been suggested that apelin can enhance insulin sensitivity and improve insulin resistance [Citation28]. In addition, it has been shown that apelin can regulate lipid metabolism by up-regulating PI3K/Akt signal pathway, and play a role in reducing CHO-concentrations and increasing HDL-C levels [Citation29]. We found that apelin was only positively correlated with HDL-C in the control-group. In our PCOS patients, there was no difference in apelin with or without IR, and there was no correlation between apelin and various relevant PCOS indicators. This may be due to the small sample size of our study, or a minor role of apelin in the glucose and lipid metabolism in PCOS. There may also be differences due to race, age, genetic characteristics, eating habits, etc., which need further exploration in the future.
Strengths and limitations
Our study has several strengths. First, we found that visfatin may be a potential adipocyte factor preventing the occurrence of IR in adolescent PCOS patients, and to a certain extent, it had a predictive value for IR. Furthermore, it can reflect the level of beneficial lipids, and has a potential protective effect on atherosclerosis. It may be a protective factor in certain stages of the development of the metabolic syndrome among PCOS patients, thus providing a new target for clinical prevention and treatment. Patients and control groups were consecutively recruited within a defined time-span, and the controls were age-matched. All patients were diagnosed by the same gynecological endocrinologist.
The weakness of this study is its small sample size, but it is consistent with the sample size calculation. Furthermore, it must be emphasized that as with every ROC-curve analysis, the cutoff value is restricted to the subgroup of investigated disease in the study, here adolescent girls.
Conclusion
The adipocyte factor visfatin has an insulin-like effect and may play an important role in preventing IR in adolescent PCOS-patients. It has a certain clinical predictive value for IR, and can regulate lipid-metabolism and reflect the level of beneficial lipids in the circulation. The negative correlation with the lipid-ratio indicates that it may be a protective factor in the development of a metabolic syndrome during PCOS. Visfatin is expected to become a new target for studying the pathogenesis and to achieve early prevention of the metabolic syndrome in PCOS patients which has started during adolescence. However, according to the conflicting results of previous studies, the relationship between apelin and PCOS still remains unclear; hence, there is a need for future research in this regard.
Acknowledgment
The authors especially thank Prof. Xingming Li of Capital Medical University (Beijing, China) for his assistance in data statistical analysis.
Disclosure statement
All authors declare that they have no conflicts of interest.
Additional information
Funding
References
- Bouzas IC, Cader SA, Leão L, et al. Menstrual cycle alterations during adolescence: early expression of metabolic syndrome and polycystic ovary syndrome. J Pediatr Adolesc Gynecol. 2014;27(6):1–5.
- Ruan X, Li M, Mueck AO. Why does polycystic ovary syndrome (PCOS) need long-term management? Curr Pharm Des. 2018;24(39):4685–4692.
- Bhattacharya SM, Jha A. Prevalence and risk of metabolic syndrome in adolescent Indian girls with polycystic ovary syndrome using the 2009 ‘joint interim criteria’. J Obstet Gynaecol Res. 2011;37(10):1303–1307.
- Chen W, Pang Y. Metabolic syndrome and PCOS: pathogenesis and the role of metabolites. Metabolites. 2021;11(12):869.
- Kumari B, Yadav UCS. Adipokine visfatin’s role in pathogenesis of diabesity and related metabolic derangements. Curr Mol Med. 2018;18(2):116–125.
- Radzicka S, Pietryga M, Iciek R, et al. The role of visfatin in pathogenesis of gestational diabetes (GDM). Ginekol Pol. 2018;89(9):518–521.
- Zhu S, Sun F, Li W, et al. Apelin stimulates glucose uptake through the PI3K/akt pathway and improves insulin resistance in 3T3-L1 adipocytes. Mol Cell Biochem. 2011;353(1–2):305–313.
- Endocrinology Group and Guidelines Expert Group of the Chinese Medical Association Obstetrics and Gynecology Branch. Guidelines for diagnosis and treatment of polycystic ovary syndrome in China. Chin J Obstet Gynecol. 2018;53:2–6.
- Yin J, Li M, Xu L, et al. Insulin resistance determined by homeostasis model assessment (HOMA) and associations with metabolic syndrome among Chinese children and teenagers. Diabetol Metab Syndr. 2013;5(1):71.
- Garruti G, de Palo R, Rotelli MT, et al. Association between follicular fluid leptin and serum insulin levels in non-overweight women with polycystic ovary syndrome. Biomed Res Int. 2014;2014:980429.
- Gen R, Akbay E, Muslu N, et al. Plasma visfatin level in lean women with PCOS: relation to proinflammatory markers and insulin resistance. Gynecol Endocrinol. 2009;25(4):241–245.
- Sun Y, Wu Z, Wei L, et al. High-visfatin levels in women with polycystic ovary syndrome: evidence from a meta-analysis. Gynecol Endocrinol. 2015;31(10):808–814.
- Farshchian F, Ramezani Tehrani F, Amirrasouli H, et al. Visfatin and resistin serum levels in normal-weight and obese women with polycystic ovary syndrome. Int J Endocrinol Metab. 2014;12(3):e15503.
- Kamińska A, Kopczyńska E, Bronisz A, et al. An evaluation of visfatin levels in obese subjects. Endokrynol Pol. 2010;61(2):169–173.
- Kim JJ, Choi YM, Hong MA, et al. Serum visfatin levels in non-obese women with polycystic ovary syndrome and matched controls. Obstet Gynecol Sci. 2018;61(2):253–260.
- Cekmez F, Cekmez Y, Pirgon O, et al. Evaluation of new adipocytokines and insulin resistance in adolescents with polycystic ovary syndrome. Eur Cytokine Netw. 2011;22(1):32–37.
- Gümüş Ü, Güzel AI, Topcu HO, et al. Plasma visfatin levels in adolescents with polycystic ovary syndrome: a prospective case-control study. J Pediatr Adolesc Gynecol. 2015;28(4):249–253.
- Asadi S, Goodarzi MT, Saidijam M, et al. Resveratrol attenuates visfatin and vaspin genes expression in adipose tissue of rats with type 2 diabetes. Iran J Basic Med Sci. 2015;18(6):537–543.
- Wang P, van Greevenbroek MM, Bouwman FG, et al. The circulating PBEF/NAMPT/visfatin level is associated with a beneficial blood lipid profile. Pflugers Arch. 2007;454(6):971–976.
- Smith J, Al-Amri M, Sniderman A, et al. Visfatin concentration in Asian Indians is correlated with high density lipoprotein cholesterol and apolipoprotein A1. Clin Endocrinol (Oxf). 2006;65(5):667–672.
- Martínez Larrad MT, Corbatón Anchuelo A, Fernández Pérez CM; Segovia Insulin Resistance Study Group (SIRSG), et al. Obesity and cardiovascular risk: variations in visfatin gene can modify the obesity associated cardiovascular risk. Results from the segovia population Based-Study. Spain. PLoS One. 2016;11(5):e0153976.
- Gören K, Sağsöz N, Noyan V, et al. Plasma apelin levels in patients with polycystic ovary syndrome. J Turk Ger Gynecol Assoc. 2012;13(1):27–31.
- Hasan MM, Abd El Hameed AA. Serum adipokine (apelin) in lean and obese polycystic ovary syndrome patients before and after metformin treatment. Middle East Fertility Society Journal. 2018;23(4):315–318.
- Chang CY, Tsai YC, Lee CH, et al. Lower serum apelin levels in women with polycystic ovary syndrome. Fertil Steril. 2011;95(8):2520–2523.e1-2.
- Altinkaya SÖ, Nergiz S, Küçük M, et al. Apelin levels in relation with hormonal and metabolic profile in patients with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2014;176:168–172.
- Benk Silfeler D, Gokce C, Keskin Kurt R, et al. Does polycystic ovary syndrome itself have additional effect on apelin levels? Obstet Gynecol Int. 2014;2014:536896.
- Olszanecka-Glinianowicz M, Madej P, Nylec M, et al. Circulating apelin level in relation to nutritional status in polycystic ovary syndrome and its association with metabolic and hormonal disturbances. Clin Endocrinol (Oxf). 2013;79(2):238–242.
- Kolahdouzi S, Baghadam M, Kani-Golzar FA, et al. Progressive circuit resistance training improves inflammatory biomarkers and insulin resistance in obese men. Physiol Behav. 2019;205:15–21.
- Zheng XD, Huang Y, Li H. Regulatory role of apelin-13-mediated PI3K/AKT signaling pathway in the glucose and lipid metabolism of mouse with gestational diabetes mellitus. Immunobiology. 2021;226(5):152135.